Abstract
Aims: The clinical outcome of patients treated with the everolimus-eluting bioresorbable vascular scaffold (Absorb BVS) in the ABSORB Cohort A and B studies using mandatory intravascular ultrasound (IVUS) imaging showed encouraging results. The ABSORB EXTEND study aimed to include longer lesions, allow overlap and did not oblige IVUS imaging. We assessed the procedural and short-term clinical outcomes in a cohort including these extended criteria.
Methods and results: Patients included in three study cohorts (ABSORB Cohort A, Cohort B and EXTEND) at two centres in Rotterdam were systematically followed for major adverse cardiac events (MACE). Clinical data were obtained for 88 patients (mean age 61.2 years, 73% male) with a total of 92 lesions. Lesion length was significantly longer in the ABSORB EXTEND cohort 11.34±4.01 mm (9.20±2.66 mm; p<0.01) and the reference vessel diameter was smaller 2.53±0.34 mm (2.87±0.38 mm; p<0.001) compared to previous cohorts. Predilatation was performed with a balloon diameter of 2.5±0.3 mm and inflated to a maximum pressure of 12.6±3.2 atm. The scaffold was successfully implanted in 90 of the 92 lesions (97.8%) with a maximum pressure of 14.1±2.8 atm. Post-dilatation was performed in 55% of the patients (53% EXTEND vs. 56% Cohort A and B; p=0.7). The acute gain was 1.21±0.37 mm. Absolute recoil was 0.16±0.20 mm with percentage acute recoil of 5.60±6.60%. At one month, none of the patients had a MACE.
Conclusions: This study, which constitutes the largest combined study cohort of patients treated with the Absorb BVS in Rotterdam, shows that treatment of longer lesions and smaller vessels without obligatory IVUS use is safe and efficacious at one month.
Introduction
The implantation of permanent metallic stents in the coronary arteries has been associated with the inability of lumen remodelling, impairment of endothelial function, limitation of appropriate evaluation by non-invasive imaging modalities, restriction of coronary revascularisation options and, most of all, the occurrence of (late) stent thrombosis1-3.
The first generation of fully bioresorbable everolimus-eluting vascular scaffolds (Absorb BVS; Abbott Vascular, Santa Clara, CA, USA) was investigated in the ABSORB Cohort A study3. This Absorb BVS, first-generation device, consisting of out-of-phase circumferential zig-zag poly-L-lactic acid struts (150 μm thickness) with a poly-D-L-lactide acid coating containing everolimus (98 μg/cm2 of surface area), appeared to be safe and efficacious in a total of 30 patients. However, at invasive six-month follow-up, in-scaffold luminal late loss was not as good as newer-generation metallic stents, most likely caused by increased late recoil and a fast resorption process of the Absorb BVS. Changes in the manufacturing process and scaffold design were applied to the second-generation Absorb BVS to reduce scaffold recoil, which resulted in an improvement of late luminal loss from 0.44 mm to 0.19 mm at six months in the ABSORB Cohort B study4.
Until now, data on the procedural and short-term clinical outcome of the Absorb BVS are scarce and based on small studies. Moreover, the ABSORB Cohort A and B studies used stringent inclusion criteria, including mandatory intravascular ultrasound (IVUS) imaging. The ABSORB EXTEND study aimed to include longer lesions, allow overlap and did not oblige IVUS imaging. The aim of our study was to assess the procedural and short-term clinical outcomes in a cohort including these extended criteria in the largest combined study cohort to date by pooling individual patient data from two centres in Rotterdam.
Methods
STUDY DESIGN, DEFINITIONS AND ENDPOINTS
The B-SEARCH registry was established to evaluate the safety and efficacy of the bioresorbable scaffolds (Absorb BVS). It was designed as a dynamic registry to conform with our previous registries including RESEARCH, T-SEARCH and X-SEARCH5-7. A second enrolling centre in Rotterdam has been included in the current registry.
Patients treated with an Absorb BVS between March 2006 and November 2011 in the Thoraxcenter Rotterdam and the Maasstad Ziekenhuis Rotterdam (from the ABSORB Cohort A, Cohort B and EXTEND trials) were enrolled in this study. The study design of these trials is described elsewhere3,4,8. Briefly, the ABSORB Cohort A and B trials were single-arm, prospective, open-label studies. All patients had (un)stable angina or silent ischaemia with single de novo lesions (max lesion length 8 mm; ABSORB Cohort A) or with a maximum of two de novo lesions (max lesion length 14 mm; ABSORB Cohort B) in a native coronary artery with a maximum diameter of 3.0 mm by visual estimate (recommended range of the vessel diameter ≥2.5 mm and ≤3.3 mm). Acute MI (and the presence of thrombus), unstable arrhythmias, left ventricular ejection fraction less than 30%, restenotic lesions, left main lesions, lesions involving a side branch more than 2 mm in diameter were major exclusion criteria. All patients enrolled in the ABSORB EXTEND had target lesion(s) with a length of ≤28 mm and were treated with 2.5×18 mm, 3.0×18 mm or 3.0×28 mm scaffolds where overlap was allowed, in a maximum of two de novo native coronary artery lesions each located in different epicardial vessels.
Device success was defined as successful delivery and deployment of the scaffold at the intended target lesion with a residual stenosis of less than 50% of the target lesion by quantitative coronary angiography. Acute absolute recoil was defined as the difference between mean diameter of the last inflated balloon at the highest pressure (X) and mean lumen diameter of the stent immediately after the last balloon deflation (Y). Acute percent recoil was defined as (X-Y)/X and expressed as a percentage.
The clinical endpoints assessed at one month included ischaemia-driven major adverse cardiac events (ID-MACE) and the individual components, namely cardiac death, any myocardial infarction or ischaemia-driven target lesion revascularisation (TLR) for a diameter stenosis of ≥50%, based on quantitative coronary angiography, either with symptoms or ischaemia or with a diameter stenosis of ≥70% at the time of (un)scheduled angiography. The elevation of the CK levels >2 times the upper limit of normal and elevated CK-MB were required for the diagnosis of non-Q-wave MI. Other endpoints included non-ischaemia-driven TLR, any revascularisation procedure and scaffold thrombosis. All angiograms were analysed by an independent core laboratory (Cardialysis BV, Rotterdam, The Netherlands).
STATISTICAL ANALYSIS
Baseline and procedural variables are presented as mean ± standard deviation (SD) for continuous variables and as percentages for categorical variables. The Student’s t-test was used to compare the unadjusted association between continuous variables. All statistical tests were performed with SPSS for Windows version 15 (SPSS Inc., Chicago, IL, USA).
Results
POPULATION CHARACTERISTICS
The baseline characteristics of the patients are shown in Table 1. Eighty-eight patients were treated with an Absorb BVS for the treatment of a total of 92 de novo native coronary lesions. A total of 16 first-generation everolimus-eluting Absorb BVS and 76 second-generation Absorb BVS were implanted. The patients were on average 61 years old and 73% of the population were male. Most patients were treated because of stable angina (82%) and the majority of the lesions involved the left anterior descending coronary artery (46%), followed by the right coronary artery (29%) and left circumflex coronary artery (25%). More than half of the treated lesions (63%) were ACC lesion type B1.
PROCEDURAL AND CLINICAL OUTCOMES
The procedural characteristics of the lesions are shown in Table 2. The QCA analysis showed that the mean reference vessel diameter was 2.67±0.39 mm with a minimum luminal diameter of 1.05±0.28 mm. The reference vessel diameter of patients in the ABSORB EXTEND (2.53±0.34 mm) cohort was significantly smaller than ABSORB Cohort A and B (2.87±0.38 mm; p<0.001) and lesions were longer (11.34±4.01 mm vs. 9.20±2.66 mm; p<0.01).
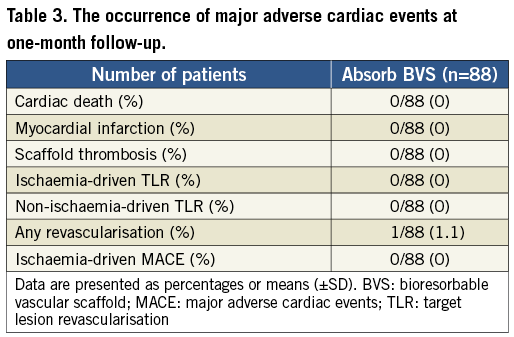
A total of 102 predilatations were performed in 92 coronary lesions. The mean diameter of the largest balloon diameter predilatation was 2.5±0.3 mm, with a mean longest balloon length of 13.3±3.1 mm and a mean maximum pressure of 12.6±3.2 atm, whereas the scaffold was implanted with a maximum pressure of 14.1±2.8 atm. The scaffold was successfully implanted in 90 of the 92 lesions (97.8%). In one case, the intended lesion in a distal left circumflex (LCx) coronary artery could not be reached due to tortuosity and calcification of the vessel. Thereafter, the Absorb BVS 1.0 was partially dislodged and implanted in the proximal circumflex coronary artery. The intended lesion was treated with three overlapping CYPHER® stents (Cordis Corporation, Johnson & Johnson, Warren, NJ, USA) (Figure 1). In the second case, two Absorb BVS did not cross the proximal right coronary artery due to severe calcification. The lesion was treated with three overlapping XIENCE PRIME stents (Abbott Vascular, Santa Clara, CA, USA) (Figure 2) delivered with deep intubation utilising a GuideLiner V2 catheter (Vascular Solutions, Minneapolis, MN, USA). A total of 48 patients (55%) had a post-dilatation, 11 of whom had two or more inflations (53% EXTEND vs. 56% Cohort A and B; p=0.7). The acute gain was 1.21 mm and the diameter stenosis improved from 60.5% to 16.9%. The mean lumen diameter absolute recoil was 0.16±0.20 mm with a percent acute recoil of 5.60±6.60% in patients included in the BVS A and B cohorts. Intravascular ultrasound data showed a mean plaque area of 8.82 mm2. At one-month follow-up, none of the patients had an ischaemia-driven major adverse cardiac event. One patient had a non-target vessel revascularisation at 33 days (Table 3). This patient had an ACC/AHA type B2 lesion in the distal left anterior descending coronary artery which was treated. A significant ostial stenosis of the circumflex coronary artery was left untreated due to small vessel size (<2 mm) and the patient had optimal medical treatment with aspirin, beta-blocker, ACE inhibitor, statin and nitrate. However, the patient had a recurrence of typical anginal symptoms and was treated 33 days later for the ostial lesion in the circumflex coronary artery with a XIENCE PRIME 2.5×12 mm stent. None of the patients had scaffold thrombosis during the follow-up period.
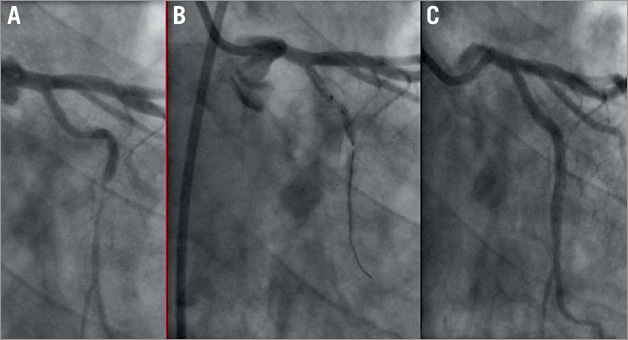
Figure 1. The lesion in the left circumflex coronary (LCx) artery could not be reached because of tortuosity and calcification of the vessel just proximal to the lesion site (A). The first-generation Absorb BVS was implanted in the proximal LCx (B). The intended lesion was treated with three overlapping drug-eluting stents with a good final angiographic result (C).
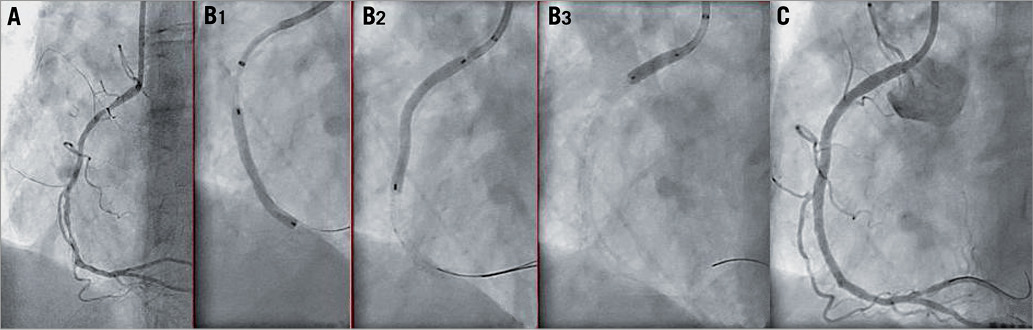
Figure 2. Two second-generation Absorb BVS did not cross the proximal right coronary artery due to diffuse disease with severe calcification (A). Delivery of the XIENCE PRIME stents was only possible after the use of a GuideLiner v2 catheter (Vascular Solutions, Minneapolis, MN, USA) which allowed deep intubation of the coronary artery (B1). Three overlapping stents were implanted (B1-B3) with a good angiographic result (C).
Discussion
The B-SEARCH describes the procedural and clinical outcomes of PCI patients treated in Rotterdam with an Absorb BVS for de novo native coronary artery lesion(s) in the largest Absorb BVS patient cohort of combined studies to date. The main findings are that the Absorb BVS appears to be associated with good procedural outcomes and short-term adverse cardiac event rates. We show that treatment of longer lesions and smaller vessels without obligatory IVUS use is safe and efficacious at one month. In summary, the scaffold was successfully implanted in 90 of the 92 lesions (97.8%). The implantation of an Absorb BVS resulted in an improvement of the minimal luminal diameter means from 1.05 mm (±0.28 mm) to 2.26 mm (±0.32 mm). Subsequently, the diameter stenosis decreased from 61% to 16%. At one-month follow-up, none of the patients had an ischaemia-driven major adverse cardiac event, and only one patient had experienced a non-target vessel revascularisation at 33 days. No scaffold thrombosis was recorded during the follow-up period while all patients were on dual antiplatelet therapy.
We previously reported that the implantation of the first-generation Absorb BVS in 30 patients resulted in excellent clinical safety up to one year after the index procedure3. Only one patient sustained a non-Q-wave myocardial infarction related to target lesion revascularisation during the follow-up period, specifically at 46 days. Although the in-scaffold luminal late loss of 0.44 mm was comparable to most metallic drug-eluting stents (DES), it was more than usually associated with the XIENCE V® (Abbott Vascular) everolimus-eluting stents (0.15 mm). Substantial changes in the manufacturing process and scaffold design directed at improving late recoil were made for the second-generation Absorb BVS. This resulted in a reduction of luminal late loss (0.19 mm) to levels comparable to DES currently utilised4.
Our previous studies have shown favourable results for the first and second-generation Absorb BVS in relatively small cohorts. The positive results of the current study are reassuring and may be good grounds for the extension of Absorb BVS use in all-comer real-world patients. Given that real-world percutaneous intervention practice involves increasingly complex and longer lesions that require multiple scaffold implantation, selective use only of IVUS and other intravascular imaging modalities and scaffold sizing by eyeballing, it is essential that the optimal safety and efficacy profile shown to date is reproduced in such settings. Larger studies are warranted to determine the safety and efficacy of the Absorb BVS compared to DES currently utilised.
Guest Editor
This paper was Guest Edited by Rafael Beyar, MD, DSc, MPH; Rambam Health Care Campus (RHCC), Haifa, Israel.
Acknowledgements
C. Simsek was supported by a research grant from the “Nederlandse Hartstichting” (2009B091).
Funding
Abbott Vascular sponsored and funded these trials.
Conflict of interest statement
C. Dorange, S. Veldhof are employees of Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

