Abstract
Aims: The study sought to compare the acute gain and two-year follow-up late lumen loss (LLL) between the Absorb bioresorbable vascular scaffold (BVS) and the analogous everolimus-eluting metallic stent (EES).
Methods and results: The current analysis included all the patients recruited in the ABSORB Cohort B and SPIRIT II trials implanted with a single 3.0×18 mm device (Absorb BVS or EES) who underwent serial angiographic examinations at baseline and at two-year follow-up. The acute gain was defined as the difference between post- and preprocedural minimal lumen diameter (MLD). The in-stent/scaffold LLL was calculated as the difference in stent/scaffold segment between the post-procedural MLD and follow-up MLD. Thirty-three patients (33 lesions) implanted with the Absorb BVS, and 26 patients (28 lesions) implanted with the EES were studied. The acute gain was similar in the Absorb BVS group (1.23±0.38 mm) compared to the EES group (1.32±0.26 mm, p=0.29). The in-stent/scaffold LLL at two-year follow-up in the Absorb BVS group (0.26±0.19 mm) was also similar compared to the EES group (0.22±0.22 mm, p=0.29). Although the two groups had similar two-year clinical outcomes (major adverse cardiac events: Absorb BVS: 6.1% vs. EES: 0.0%), patients treated with the Absorb BVS exhibited a significantly lower two-year in-stent/scaffold MLD compared to the EES (2.02±0.26 mm vs. 2.22±0.34 mm, p=0.01).
Conclusions: Although BVS and EES demonstrated similar two-year clinical outcomes, patients treated with the Absorb BVS exhibited a significantly lower two-year in-stent/scaffold MLD compared to patients treated with the EES. Appropriately powered randomised trials are necessary to confirm these exploratory results and evaluate their prognostic and clinical significance.
Introduction
Coronary metallic stents have been widely used as the standard endoluminal device for the treatment of patients with coronary artery disease (CAD)1. The introduction of drug-eluting stents (DES) has markedly reduced the risk of restenosis, particularly in complex anatomical cases and high-risk patients2. Recent advances in stent technology, with the introduction of biocompatible or biodegradable polymers, have minimised the risk of complications, in particular stent thrombosis, evident with the first-generation DES3,4. Despite these improvements, newer-generation DES have not managed to address all the limitations of coronary stents, such as the risk of neoatherosclerosis, preclusion of late lumen enlargement and the lack of reactive vasomotion5. Furthermore, the risk of stent thrombosis and its clinical sequelae, although substantially reduced in incidence with newer-generation DES, still remains.
Bioresorbable scaffolds were introduced to overcome the abovementioned drawbacks, as they potentially have the ability to restore the patency of the vessel and then gradually disappear, thus allowing the artery to maintain its physiological integrity and responsiveness6. Today, more than 14 bioresorbable scaffolds are either being investigated in clinical trials or undergoing preclinical evaluation7. Three biodegradable scaffolds currently have Conformité Européenne (CE) mark approval: the Igaki-Tamai® (Kyoto Medical Planning Co., Ltd, Kyoto, Japan) scaffold for the treatment of peripheral vascular disease, the DESolve® scaffold (Elixir Medical Corporation, Sunnyvale, CA, USA), and the Absorb bioresorbable vascular scaffold (BVS; Abbott Vascular, Santa Clara, CA, USA) for the treatment of CAD. The latter consists of a poly-L-lactic acid (PLLA) backbone, coated with a bioresorbable polymer that contains and controls the release of the antiproliferative drug everolimus8. The differential mechanical properties of PLLA, compared to conventional metallic stents, are likely to affect device expansion and recoil during implantation. Furthermore, a higher acute gain post device implantation, and a relatively lower late lumen loss (LLL) at follow-up, have been shown to be predictors of a more favourable long-term prognosis9,10. The purpose of this study was to compare the post device implantation acute gain, and the two-year LLL, in patients treated with the Absorb BVS and the analogous metallic platform drug-eluting stent (XIENCE V; Abbott Vascular, Santa Clara, CA, USA).
Methods
STUDY DESIGN AND POPULATION
The present study represents a pooled patient analysis of the ABSORB Cohort B and the SPIRIT II clinical trials, recruiting patients treated with a single 3.0×18 mm device, who underwent angiographic examination at baseline and at two-year follow-up. The patients recruited in this analysis had different baseline and angiographic characteristics, and therefore a further analysis was conducted after matching the two populations for the following variables: diabetes mellitus, reference vessel diameter (RVD), preprocedural minimal lumen diameter (MLD), and lesion length.
ABSORB COHORT B
The ABSORB Cohort B (a clinical evaluation of the bioresorbable everolimus-eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions) trial is a non-randomised, multicentre, single-arm study (ClinicalTrials.gov NCT00856856). All patients over the age of 18 years with evidence of myocardial ischaemia were suitable for inclusion. The treated lesions were native de novo stenoses with an estimated diameter of 3.0 mm, a length <14 mm, and a percentage diameter stenosis (DS) of >50% and <100%. One hundred and one patients (102 lesions) treated with an Absorb BVS device were enrolled and divided into two groups (Cohort B1 and B2). Patients enrolled in Cohort B1 (n=45) underwent quantitative coronary angiography (QCA) and intravascular ultrasound (IVUS) examinations at baseline, six months and two years. Patients enrolled in Cohort B2 (n=56) underwent the same invasive tests, at baseline, one year, and three years (study ongoing).
SPIRIT II
SPIRIT II was a multicentre, randomised two-arm trial, which recruited 300 patients with de novo lesions (ClinicalTrials.gov NCT00180310). Study patients were randomised in a 3:1 ratio to either everolimus-eluting (EES, n=233) or paclitaxel-eluting (n=77) stents11. The study had similar clinical inclusion criteria to the ABSORB Cohort B trial. The treated de novo lesions had an RVD 2.25-4.25 mm, and a percentage DS >50% and <100%. Angiographic follow-up was performed in all patients at six months. One hundred and fifty-two patients (113 patients in EES group) underwent angiographic and/or IVUS examinations at two years.
QUANTITATIVE CORONARY ANALYSIS ASSESSMENT
QCA analyses were undertaken in corresponding end-diastolic angiographic frames acquired pre and post device implantation, and at two-year follow-up12. The following measurements were obtained: lesion length, MLD, RVD derived by an interpolated method, in-stent/scaffold acute gain, LLL, net gain, percentage DS and minimum lumen area (MLA). The in-stent/scaffold acute gain was defined as the difference between pre- and post-procedural MLD. The LLL was defined as the difference between post-procedural and follow-up MLD. The net gain was defined as the sum of the offsetting effects of the acute gain and LLL. Two different MLA metrics, based on video-densitometry (VID) and edge-detection (ED) techniques, were analysed13,14. The basic principles of the video-densitometric technique have been described previously14. This technique is a non-geometric approach to the analysis of coronary angiography, based on the relationship between the attenuating power of the lumen filled with contrast medium and the x-ray image intensifier. An absolute reference for densitometric area is calculated using the diameter measurements obtained from the edge-detection technique, assuming circular vessel geometry in a user-defined reference segment outside the stenosis.
INTRAVASCULAR ULTRASOUND GREYSCALE ANALYSIS
IVUS examinations were undertaken post-procedurally and at two-year follow-up, either with a mechanical (Atlantis; Boston Scientific, Natick, MA, USA) or a phased array (Eagle Eye; Volcano Corp., Rancho Cordova, CA, USA) catheter12. The following metrics were calculated: mean lumen area, minimum lumen area (MLA), mean scaffold/stent area, mean neointimal hyperplasia (NIH) area, and mean vessel area. In addition, the “projected MLD” was used to assess the lumen diameter, estimated by rotating the object representing the lumen around the centre of gravity three times in 30° steps. For each rotation, the projection distances on the x and y axes were calculated and compared with the previous diameter15.
CLINICAL ENDPOINTS
Composite ischaemia-driven major adverse cardiac events (MACE) included cardiac death, any myocardial infarction (MI), and ischaemia-driven target lesion revascularisation (ID-TLR). The ID-TLR was defined as a QCA percentage DS of ≥50%, with symptoms of ischaemia, or DS ≥70% at the time of scheduled or unscheduled angiography. An event was classified as non-Q-wave MI only if there was an elevation of creatinine kinase (CK) levels ≥2 times the upper limit of normal, with an elevated CK-MB. All events were adjudicated by an independent clinical events committee.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviation (SD). Binary variables are presented as counts and percentages. The p-value was calculated using the Mann-Whitney U test (two-sided) if the variable was continuous. Fisher’s exact test was used if the variable was categorical. Matching was processed at the lesion level using the optimal method minimising the overall distance. The distance between cases and controls, Dij, is defined as the weighted sum of the absolute differences between the case and control matching factors, i.e., where Wk=the weight assigned to matching factor k and Xik=the value of variable X(k) for subject i.
![]()
Variables used for the matching were standardised variables and equally weighted for this analysis. Statistical significance was assumed at p<0.05. All statistical analyses were performed with SAS 9.1.3 (SAS Institute Inc., Cary, NC, USA).
Results
BASELINE CHARACTERISTICS
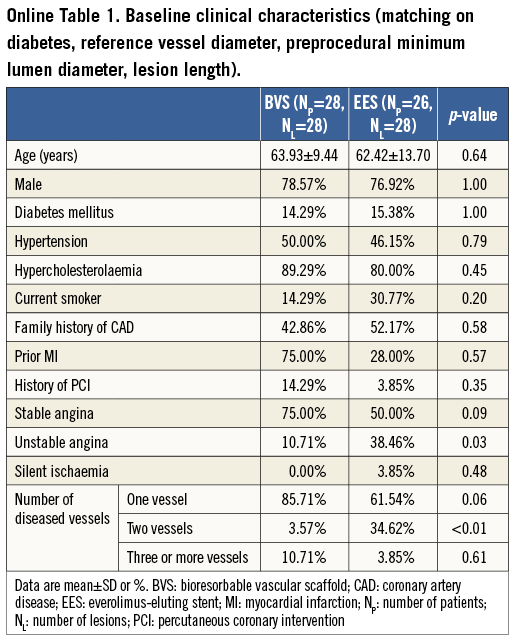
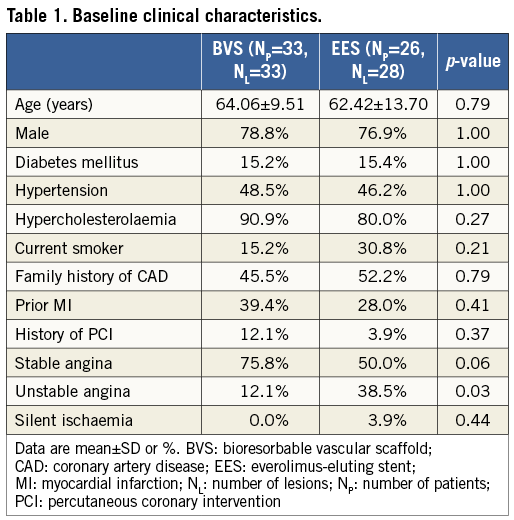
A flow chart summarising patient selection is shown in Figure 1. In total, 59 patients (61 lesions) were included in the final analysis. Thirty-three patients (33 lesions) were implanted with 3.0×18 mm Absorb BVS, and 26 patients (28 lesions) with 3.0×18 mm EES. No significant differences in a history of diabetes mellitus (p=1.00), hypertension (p=1.00), or hypercholesterolaemia (p=0.27) were evident between the two groups. Patients treated with an EES were more likely to have two-vessel CAD (p=0.01) and to have presented with unstable angina (p=0.03) (Table 1).
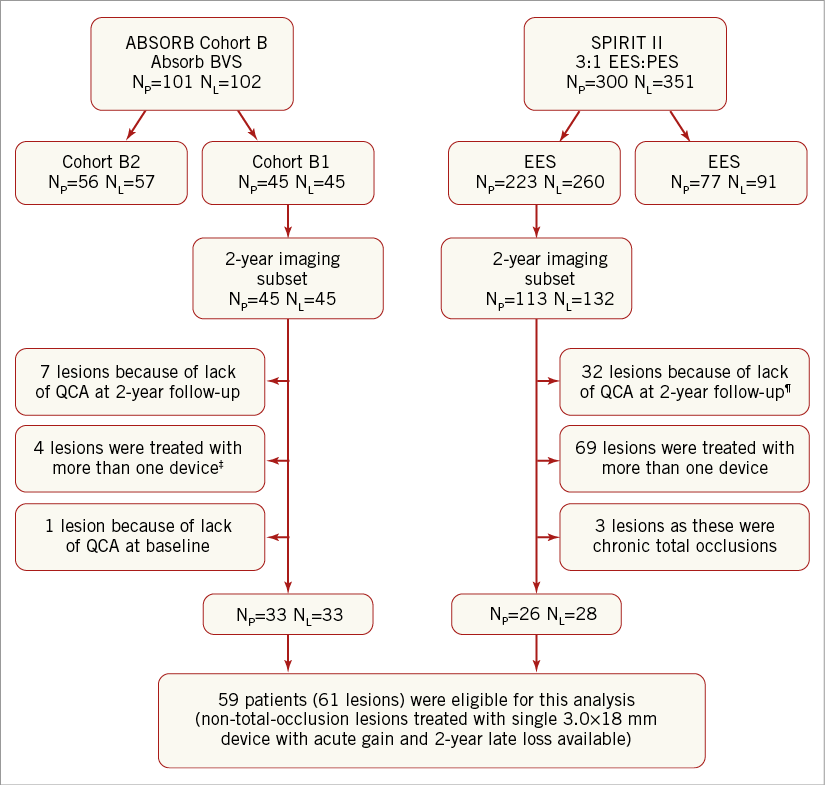
Figure 1. Flow chart of patient selection. 4 patients refused, 2 patients were not suitable for angiographic follow-up while in 1 case post-nitrate angiographic images were not available for QCA analysis. ¶18 patients (21 lesions) refused follow-up coronary angiography, 2 patients were deemed unsuitable for invasive follow-up, 5 were withdrawn from the study, 3 died from non-cardiac death and in 1 case the QCA was not feasible. ‡3 patients had additional bail-out stenting and 1 patient was not included, who received 1 non-study stent in the target vessel during procedure. BVS: bioresorbable vascular scaffold; EES: everolimus-eluting stent; NL: number of lesions; NP: number of patients; PES: paclitaxel-eluting stent; QCA: quantitative coronary angiography
ANGIOGRAPHIC CHARACTERISTICS
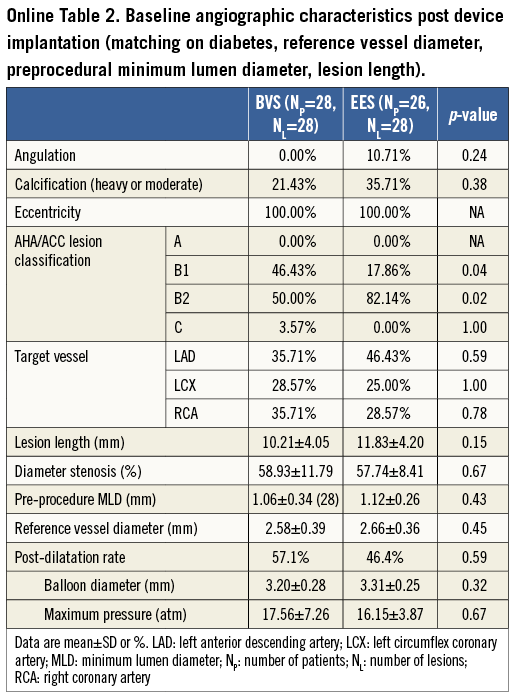
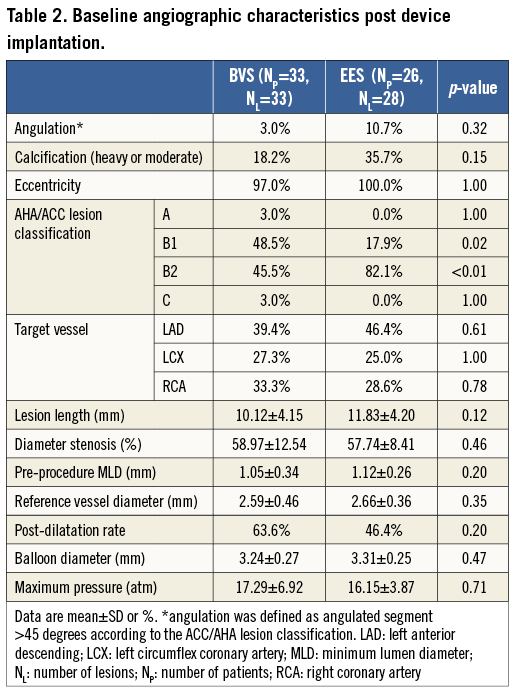
The preprocedural angiographic characteristics were similar in the BVS and EES groups (Table 2). The majority of treated stenoses were type B lesions (AHA/ACC classification, BVS: 94.0%, EES: 100.0%)16. No statistically significant differences were evident between the BVS and EES groups for DS, MLD and RVD. The percentage of post-dilatation (63.64% vs. 46.43%, p=0.20), and the maximum pressure of post-dilation (17.29±6.92 atm vs. 16.15±3.87 atm, p=0.71) were comparable between the BVS and EES groups.
ACUTE GAIN, LLL AND OTHER ANGIOGRAPHIC MEASUREMENTS
The results of the QCA analysis are shown in Table 3. There was a similar acute gain in the BVS group (BVS: 1.23±0.38 mm, EES: 1.32±0.26 mm, p=0.29) (Figure 2). The post-procedural in-stent/scaffold MLD in the BVS group was similar compared to the EES group (BVS: 2.28±0.27 mm, EES: 2.44±0.26 mm, p=0.02). The two-year in-stent/scaffold LLL in the Absorb BVS group was similar compared to the EES group (BVS: 0.26±0.19 mm vs. EES: 0.22±0.22 mm, p=0.29). However, the two-year in-stent/scaffold MLD was significantly lower in the Absorb BVS compared to the EES (2.02±0.26 mm vs. 2.22±0.34 mm, p=0.02). Notably, the reported net gain did not differ statistically between the two groups at two-year follow-up (0.97±0.40 mm vs. 1.10±0.35 mm, p=0.22).
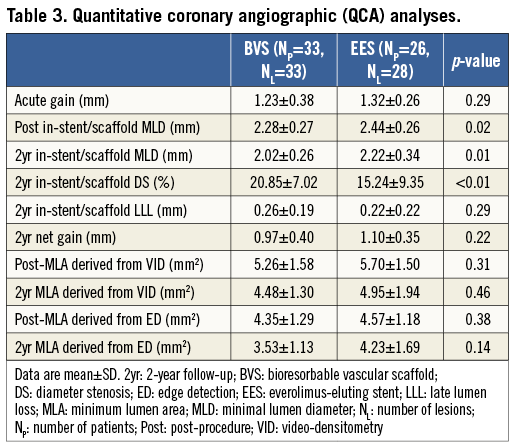
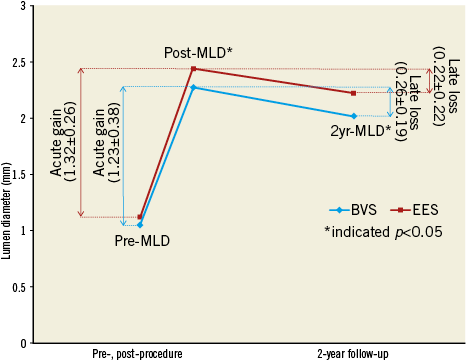
Figure 2. Schematic representation of the QCA-derived measurements pre- and post-procedure, and at two-year follow-up. BVS: bioresorbable vascular scaffold; EES: everolimus-eluting stent; MLD: minimum lumen diameter; Post: post-procedure; Pre: pre-procedure
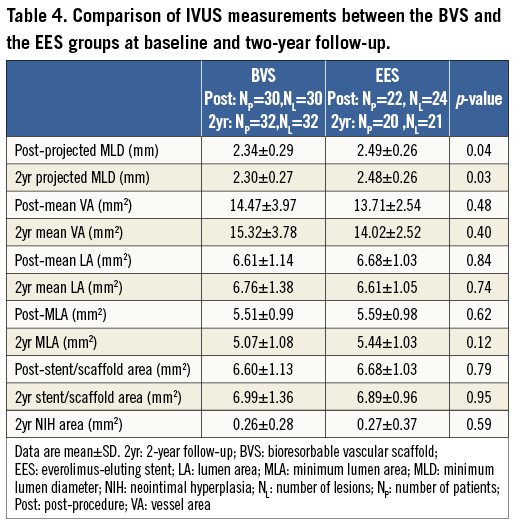
INTRAVASCULAR ULTRASOUND ANALYSIS
Thirty patients (30 lesions) from the Absorb BVS group and 22 patients (24 lesions) from the EES group had IVUS examinations post device implantation. IVUS assessments were undertaken in 32 patients (32 lesions) treated with an Absorb BVS, and in 20 patients (21 lesions) implanted with an EES, at two-year follow-up. The mean vessel area, mean lumen area, MLA, and stent/scaffold areas, post procedure and at follow-up were similar between the two groups (Figure 3, Table 4). In addition, there were no significant differences between the two groups in the mean NIH area at follow-up (0.26±0.28 mm2 vs. 0.27±0.37 mm2, p=0.59). The IVUS-derived “projected MLD” was significantly lower in the BVS group compared to the EES group (post procedure: 2.34±0.29 mm vs. 2.49±0.26 mm, p=0.04; two-year follow-up: 2.30±0.27 mm vs. 2.48±0.26 mm, p=0.03, respectively).
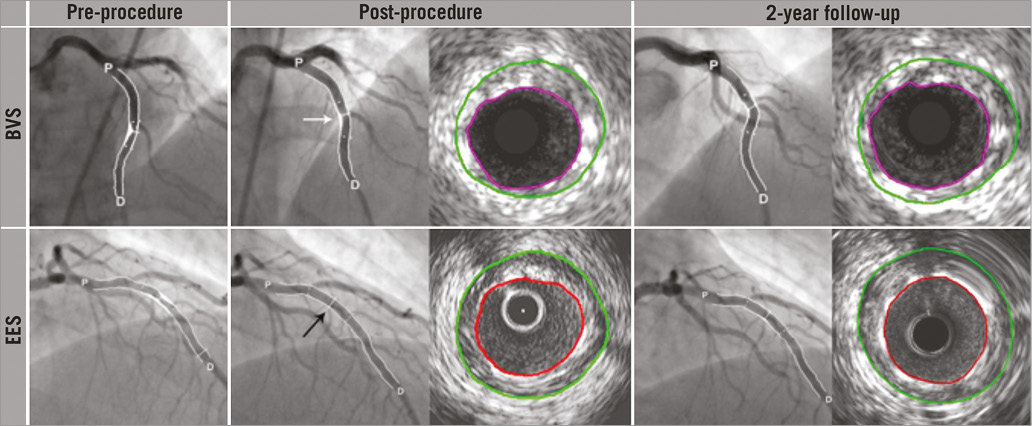
Figure 3. Serial cineangiograms pre-procedurally, post-procedurally and at two-year follow-up, for the Absorb BVS and the EES. From left to right in the upper panels, coronary angiograms of a mid-left anterior descending (LAD) coronary lesion treated with Absorb BVS, pre-procedurally, post-procedurally, and at 2-year follow-up, and corresponding IVUS cross-sectional views for the projected MLD. Post-procedurally, the scaffold was insufficiently expanded (white arrow) and consequently associated with a lower acute gain. In the lower panels, from left to right, angiogram of a mid-LAD coronary lesion treated with an EES, pre-procedurally, post-procedurally, and at 2-year follow-up, and corresponding IVUS cross-sectional views for the projected MLD. Post-procedurally, the EES was well expanded with a consequent greater acute gain (black arrow). BVS: bioresorbable vascular scaffold; D: distal; EES: everolimus-eluting stent; IVUS: intravascular ultrasound; P: proximal; QCA: quantitative coronary angiography
FURTHER ANALYSIS AFTER MATCHING
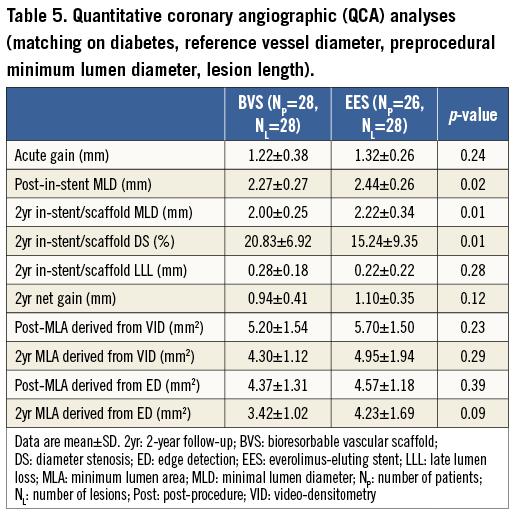
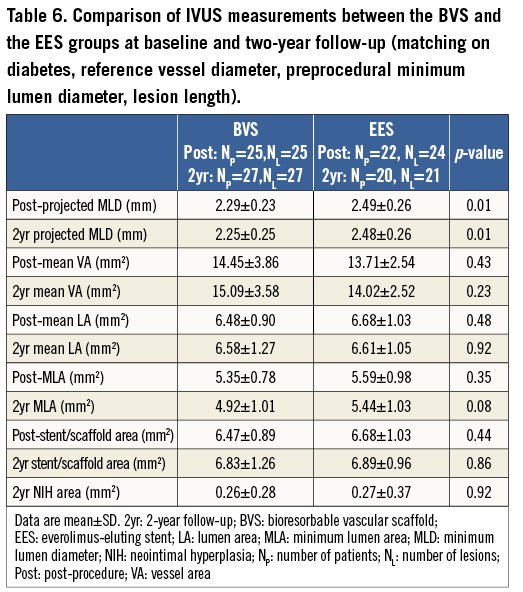
After matching, 28 patients with 28 lesions in the BVS group, and 26 patients with 28 lesions in the EES group were analysed. The main results of the QCA and IVUS analyses are shown in Table 5 and Table 6. The evaluation of the acute gain and LLL by QCA showed a trend in the same direction as seen in the entire population. At two-year follow-up, angiographic in-stent/scaffold MLD by QCA and projected MLD by IVUS remained statistically lower in the BVS group than in the EES group (p=0.01, p=0.01, respectively).
CLINICAL OUTCOMES
During two-year follow-up, two patients developed ID-TLR in the Absorb group. One patient had in-scaffold restenosis (ISR) at 168 days of follow-up, showing a Type 1B ISR at the proximal edge of the scaffold (QCA MLD: 0.89 mm, %DS: 63.5%, in-scaffold LLL: 0.50 mm); the other patient had ISR at 383 days of follow-up, also showing a Type 1B ISR in the segment proximal to the scaffold (QCA MLD: 0.90 mm, %DS: 67.0%, in-scaffold LLL: 1.20 mm). Two patients in the Absorb BVS group but no patients in the EES group experienced non-Q-wave MI at two-year follow-up. No death was reported in either the Absorb BVS or the EES group.
Discussion
The main findings of the study are that: 1) there was similar acute gain in patients implanted with an Absorb BVS, but the difference between the two groups did not reach statistical significance; 2) the two-year follow-up in-stent/scaffold LLL in the Absorb BVS was also similar compared to the EES; 3) although the two groups had similar two-year clinical outcomes, patients treated with the Absorb BVS exhibited a significantly lower two-year in-stent/scaffold MLD compared to the EES.
COMPARISON OF ACUTE GAIN BETWEEN THE ABSORB BVS AND EES
QCA has been extensively used to study the performance of new endovascular treatments. The immediate benefit of a coronary intervention, as expressed by the acute gain, has historically been used as a surrogate endpoint to compare plain old balloon angioplasty, rotational atherectomy, and bare metal stents (BMS)17. In the BMS era, it was shown that increased acute gain and a larger post-procedural MLD were predictors of a more favourable prognosis, thus suggesting device post-dilation to be of significant clinical value18. This effect appeared to be maintained in the DES era9. Specifically, in a substudy of the STLLR (Stent deployment Techniques on clinical outcomes of patients treated with the Cypher™ stent) trial, it was demonstrated that a lower acute gain, after implantation of a sirolimus-eluting stent, was the only predictor of TLR9.
In the present study, the acute gain associated with the Absorb BVS was demonstrated to be numerically lower compared to the EES. However, this difference did not reach statistical significance, probably secondary to the relatively small number of patients. Conversely, bench studies have demonstrated that the two devices have comparable radial strength, and exhibit a similar acute recoil post implantation19. The numerically higher acute gain associated with the EES may be secondary to the higher size of post-dilation balloon during device deployment, which is not recommended in the Absorb BVS because of the limited distensibility of the Absorb BVS, and the risk of inducing acute strut fracture if dilated beyond its design limits19. However, the percentage of post-dilatation used and maximum pressure of post-dilatation in the BVS group are higher than seen in the EES group, though they did not differ significantly between the Absorb BVS and EES groups (Table 2). In addition, the different strut thicknesses of these two devices may also influence the acute gain; the advent of optical coherence tomography with a high resolution imaging technique is expected to provide a precise assessment of this parameter.
COMPARISON OF LLL BETWEEN THE ABSORB BVS AND EES
The in-stent/scaffold LLL constitutes a traditional measure to evaluate in-stent/scaffold restenosis, and to examine the long-term efficacy of DES20. Numerous studies have shown that LLL is a predictor of future cardiovascular events, and it has been used as a surrogate endpoint to compare different devices10. In permanent metallic stent platforms, the LLL is solely due to neointimal proliferation, and thus the LLL provides an indirect angiographic evaluation of the vessel wall response to the endovascular device21. In contrast, the LLL in the Absorb BVS depends not only on neointimal formation, but also on the late scaffold expansion, which may potentially occur as early as one year post device implantation22. Although the current analysis demonstrated no differences between the LLL in the EES and the Absorb BVS, the patients implanted with an Absorb BVS had a smaller MLD at two-year follow-up. This finding may be attributed to the smaller MLD reported post-Absorb BVS implantation.
COMPARISONS OF THE MEASUREMENTS DERIVED FROM QCA AND IVUS
Notably, the significant differences in the QCA-derived MLD between the BVS and EES groups were confirmed by the IVUS-derived projected MLDs post procedure and at two-year follow-up. Moreover, the MLA derived from two different QCA methods (VID and ED) demonstrated consistent, non-significant differences between the QCA and IVUS analyses post procedure and at two years. Several reasons may be involved in this paradox between the MLA and MLD in the two groups. It should be noted that a few patients did not undergo IVUS examinations post-procedurally (three in the Absorb BVS and four in the EES group) and at two-year follow-up (one in the Absorb BVS and seven in the EES group). Furthermore, the two devices have different mechanical properties (e.g., conformability and flexibility) which potentially affected their expansion pattern, with the Absorb BVS shown to have a more eccentric expansion when compared to the metallic EES23. An asymmetric expansion of the Absorb BVS is likely to have been underestimated by the QCA, since this MLD is based on the analysis of two-dimensional images. Moreover, different patterns of restenosis were possibly involved after implantation of an Absorb BVS or an EES, something which should be investigated in the near future24. Therefore, it may be argued that, in patients treated with an Absorb BVS, the operator should assess the results of QCA with caution and, in cases of scaffold underexpansion, should consider further evaluation of its deployment, either with intravascular imaging modalities or with three-dimensional QCA, before attempting post-dilatation.
Clinical relevance
Traditionally, metallic stent implantation is associated with an increased incidence of post-procedural chest pain (PPCP) compared with angioplasty25. The majority of PPCP after stenting is identified as non-ischaemic and argued to be benign26. However, a study from Kini et al showed that non-ischaemic PPCP was probably induced by micromyonecrosis and vessel stretching after stent implantation, and patients with PPCP had a significantly higher restenosis rate compared with no PPCP at nine-month follow-up27. The aetiology of non-ischaemic PPCP has been attributed to oversizing stents and high inflation pressures. A higher stent-to-vessel ratio together with a raised inflation pressure may achieve a larger post-procedure MLD, but also results in irritation of sensory nerves located in the adventitia, causes deep adventitia injury, and increases the incidence of PPCP28. In our study, the patients had a significantly lower post-procedure MLD in the Absorb BVS group than that seen in the XIENCE V group (2.28±0.27 mm vs. 2.44±0.26 mm, p=0.02), which could be accounted for in the Absorb BVS group by less continuous stretching of the treated vessel segment. However, the exact prevalence of PPCP after Absorb BVS implantation and its correlation with clinical events at follow-up require further investigation.
Recently, the concept of a “bioresorbable scaffold” has been highlighted29. The feasibility of this concept was proven in the early 1990s and, over the last 20 years, a considerable effort has been made to develop new fully bioresorbable scaffolds6. Strikingly however, as previously discussed, the reported numerically lower acute gain and higher late loss in the Absorb BVS group potentially resulted in a significantly different MLD post procedure and at two-year follow-up compared to the analogous metallic drug-eluting stent. The findings of this observational study were hypothesis-generating and initiated prior to a randomised trial which is necessary to confirm these exploratory results as well as to evaluate their prognostic and clinical significance.
Limitations
A considerable limitation of the present study is that the Absorb BVS and EES groups had different baseline characteristics, which may have affected the reported outcomes. In order to address this pitfall, we conducted a separate analysis after matching patients (Online Table 1, Online Table 2), based on the history of diabetes and on angiographic variables including RVD, pre-procedure MLD and lesion length. The repeated analysis demonstrated similar results. Most of the stenoses treated by the two devices were simple lesions and hence the reported findings cannot be extrapolated to the general population. A further precise assessment regarding QCA-derived variables between these two devices, to be conducted by adjusting procedural and lesion characteristics, is warranted. Moreover, further evaluation is also required in larger populations and in complex lesions, in order to examine the differences in the QCA measurements between the two devices and their prognostic implications.
Conclusions
Patients implanted with an Absorb BVS appear to exhibit similar acute gain and in-stent/scaffold LLL compared to those treated with the analogous EES. Although the two groups had similar two-year clinical outcomes, patients treated with the Absorb BVS exhibited a significantly lower two-year in-stent/scaffold MLD compared to the EES group. Appropriately powered randomised trials are required to assess the reported results and to evaluate the impact of QCA-derived metrics on clinical outcomes.
| Impact on daily practice A higher acute gain post-stent implantation, and a relatively lower late lumen loss (LLL) at follow-up have been shown to be predictors of a more favourable long-term prognosis. The comparable acute gain and two-year LLL between the Absorb BVS and EES are for the first time reported in this study. However, the numerically lower acute gain and higher late loss in the Absorb BVS group potentially resulted in a significantly different MLD post-procedure and at two-year follow-up, compared with the EES group. |
Guest Editor
This paper was guest edited by Andreas Baumbach, MD, Division of Cardiology, Bristol Heart Institute, Bristol, United Kingdom.
Funding
This study was sponsored by Abbott Vascular, Santa Clara, CA, USA.
Conflict of interest statement
The authors have no conflicts of interest to declare. The Guest Editor has received research support from Abbott Vascular.
Online data supplement