
Introduction
Previous midterm follow-up reports after implantation of the Absorb™ everolimus-eluting bioresorbable scaffold (BRS; Abbott Vascular, Santa Clara, CA, USA) in stable coronary artery disease and acute coronary syndrome have shown an increase of scaffold thrombosis leading to an excess of the device-oriented composite endpoint (DOCE: a composite of cardiac death, target vessel myocardial infarction [TVMI], and clinically driven target lesion revascularisation [CD-TLR])1. In contrast, in the six-month primary report of ABSORB STEMI: the TROFI II Study (NCT01986803)2, which randomised patients with ST-elevation myocardial infarction (STEMI) to receive either the Absorb BRS or the XIENCE metallic everolimus-eluting stent (EES; Abbott Vascular), optical frequency domain imaging (OFDI)-derived healing score was comparable between the BRS arm and the EES arm. The aim of this report was to present the three-year clinical outcome results of the BRS and the metallic EES at the time when full resorption of the scaffold device can be expected.
Methods
The trial design and methods, as well as the study population, have been described in previous reports2. Follow-up information at three years was available in 100% of the study population (Figure 1). All clinical events were adjudicated by an independent clinical events adjudication committee. Time-to-event variables were compared by log-rank test.
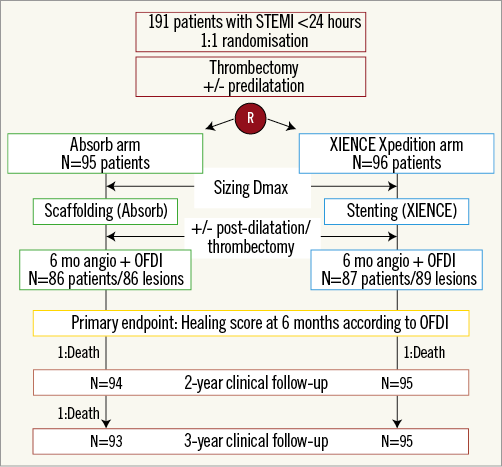
Figure 1. Study flow chart. OFDI: optical frequency domain imaging; STEMI: ST-segment elevation myocardial infarction
Results
Clinical outcomes at three years are tabulated in Table 1. At three years, the rates of DOCE were 5.3% (5/95) in the BRS arm and 3.1% (3/96) in the EES arm without a statistically significant difference (p=0.465).
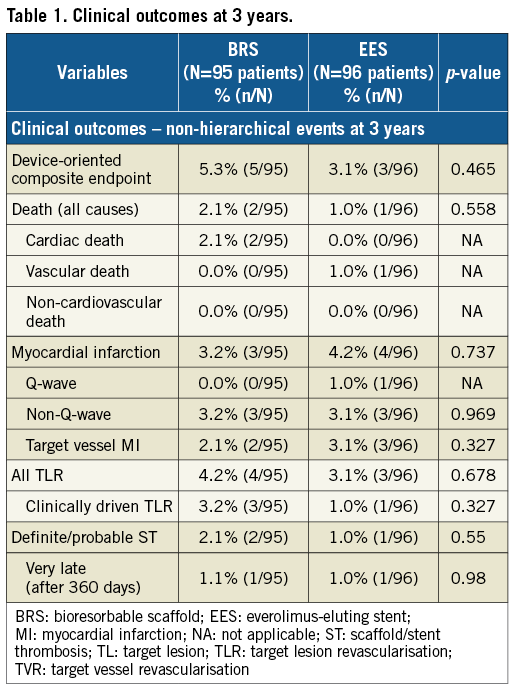
There were two cardiac deaths (2.1%) in the BRS arm: one was adjudicated as cardiac death on day 280 since no information was available as the patient died overseas. The autopsy of the second patient who died on day 999 revealed no evidence of scaffold thrombosis. There were no cardiac deaths in the EES arm.
Definite/probable very late scaffold/stent thrombosis (VLST) occurred in 1.1% (1/95) and 1.0% (1/96) in the BRS and EES arm, respectively (Supplementary Figure 1).
Discussion
Three years after implantation of BRS or EES, in the setting of primary PCI, the rates of DOCE and ST were low and not significantly different between both arms. It is of note that there were no cases of ST after two years, while most of the patients in both arms discontinued dual antiplatelet therapy between one and two years (Supplementary Table 1). The three-year clinical results are in line with the comparable OFDI-derived healing scores2 and neointimal quality3 at six months. In addition, the recent report in the subpopulation of the current cohort showed a better vasomotion in BRS than in EES and similar microcirculatory function as well as healing score at three years4.
Although routine thrombectomy is not recommended in the recent guidelines, in treating STEMI patients with BRS, thrombectomy could facilitate better scaffold sizing to achieve better long-term outcome.
In the substudy of the present trial4, intraluminal dismantling was observed by OCT in 26.3%. However, the fact that the substudy only included event-free patients precludes the understanding of the clinical implication of intraluminal dismantling.
Limitations
The trial was not powered to evaluate clinical endpoints. The implantation technique reflects the state of the art at the time of enrolment in 2014 and did not take into account the more recent findings on BRS usage in terms of size selection, lesion preparation and deployment technique. It remains to be elucidated whether the comparative clinical outcomes in this study performed in STEMI patients will be maintained after three years.
Conclusions
Three years after implantation of BRS or EES, in the setting of primary PCI, the rates of DOCE and ST were low and not significantly different between both arms. The three-year clinical results are in line with the comparable healing scores observed at six months. However, the study was not powered to assess clinical endpoints.
| Impact on daily practice Previous midterm follow-up reports after BRS implantation have shown an increase of ST leading to an excess of DOCE. The three-year follow-up of the TROFI II trial is the first observation of midterm clinical outcomes in a STEMI population treated with BRS in the context of a randomised trial. The rates of DOCE and ST were low and not different between BRS and EES, and no excess risk in BRS was observed. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Funding
The TROFI II trial was sponsored by the ECRI and supported with unrestricted grants from Terumo Europe N.V. and Abbott Vascular.
Conflict of interest statement
Y. Onuma is a member of the Advisory Board for Abbott Vascular and has received speaker honoraria from Terumo. L. Jensen has received research grants to the institution from Terumo, Biotronik and Biosensors. S. Hofma declares non-study-related unrestricted educational research grants from Abbott Vascular to the institution. L. Räber has received grants to the institution from Abbott, Sanofi and Regeneron and speaker or consulting fees from Abbott, Amgen, AstraZeneca, Biotronik, and CSL Behring. M. Sabaté reports personal fees from Abbott Vascular. S. Windecker reports having received research contracts to the institution from Abbott, Amgen, Bayer, Biotronik, Boston Scientific, Medtronic, Edwards, Terumo and St. Jude. P.W. Serruys is a member of the Advisory Board for Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
Supplementary data
Supplementary Figure 1. Very late scaffold/stent thrombosis cases in BRS and EES.
Supplementary Table 1. Proportion of patients on dual antiplatelet therapy at 1-, 2-, and 3-year follow-up.
To read the full content of this article, please download the PDF.

