
Abstract
Aims: Bioresorbable scaffolds (BRS) were conceived to ensure transient coronary artery support during antiproliferative drug delivery. However, the Absorb everolimus-eluting bioresorbable scaffold was found to be inferior to everolimus-eluting metallic stents (EES) in moderately complex coronary anatomies. We sought to investigate whether the Absorb represents a valuable option for the percutaneous treatment of patients with ST-elevation myocardial infarction (STEMI).
Methods and results: We pooled the individual patient data of two randomised trials specifically designed to investigate the performance of Absorb versus EES in patients with acute myocardial infarction (MI). The primary outcome was lesion (in-segment) diameter stenosis at angiographic follow-up. The main secondary outcome was the device-oriented composite endpoint (DOCE) of cardiac death, target vessel MI and target lesion revascularisation at one year. A total of 388 patients with STEMI were allocated to Absorb (n=227) or EES (n=161). Angiographic follow-up at one year was available for 332 (85.6%) patients. Lesion diameter stenosis was comparable between Absorb and EES (22.8±9.8% versus 23.6±11.2%; mean difference –0.8%, 95% confidence interval [CI]: –3.18-1.48, p=0.47). DOCE occurred in 21 patients at one year, with similar distribution between the Absorb and EES groups (5.3% versus 5.6%; hazard ratio 0.95, 95% CI: 0.40-2.26, p=0.91).
Conclusions: This pooled analysis provides evidence for a comparable angiographic performance and suggests similar clinical performance of Absorb and EES in STEMI patients undergoing percutaneous revascularisation. The long-term durability of Absorb and the extent to which newer BRS platforms might have a potential role in STEMI deserve further investigation. Both trials were registered at www.clinicaltrials.gov (NCT01942070 and NCT01986803).
Introduction
Bioresorbable scaffolds (BRS) are percutaneous coronary prostheses designed to offer a transient support for the dilated vessel and to dissolve into inert breakdown products over time, once the antiproliferative function is completed1.
The fully bioresorbable, Absorb™ everolimus-eluting scaffold (Abbott Vascular, Santa Clara, CA, USA) represents the most studied bioresorbable platform to date. Initial data on percutaneous coronary intervention (PCI) with the Absorb in selected patients were encouraging, though not confirmed in subsequent randomised trials2. In fact, up to one year the Absorb displayed a twice as high thrombotic risk in comparison with the XIENCE metallic everolimus-eluting metallic stent (EES) (Abbott Vascular). More disappointingly, follow-up data beyond one year revealed that the risk of failure of the Absorb continued to accrue during longer-term follow-up3. In response to this, in September 2017 the manufacturer withdrew the Absorb from the market, though other BRS are approved for clinical use in Europe and available for clinical use.
Although individuals with ST elevation myocardial infarction (STEMI) were excluded from most randomised trials investigating the Absorb, these patients represent a subset that may derive greater benefit from treatment with BRS technology. In fact, the lesions of STEMI patients generally consist of soft, lipid-rich, thrombotic plaques located in larger vessel segments, with less resistance to dilation and more favourable healing patterns4. To shed more light on the angiographic and clinical performance of the Absorb versus EES in patients with STEMI, we performed a pooled analysis of individual patient data from the Intracoronary Scaffold Assessment a Randomised Evaluation of Absorb in Myocardial Infarction (ISAR-Absorb MI) and the Comparison of the ABSORB™ Everolimus Eluting Bioresorbable Vascular Scaffold System With a Drug-Eluting Metal Stent (Xience™) in Acute ST-Elevation Myocardial Infarction (ABSORB STEMI TROFI II) randomised trials.
Methods
Full details of the study population, methods, endpoints and primary analyses of the ISAR-Absorb MI5 and ABSORB STEMI TROFI II4 clinical trials have been reported previously. In brief, both were multicentre, open-label, randomised trials of patients with acute myocardial infarction (MI) undergoing PCI with either Absorb or EES. Between September 2013 and March 2017, the ISAR-Absorb MI trial enrolled 262 patients with STEMI (or NSTEMI with visible thrombus at baseline angiography) in five centres: 173 participants were allocated to Absorb and 89 to EES. Between January and September 2014, the ABSORB STEMI TROFI II trial enrolled 191 patients with STEMI in eight centres: 95 participants received Absorb and 96 received EES.
Inclusion criteria were broadly comparable between the studies. To be included, patients should be aged ≥18 years, present with MI and planned to receive a stent in de novo lesions in native vessels or coronary bypass grafts with a reference vessel diameter ≥2.25 mm and ≤3.9 mm. Patients were considered ineligible for the studies if they had a target lesion located in an unprotected left main trunk, cardiogenic shock, malignancies or other comorbid conditions with life expectancy <12 months or that may result in protocol non-compliance or had contraindications or a known allergy to antiplatelet therapy, stent components or pregnancy (present, suspected or planned). Patient allocation to each of the treatment groups was in a 2:1 proportion in the ISAR-Absorb MI trial and in equal proportion in the ABSORB STEMI TROFI II trial. The primary endpoints of the ISAR-Absorb MI and ABSORB STEMI TROFI II trials were percentage diameter stenosis at six- to eight-month coronary angiography and neointimal healing score at six-month optical coherence tomography, respectively. In both trials, a non-inferiority design served to test the primary study hypothesis.
For patients treated with the Absorb, the protocol of ISAR-Absorb MI had no explicit recommendation for lesion preparation, vessel and device sizing or for scaffold post-dilation, though predilation was strongly encouraged. In the ABSORB STEMI TROFI II trial, manual thrombus aspiration with at least two passages was mandatory to reduce thrombus burden. All patients were pre-treated with aspirin (250 to 500 mg) before PCI in both trials. In all cases, anticoagulation during PCI was accomplished by intra-arterial or intravenous administration of heparin up to a total amount of 100 U/kg body weight or bivalirudin (intravenous bolus of 0.75 mg/kg prior to the start of the intervention, followed by infusion of 1.75 mg/kg per hour for the duration of the procedure). After the intervention, all patients received dual antiplatelet therapy (DAPT) according to the recommendations of guideline-writing authorities6. Other cardioactive drugs were prescribed according to standard practice.
COLLECTION OF PATIENT-LEVEL DATA
For the purpose of this study, the principal investigators of the ABSORB STEMI TROFI II trial were contacted to provide individual data of participants. After agreement, anonymised data were transferred to the Deutsches Herzzentrum München, Technische Universität München, Munich, Germany, and merged with those of ISAR-Absorb MI in a single dedicated database. The final data set was checked for completeness and consistency and compared with the results from prior publications. The principal investigators were directly contacted in case of inconsistencies with the original publications or requirement for additional data. Divergences were resolved by consensus. Data were analysed according to the intention-to-treat principle. The institutional review board or ethics committee at each participating centre approved the studies included in the present analysis, and all patients signed informed, written consent before receiving the assigned treatment in each trial.
OUTCOME VARIABLES
The primary outcome of this analysis was lesion (in-segment) percentage diameter stenosis at repeat coronary angiography six to eight months after intervention. The main secondary outcome was the device-oriented composite endpoint (DOCE) of cardiac death/target vessel MI/target lesion revascularisation (TLR). Other angiographic endpoints of interest were in-device percentage diameter stenosis, late lumen loss (LLL) and binary restenosis. Other clinical endpoints of interest were the composite of death/any MI/all revascularisation (patient-oriented composite endpoint [POCE]), each individual component of the main secondary outcome and the incidence of definite/probable scaffold or stent thrombosis. Study definitions have been described in detail previously4,5. Clinical follow-up was up to 12 months.
STATISTICAL ANALYSIS
Statistical analysis is shown in Supplementary Appendix 1.
Results
A total of 453 patients were enrolled in the two trials (Supplementary Figure 1). Of these, 65 patients with NSTEMI and visible thrombus at coronary angiography enrolled in the ISAR-Absorb MI trial were excluded, leaving 388 individuals with STEMI (227 assigned to Absorb and 161 to EES) available for final analyses. Baseline characteristics were well balanced between the treatment groups and matched those typically associated with STEMI patients. In fact, participants were relatively young, overweight, the overwhelming majority being male, and a high proportion having hyperlipidaemia and a smoking habit (Table 1).
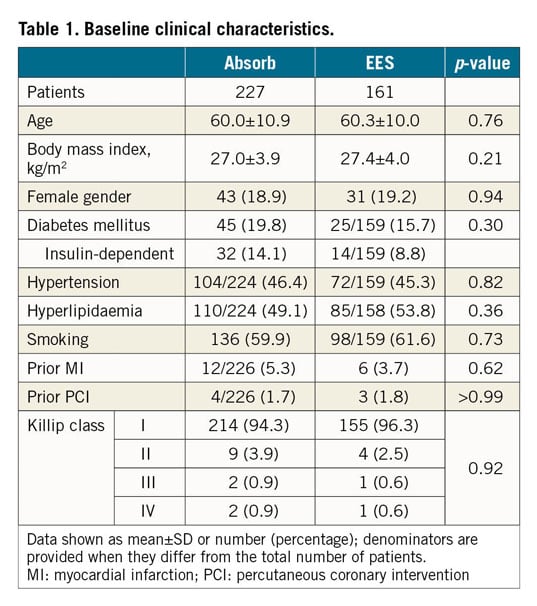
Baseline lesion and procedural characteristics were well balanced between the treatment groups (Table 2). The infarct-related vessels more frequently comprised the left anterior descending or the right coronary artery. A complete occlusion of the infarct-related vessel was observed in circa 60% of patients. Predilation (78.3% and 63.1%, p=0.001) and post-dilation (53.3% and 32.3%, p<0.001) were more common in patients treated with Absorb versus EES, respectively. After PCI, the minimal lumen diameter was non-significantly smaller with Absorb versus EES (2.54±0.41 mm versus 2.60±0.43 mm, p=0.22), whilst the residual percentage diameter stenosis was significantly higher with Absorb compared with EES (14.1±8.6% versus 12.6±5.5%, p=0.03). Six (2.6%) patients allocated to the Absorb did not receive the assigned stent and were treated with EES. Two (1.2%) patients allocated to EES did not receive the assigned stent and were treated with the Absorb. At discharge, all patients received thienopyridines (ticagrelor: 219 [56.7%]; prasugrel: 128 [33.2%]; clopidogrel: 39 [10.1%]). The discharge therapy was unknown in two (0.5%) patients.
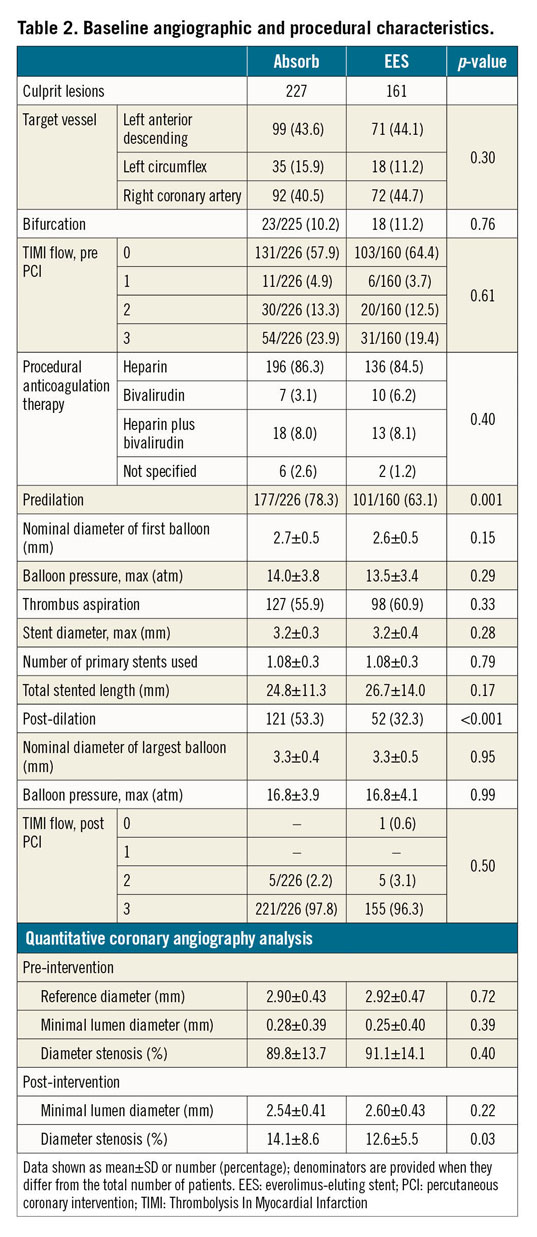
Angiographic follow-up was available for 332 (85.6%) patients without significant difference between treatment groups (p=0.72). The median time to angiographic follow-up was shorter with Absorb – 230 (208, 278) days – as compared to EES – 241 (211, 307) days – (p=0.03). Table 3 resumes the angiographic outcomes of the study. The primary outcome of lesion diameter stenosis was 22.8±9.8% with Absorb versus 23.6±11.2% with EES, with a mean difference of –0.8% (–3.18, 1.48), p=0.47 (Figure 1). The analysis stratified by trial revealed a significant interaction between the treatment effect and the primary angiographic outcome (p=0.002). In fact, lesion diameter stenosis was 23.7±11.4% with Absorb versus 29.3±12.1% with EES (p=0.006) in the ISAR-Absorb MI trial, and 21.6±7.3% with Absorb versus 20.2±9.0% with EES (p=0.27) in the ABSORB STEMI TROFI II trial.
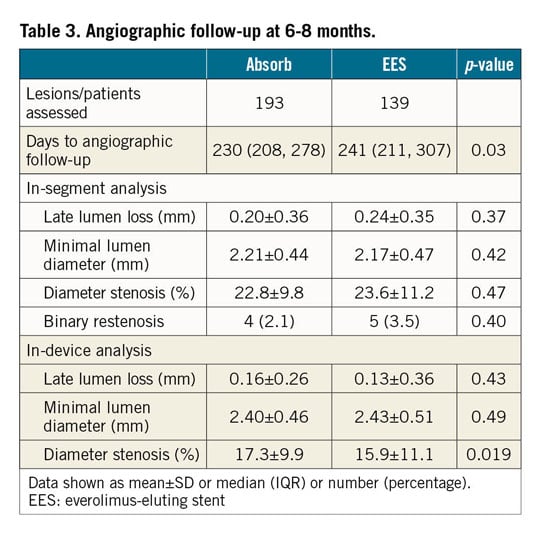
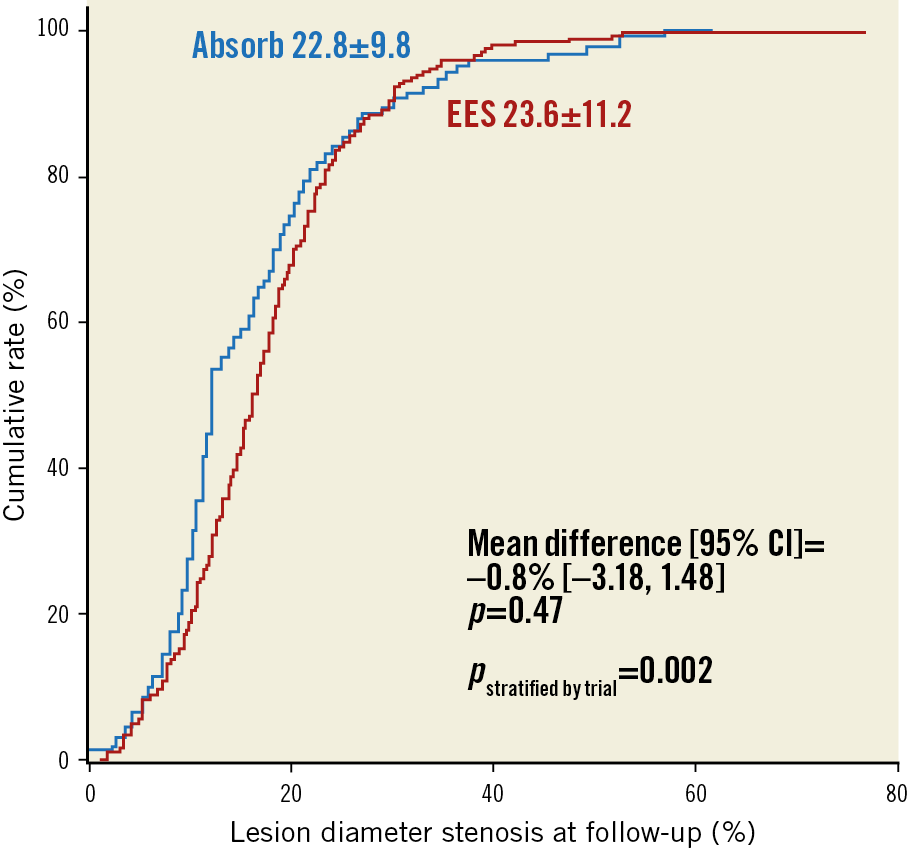
Figure 1. Primary outcome: lesion percentage diameter stenosis at six- to eight-month angiographic follow-up. Cumulative frequency distribution for lesion diameter stenosis at follow-up angiography. P-values are presented unadjusted and stratified by trial.
Absorb was associated with a higher degree of in-device diameter stenosis as compared to EES (17.3±9.9% versus 15.9±11.1%, mean difference 1.4% [–0.89, 3.78], p=0.019). LLL was comparable for in-segment (0.20±0.36 mm with Absorb versus 0.24±0.35 mm with EES, p=0.37) (Supplementary Figure 2A) and in-device measurements (0.16±0.26 mm with Absorb versus 0.13±0.36 mm with EES, p=0.43). Overall, binary restenosis was observed in nine patients (four patients with Absorb and five patients with EES, p=0.40). There were no cases of complete restenotic occlusion at follow-up.
Clinical follow-up up to 12 months was available in all patients, with a similar duration among treatment groups (p=0.59). The clinical outcomes are summarised in Table 4. DOCE occurred in 12 (5.3%) patients treated with Absorb versus 9 (5.6%) with EES (HR 0.95, 95% CI: 0.40-2.26, p=0.91) (Figure 2). Findings were consistent in the analysis stratified by trial (p=0.67).
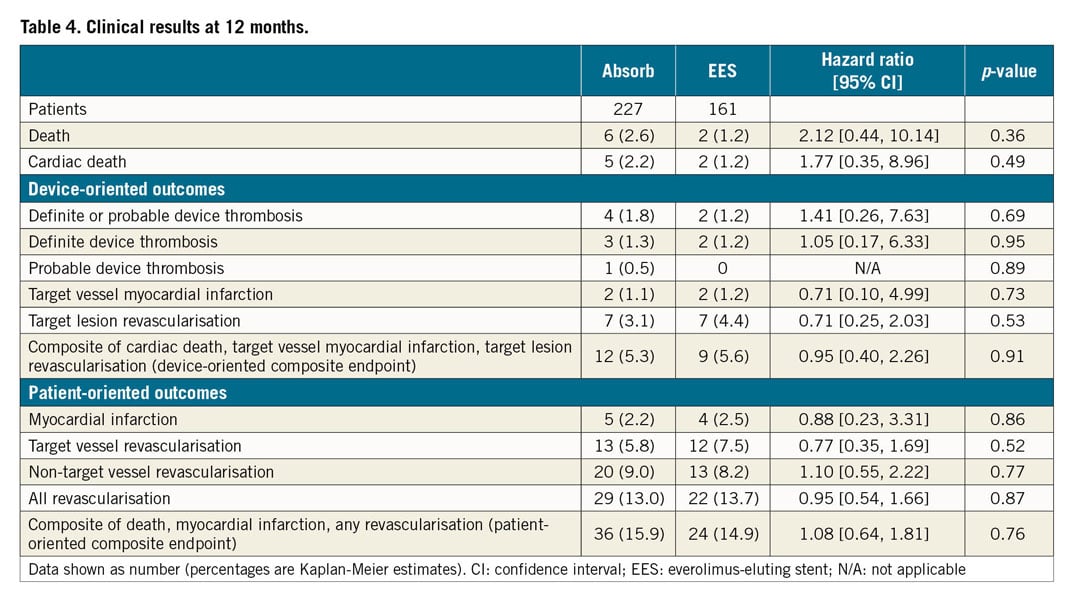
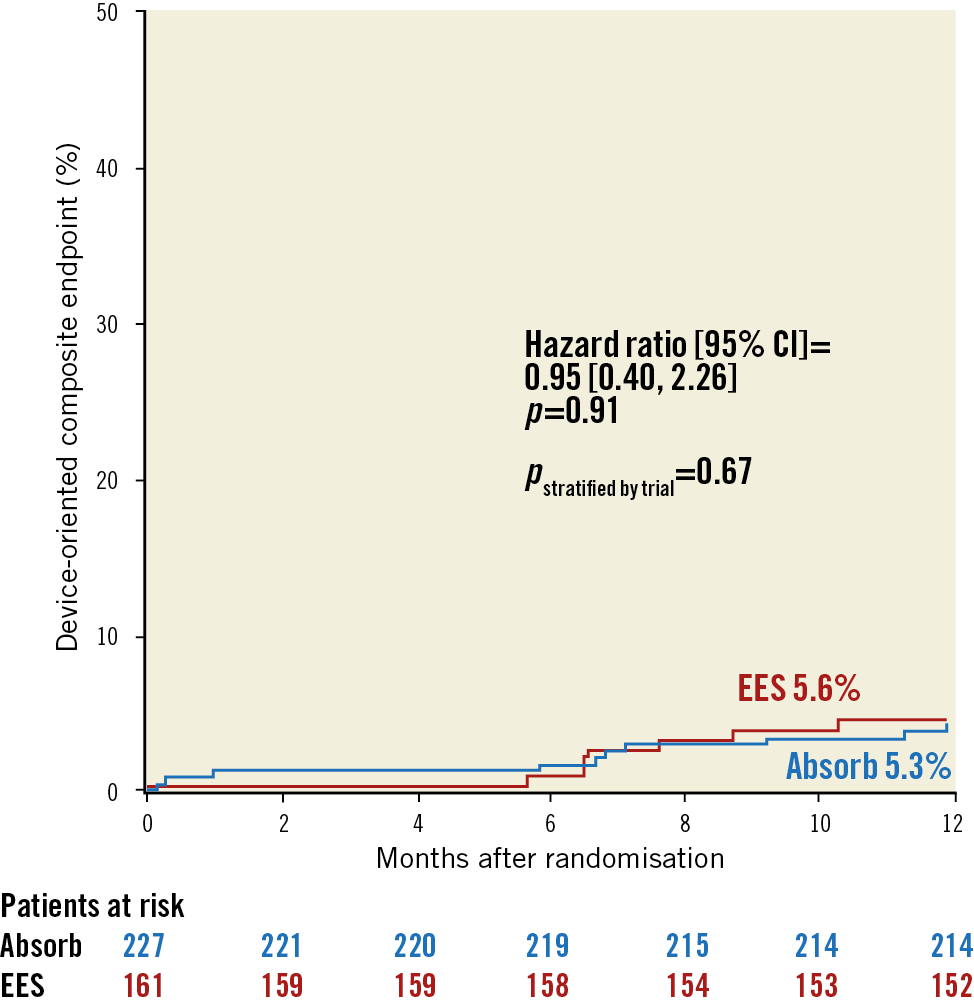
Figure 2. Main secondary outcome: device-oriented composite endpoint. Survival analysis curves for the composite of cardiac death, target vessel myocardial infarction and target lesion revascularisation. P-values are derived from Cox proportional hazards models and are presented unadjusted and stratified by trial.
POCE occurred in 36 (15.9%) patients treated with Absorb versus 24 (14.9%) with EES (HR 1.08, 95% CI: 0.64-1.81, p=0.76) (Supplementary Figure 2B). Cardiac death occurred in 5 (2.2%) patients treated with Absorb versus 2 (1.2%) with EES (HR 1.77, 95% CI: 0.35-8.96, p=0.49). Target vessel MI occurred in 2 (1.1%) patients treated with Absorb versus 2 (1.2%) with EES (HR 0.71, 95% CI: 0.10-4.99, p=0.73). TLR occurred in 7 (3.1%) patients with Absorb versus 7 (4.4%) patients with EES (HR 0.71, 95% CI: 0.25-2.03, p=0.53) (Supplementary Figure 2C). Definite/probable stent or scaffold thrombosis occurred in 4 (1.8%) patients with Absorb versus 2 (1.2%) patients with EES (HR 1.41, 95% CI: 0.26-7.63, p=0.69).
The treatment effect for the primary angiographic and main secondary clinical outcomes had no interaction with age (p for interaction – pint≥0.24), gender (pint≥0.37), diabetic status (pint≥0.15), thienopyridines at discharge (pint≥0.66), presence or absence of Thrombolysis In Myocardial Infarction (TIMI) 0 flow pre PCI (pint≥0.26), thrombus aspiration (pint≥0.51), predilation (pint≥0.06), post-dilation (pint≥0.19) and total stented length (pint≥0.69) (Supplementary Table 1).
Discussion
In this analysis, we pooled the largest cohort of STEMI patients receiving a PCI with either Absorb or EES among randomised trials with angiographic follow-up. The main findings were that: i) Absorb was comparable to EES in terms of angiographic outcomes at six to eight months and in terms of clinical outcomes at 12 months; ii) in the subgroup analysis there was no evidence of interaction between several clinical, angiographic and procedural features and treatment effect for primary angiographic and main secondary outcomes. However, some issues need to be considered when interpreting the data.
First, lesion diameter stenosis was chosen as the primary angiographic outcome. Previous investigations have shown that this surrogate endpoint represents a reliable parameter of device efficacy7. In this regard, the overall comparable angiographic performance of Absorb and EES observed in this study is noteworthy. Indeed, earlier trials including patients with predominantly stable coronary artery disease and/or moderately complex anatomies found inferior angiographic efficacy of Absorb versus EES after a follow-up duration comparable to that accumulated for the present study2. The mechanical properties of Absorb are likely to play a major role. In particular, the expansion capability of the current Absorb could not approximate that of metallic stents8, failing more often in complex coronary anatomies9. In contrast, STEMI lesions typically consist of less bulky, lipid-rich plaques with a necrotic core and superimposed thrombi, without relevant calcifications. By expanding more easily, these lesions appear more suitable to scaffolding with BRS. Consistent with previous data10, we found a lower minimum lumen diameter after PCI with Absorb as compared to EES, reflecting the intrinsic limitation of this technology. However, the treatment groups did not differ for this parameter at angiographic follow-up, suggesting a relatively stable mechanical behaviour of Absorb in STEMI patients, without instances of late recoil as previously observed11.
Second, the risk for DOCE at 12 months was similar with Absorb and EES. However, this analysis is underpowered to detect potential clinical differences between the treatment groups. Previous registry data demonstrated a poor one-year clinical performance with Absorb in STEMI, mainly attributable to more frequent scaffold thrombosis within 30 days after implantation12. The lack of an optimised technique for Absorb implantation (predilation, appropriate vessel sizing, and high-pressure post-dilation) was deemed responsible for this increased risk13. At the same time, some experts proposed to intensify DAPT after Absorb implantation14. Although we recognise the importance of a proper implantation technique to improve the acute and late performance of stents and scaffolds, this pooled analysis of randomised trials did not find a significant interaction between pre- and post-deployment dilation rates and the treatment effect for main outcomes. Moreover, the use of more potent ADP-receptor antagonists did not impact on the angiographic and clinical efficacy of Absorb versus EES in this study, though approximately 90% of our cohort received highly effective antiplatelet drugs as standard treatment for STEMI.
Third, our data lend support to device iteration and appropriate lesion selection as a prerequisite for future BRS technologies. Indeed, two recent randomised trials including patients with higher anatomical complexity (30-day and 1-year results from the ABSORB IV randomised trial15 and COMPARE-ABSORB 1-year results, presented by P.C. Smits at the Transcatheter Cardiovascular Therapeutics meeting [TCT 2018], San Diego, CA, September 25, 2018) found Absorb to be associated with a higher risk of thrombosis at one year despite the adoption of specific implantation protocols and relatively high proportions of DAPT after PCI. In this regard, although the platform investigated in this analysis is no longer available for clinical use, several BRS are in development or under investigation16. Thus, the present study may serve as an evidence base for future trials investigating improved or new BRS in STEMI, pending the demonstration of at least non-inferiority in comparison with current high-performance metallic drug-eluting stents (DES)17.
Study limitations
The current study presents a number of limitations. First, this analysis has the limitations inherent to pooled analyses and reflects the flaws of the original trials. Amongst others, the studies included were open-label, which represents a source of bias. In addition, they focused on a single BRS platform. Second, angiographic data were collected by two different core labs; this may partially account for the significant interaction observed between treatment effect and primary angiographic outcome. Third, this study was not powered to evaluate the performance of Absorb versus EES in specific subgroups of patients; in this regard, the present analysis remains exploratory in nature. Finally, the clinical follow-up was limited to one year. Longer follow-up remains crucial for two reasons – to ascertain definitively the durability of the Absorb and to address whether BRS technology has late advantages compared to current metallic DES in STEMI.
Conclusions
In STEMI patients undergoing a percutaneous revascularisation, this pooled analysis of individual participant data from two randomised trials suggests comparable performance of Absorb and EES at angiographic and clinical follow-up. The results remained consistent across several subgroups of patients. The long-term durability of the Absorb and the extent to which newer BRS platforms might have a potential role in STEMI remains to be studied further.
|
Impact on daily practice This study provides evidence for a comparable performance of Absorb and everolimus-eluting metallic stents in STEMI patients undergoing percutaneous revascularisation. Although the bioresorbable platform investigated in this analysis is no longer available for clinical use, several scaffolds are in development or under investigation. Thus, the present study may serve as an evidence base for future trials investigating improved or new bioresorbable scaffolds in STEMI. |
Appendix. Authors’ affiliations
1. Deutsches Herzzentrum München, Technische Universität München, Munich, Germany; 2. Amsterdam University Medical Centre, Amsterdam, the Netherlands; 3. DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany; 4. Hospital Clinic, Institut Clinic Cardiovascular, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), University of Barcelona, Barcelona, Spain; 5. Department of Cardiology, Hospital Universitario de La Princesa Madrid, Madrid, Spain; 6. Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland; 7. Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark; 8. Complejo Hospitalario Universitario, Hospital Alvaro Cunqueiro, Vigo, Spain; 9. E.N. Meshalkin National Medical Research Center, Novosibirsk, Russia; 10. Thoraxcenter, Erasmus Medical Center, Rotterdam, the Netherlands; 11. Clinic and Policlinic Internal Medicine I (Cardiology and Angiology), Klinikum rechts der Isar, Technische Universität München, Munich, Germany; 12. NHLI, Imperial College London, London, United Kingdom
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Conflict of interest statement
R.A. Byrne reports receiving lecture fees/honoraria from B. Braun Melsungen AG, Biotronik, Boston Scientific and Micell Technolgies and research grants to the institution from Boston Scientific and CeloNova Biosciences. L. Räber has received grants to the institution from Abbott Vascular, Sanofi and Regeneron, and speaker or consulting fees from Abbott Vascular, Amgen, AstraZeneca, Biotronik, and CSL Behring. Y. Onuma is a member of the Advisory Board for Abbott Vascular and has received speaker honoraria from Terumo. M. Joner reports being a consultant for and receiving speaker fees from Biotronik and OrbusNeich. M. Sabaté reports receiving consultancy fees from Abbott Vascular. S. Windecker reports receiving research grants to the institution from Abbott Vascular, Amgen, Biotronik, Boston Scientific, Medtronic, Edwards Lifesciences, St. Jude and Terumo. P.W. Serruys is a member of the Advisory Board for Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
Supplementary data
To read the full content of this article, please download the PDF.

