Introduction
In patients with stable coronary artery disease, drug-eluting bioresorbable scaffolds (BRS) displayed inferior clinical results in comparison to metallic drug-eluting stents1. However, initial evidence on BRS implantation in patients with acute myocardial infarction (AMI) was encouraging (Sabaté M. Biodegradable scaffolds in STEMI: rationale, registry outcomes and TROFI II 2-year results. Presented at Transcatheter Therapeutics [TCT]. Washington, DC, Oct 31, 2016) and lends support to further investigation. The Intracoronary Scaffold Assessment a Randomized evaluation of Absorb in Myocardial Infarction (ISAR-Absorb MI) trial demonstrated that BRS implanted in the setting of AMI have a similar antirestenotic efficacy compared to everolimus-eluting stents (EES), as assessed by angiographic follow-up after 6-9 months2. The aim of the present analysis was to report the two-year clinical follow-up of the ISAR-Absorb MI trial.
Methods
The ISAR-Absorb MI trial is an investigator-initiated, prospective, randomised, multicentre, non-inferiority, clinical trial. Patients with AMI were randomised in a 2:1 ratio to be treated with either an everolimus-eluting BRS (Absorb; Abbott Vascular) or an EES (XIENCE; Abbott Vascular). A detailed description of the study has been published previously2. The endpoints of interest for this analysis were the device-oriented composite endpoint (DOCE) of cardiac death, target vessel myocardial infarction and target lesion revascularisation (TLR), and the patient-oriented composite endpoint (POCE) of all-cause death, any myocardial infarction and any revascularisation). Device thrombosis was defined according to Academic Research Consortium criteria. All events relevant for the primary and secondary endpoints were blindly adjudicated by an independent event adjudication committee.
Results
Baseline characteristics were described previously2. Two-year clinical follow-up was available for 164 patients in the BRS group and 87 patients in the EES group. DOCE occurred in 10.0% in the BRS group and in 7.9% in the EES group (hazard ratio [HR] 1.28, 95% confidence interval [CI]: 0.53-3.09, p=0.58) and POCE occurred in 21.6% and 16.9% (HR 1.28, 95% CI: 0.70-2.33, p=0.42), in the BRS group and EES group, respectively. The two-year all-cause mortality rate was not statistically different between patients treated with BRS and EES (5.3% versus 2.2%; HR 2.32, 95% CI: 0.50-10.75, p=0.28). The rate of TLR was 7.2% in the BRS group and 6.8% in the EES group (HR 1.06, 95% CI: 0.40-2.82, p=0.91). The rate of definite device thrombosis was 2.4% in the BRS group and 2.3% in the EES group (HR 1.04, 95% CI: 0.19-5.65, p=0.97). A summary of the clinical outcomes is displayed in Table 1 and Kaplan-Meier curves are shown on Supplementary Figure 1. Data regarding antiplatelet and anticoagulant therapies were available for 98.4% of the patients who were alive at the two-year follow-up. Of these patients, 97.2% were on aspirin, 18.6% on a P2Y12 inhibitor and 2.4% on oral anticoagulation.
The analysis of clinical outcomes in prespecified subgroups including age, gender, diabetic status, presentation diagnosis and vessel size showed comparable results between BRS and EES regarding DOCE and TLR. With respect to POCE, EES showed more favourable results than BRS in the subgroup of non-ST-elevation myocardial infarction (NSTEMI) patients (pinteraction=0.03), without significant differences in other subgroups (Supplementary Figure 2).
Discussion
AMI lesions are typically characterised by predominantly thrombotic, soft, lipid-rich plaques with little calcification and evidence of plaque rupture and therefore are associated with less resistance to stent expansion3. In addition, patients with AMI are also typically younger with fewer comorbidities at the time of diagnosis4. Taking into account also the mechanical properties of BRS, with less radial force compared to metallic stents, AMI patients are probably well-suited for interventional treatment with BRS technologies. Thus, further research is justified, and the ISAR-Absorb MI trial aims to address this need.
Overall, the two-year clinical outcomes in AMI patients treated with either BRS or EES were comparable in the ISAR-Absorb MI trial. These findings are in line with other randomised studies (Figure 1), e.g., the TROFI II study included 191 patients with STEMI and the primary endpoint was a healing score assessed by optical coherence tomography after six months, which was similar in both groups. The two-year clinical outcomes showed statistically comparable rates of DOCE (3.2% versus 3.2%; p=0.97), TLR (2.1% versus 1.0%; p=0.55) and definite/probable device thrombosis (2.1% versus 1.0%; p=0.55) between BRS and EES (Sabaté M. Biodegradable scaffolds in STEMI: rationale, registry outcomes and TROFI II 2-year results. Presented at Transcatheter Therapeutics [TCT]. Washington, D.C., Oct 31, 2016). Furthermore, a prespecified subgroup analysis of the randomised controlled AIDA study did not find statistically relevant differences between patients with STEMI undergoing BRS (n=240) or EES (n=225) implantation regarding DOCE (8.4% versus 7.7%; p=0.722), TLR (6.8% versus 5.0%; p=0.394) and definite/probable device thrombosis (5.5% versus 2.7%; p=0.135) after two years4.
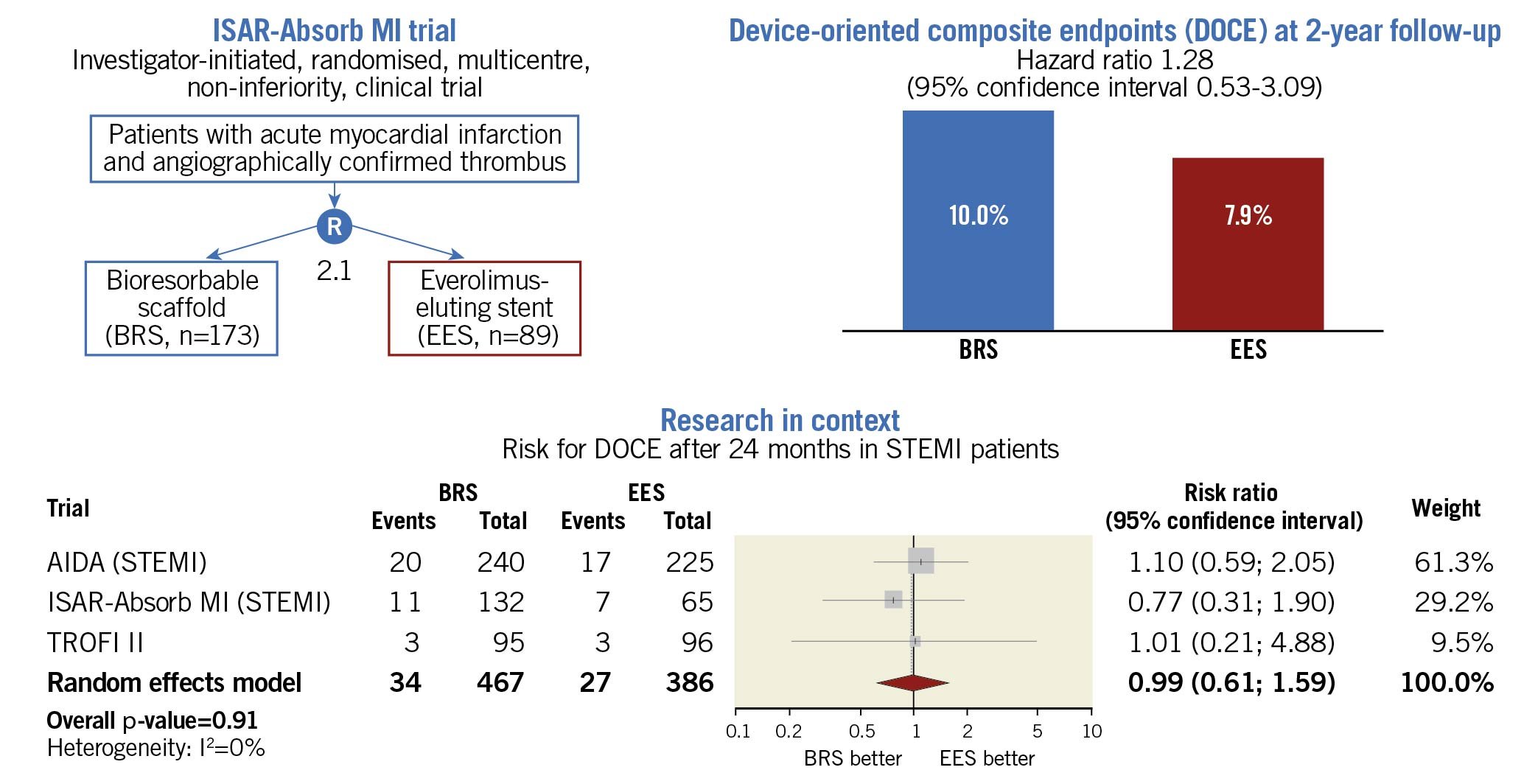
Figure 1. Design, clinical outcome and research in the context of the ISAR-Absorb MI trial. Only data from randomised studies were considered for the meta-analysis. Risk ratio with 95% confidence intervals was used as summary statistic to compare the risk of device-oriented composite endpoints (DOCE) in patients receiving a coronary revascularisation with either bioresorbable scaffolds (BRS) or everolimus-eluting stents (EES). The random effects model with the Hartung-Knapp adjustment served for overall calculations. A p-value <0.05 was considered statistically significant. The I2 statistic tested the heterogeneity across trials. In all trials, risk estimates refer to the 24-month follow-up time point (Sabaté M. Biodegradable scaffolds in STEMI: rationale, registry outcomes and TROFI II 2-year results. Presented at Transcatheter Therapeutics [TCT]. Washington, D.C., Oct 31, 2016)4. For the AIDA and ISAR-Absorb MI trials, summary estimates were obtained from patients with ST-elevation myocardial infarction (STEMI). AIDA: Amsterdam Investigator-initiateD Absorb Strategy All-comers Trial; ISAR-Absorb MI: Intracoronary Scaffold Assessment a Randomized evaluation of Absorb in Myocardial Infarction trial
Limitations
1) Although we found no significant differences between the treatment groups, the trial was not powered to assess clinical outcomes. 2) According to the trial protocol, the clinical follow-up was limited to two years. A longer follow-up is desirable to address the risk of late adverse events associated with BRS.
Conclusion
The ISAR-Absorb MI trial found that, in patients with AMI, PCI with either BRS or EES is associated with a comparable clinical performance at two-year follow-up. The potential role of contemporary BRS technology for AMI patients needs to be assessed in future trials.
Funding
The trial was sponsored by the Deutsches Herzzentrum München. The study was predominantly funded by the Deutsches Herzzentrum München and in part by a grant from Abbott Vascular.
Conflict of interest statement
R.A. Byrne reports research funding to the institution of prior employment from CeloNova BioSciences, and research or educational funding to the institution of current employment from Abbott Vascular, Biosensors, Biotronik and Boston Scientific. M. Joner reports grants and personal fees from Edwards, personal fees from Recor, AstraZeneca, Amgen, Biotronik, Orbus Neich, Boston Scientific and Abbott, and grants from Boston Scientific. J. Wiebe reports other from AstraZeneca, outside the submitted work. H. Schunkert reports personal fees from Amgen, grants and personal fees from AstraZeneca, personal fees from Bayer Vital GmbH, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, MSD Sharp & Dohme, Novartis, Sanofi-Aventis, Servier and SYNLAB. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

