Abstract
Background: Absorb bioresorbable vascular scaffold (BVS)-related events have been reported between 1 and 3 years – the period of active scaffold bioresorption. Data on the performance of the Absorb BVS in daily clinical practice beyond this time point are scarce.
Aims: This report aimed to provide the final five-year clinical follow-up of the Absorb BVS in comparison with the XIENCE everolimus-eluting stent (EES). In addition, we evaluated the effect of prolonged dual antiplatelet therapy (DAPT) administration on events in the scaffold group.
Methods: AIDA was a multicentre, investigator-initiated, non-inferiority trial, in which 1,845 unselected patients with coronary artery disease were randomly assigned to either the Absorb BVS (n=924) or the XIENCE EES (n=921). Target vessel failure (TVF), a composite of cardiac death, target vessel myocardial infarction or target vessel revascularisation, was the primary endpoint. Scaffold thrombosis cases were matched with controls and tested for the effect of prolonged DAPT.
Results: Up to five-year follow-up, there was no difference in TVF between the Absorb BVS (17.7%) and the XIENCE EES (16.1%) (hazard ratio [HR] 1.31, 95% confidence interval [CI]: 0.90-1.41; p=0.302). Definite or probable device thrombosis (DT) occurred in 43 patients (4.8%) in the scaffold group compared to 13 patients (1.5%) in the stent group (HR 3.32, 95% CI: 1.78-6.17; p<0.001). DT between 3 and 4 years occurred six times in the Absorb arm versus three times in the XIENCE arm. Between 4 and 5 years, the incidence was three versus two, respectively. Of those three DT in the scaffold group, two occurred in XIENCE EES-treated lesions. The odds ratio of scaffold thrombosis in patients on DAPT compared to off DAPT throughout five-year follow-up was 0.36 (95% CI: 0.15-0.86).
Conclusions: The excess risk of the Absorb BVS on late adverse events, in particular device thrombosis, in routine PCI continues up to 4 years and seems to plateau afterwards. Clinical Trial Registration ClinicalTrials.gov: NCT01858077.
Introduction
Drug-eluting stents have an ongoing risk of device-related adverse events long after implantation1. The pathogenesis of this ongoing annual hazard is thought to be the permanent presence of a metallic implant. To liberate the coronary artery from its permanent metallic cage, and therefore remove the potential cause of restenosis and stent thrombosis, bioresorbable vascular scaffolds (BVS) were developed. Theoretically, the function of the BVS is to scaffold the arterial wall after balloon dilatation to prevent acute vessel closure and late constrictive remodelling. Afterwards, it should dissolve over approximately three years to restore the native structure of the coronary artery. The most widely studied coronary scaffold is the Absorb BVS (Abbott Vascular), which, in a porcine model, completely resorbs and integrates in approximately three years2. However, in clinical practice, the Absorb BVS was found to be associated with an increased risk of target vessel myocardial infarction (TV-MI) and device thrombosis (DT) during the time of reabsorption compared to the everolimus-eluting metallic XIENCE stent (Abbott Vascular)345. Beyond the three-year time-point, data on the safety and efficacy of the Absorb BVS are scarce6. In addition, it is unknown whether prolonged dual antiplatelet therapy (DAPT) benefits patients treated with the Absorb BVS. Therefore, long-term outcomes are of interest. The Amsterdam Investigator-initiateD Absorb strategy (AIDA) randomised clinical trial compared the Absorb BVS with the everolimus-eluting metallic XIENCE EES stent (Abbott Vascular) in daily clinical practice7. We report the final five-year clinical outcomes of the Absorb BVS in comparison with the XIENCE EES. In addition, we evaluate whether prolonged DAPT regimes mitigate the occurrence of scaffold thrombosis (ScT).
Methods
The study design, endpoint definitions, and results up to three years have been described in detail previously3789. Briefly, the AIDA trial was an all-comer, multicentre, investigator-initiated, randomised controlled trial. Between August 2013 and December 2015, 1,845 consecutive patients with coronary artery disease undergoing percutaneous coronary intervention (PCI) of one or more target lesions suitable for drug-eluting stent implantation were enrolled. Follow-up was performed at regular intervals up to five years. Quantitative coronary angiographic analyses were performed at a core laboratory. An independent clinical events committee adjudicated all major adverse cardiac events (MACE) according to either the Third Universal Myocardial Infarction definitions10, or the Academic Research Consortium definitions11. The primary study endpoint was target vessel failure (TVF), powered for non-inferiority at two years. TVF is a composite of cardiac death, target vessel myocardial infarction (TV-MI), or target vessel revascularisation. Secondary endpoints included TVF, its components, and DT at each follow-up period.
The protocol mandated use of DAPT for at least one year post-PCI. In January 2017, the data and safety monitoring board (DSMB) noted a higher rate of early and late ScT and recommended considering prolonged DAPT in all patients treated with the Absorb BVS. Subsequently, this recommendation was implemented and referring cardiologists were advised to prescribe DAPT up to three years in all patients treated with the Absorb BVS.
The study design was in concordance with the provisions of the Declaration of Helsinki. The research ethics committee of the Academic Medical Centre, Amsterdam, approved the study protocol for all participating centres. All enrolled patients provided written informed consent.
Effect of DAPT
To assess the effect of DAPT on the occurrence of ScT, every case with definite ScT was matched with one or two control case(s) based on age, sex, presentation with acute coronary syndrome, total number of stents, total stent length and enrolment date before 1 October 2014. At the time of ScT, use of DAPT was recorded as yes or no for the cases and their controls.
Statistical analysis
The current paper reports the prespecified major outcomes at five-year follow-up. All analyses were performed according to the intention-to-treat principle. Time-to-event curves were constructed by the Kaplan-Meier method and compared with log-rank test. Hazard ratios were calculated using Cox regression. Landmark analyses were performed at three and four years after the index procedure. All ScT cases were matched fuzzy (1:2). Fuzz of 10 for age, 14 for days, 0.8 for total number of stents and 19 for total stent length were allowed. The effect of DAPT on the occurrence of ScT was assessed by calculating the odds ratio, using multivariable logistic regression adjusting for age, total number of stents and total stent length. A p-value of ≤0.05 was considered statistically significant. All statistical analyses were performed with SPSS software, Version 26.0 (IBM Corp.).
Results
From August 2013 until December 2015, 1,845 patients were enrolled at five sites throughout the Netherlands. In total, 924 patients were randomised to the Absorb BVS and 921 patients were randomised to the XIENCE EES. Baseline patient, procedural and lesion characteristics have been described in detail in previous reports15, and are shown in Supplementary Table 1 and Supplementary Table 2. Briefly, baseline characteristics were well balanced between the two groups. A total of 54% of patients presented with acute coronary syndrome at baseline; 25.2% ST-segment myocardial infarction, 20.4% non-ST-segment myocardial infarction, and 8.5% unstable angina. SYNTAX score was available for 1,661 patients (90.0%), with a median of 11 (IQR 7-18). In total, 2,446 lesions were treated.
Clinical endpoints
Complete five-year follow-up was obtained in 95.1% of patients. A study flow chart is provided in Supplementary Figure 1. Clinical outcomes up to five-year follow-up are shown in Table 1. Throughout five years, no significant difference in the rate of TVF was found between patients treated with the Absorb BVS (17.7%) versus the XIENCE EES (16.1%) (HR 1.13, 95% CI: 0.90-1.41; p=0.302) (Central illustration). The rates of TV-MI and target lesions revascularisation (TLR) remained significantly higher in the Absorb arm compared to the XIENCE arm, with five-year follow-up rates of TV-MI of 7.7% versus 5.0% (HR 1.57, 95% CI: 1.08-2.30; p=0.018) and TLR 10.1% versus 7.3% (HR 1.41, 95% CI: 1.02-1.94; p=0.034), respectively.
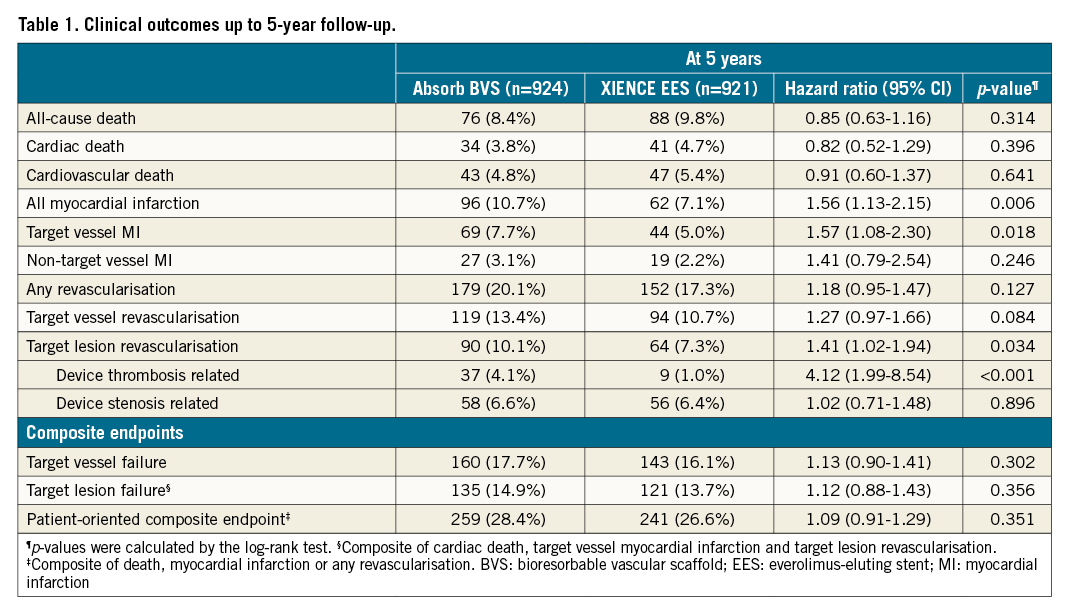
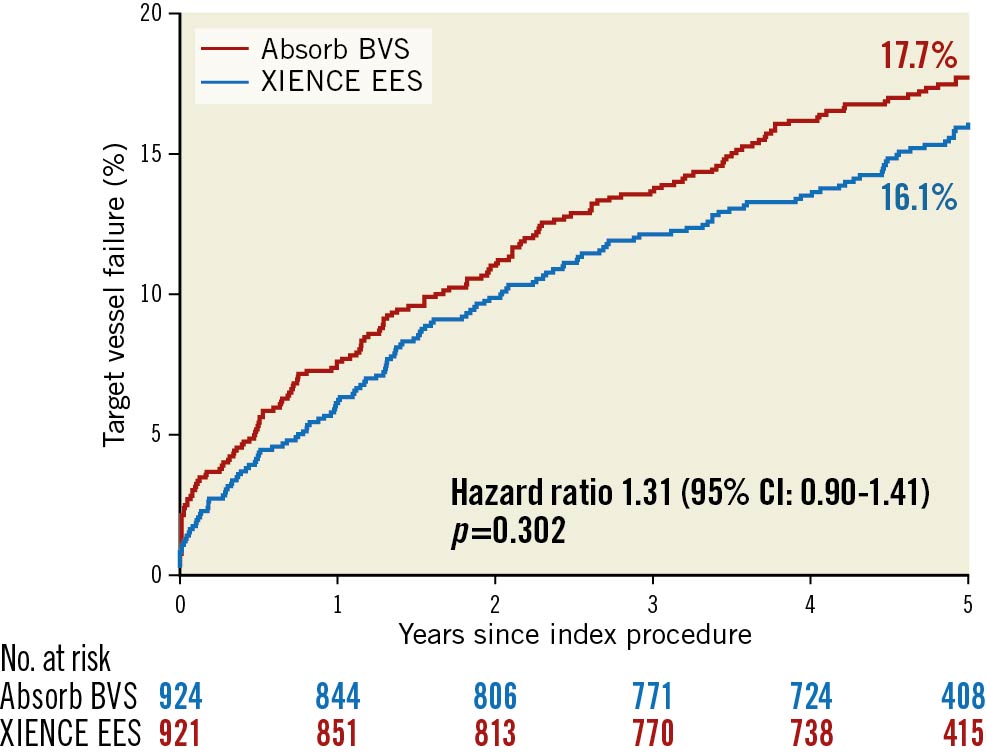
Central illustration. Kaplan-Meier curves for target vessel failure up to five-year follow-up per study arm.
Landmark analyses of clinical outcomes between 3- and 4-year, and 4- and 5-year follow-up are shown in Table 2. Clinical outcomes at four-year follow-up are shown in Supplementary Table 3. Between 3 and 4 years, the rates of TV-MI were numerically higher in the Absorb BVS compared to the XIENCE EES, 1.1% versus 0.4%, respectively (HR 3.01, 95% CI: 0.82-5.76; p=0.082). The rates of TLR were significantly higher in the Absorb BVS arm compared to the XIENCE EES arm, 1.6% versus 0.5%, respectively (HR 3.27, 95% CI: 1.07-10.02; p=0.028). This difference was mainly driven by TLR due to restenosis, 1.4% versus 0.4%, respectively (HR 3.61, 95% CI: 1.01-12.93; p=0.035).
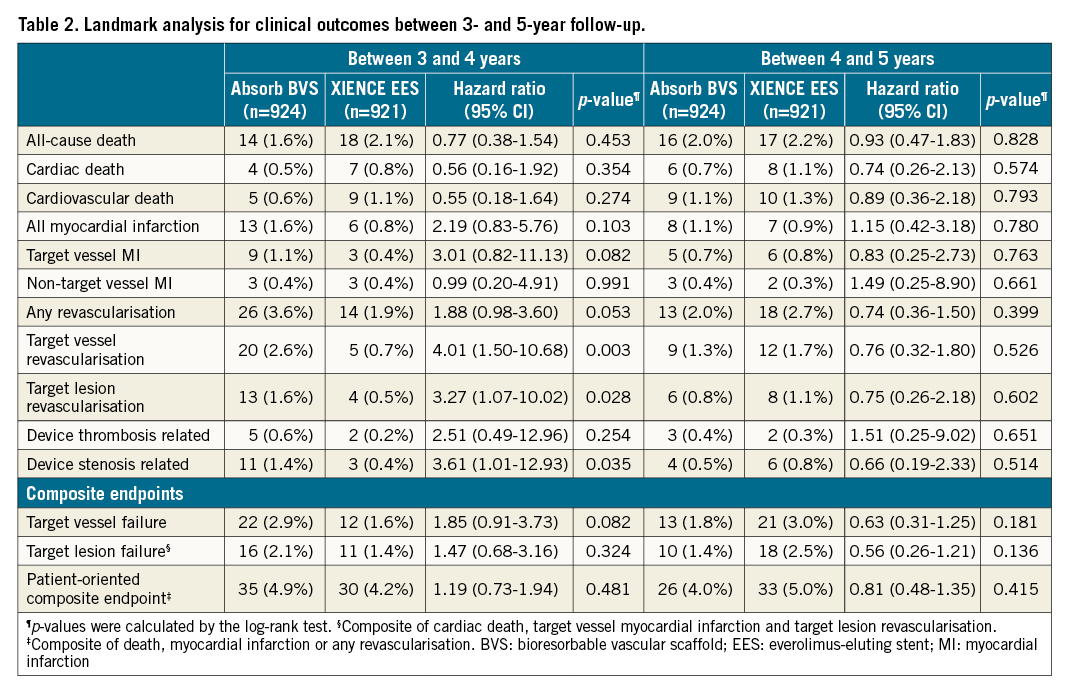
In contrast, between 4 and 5 years, the rates of TV-MI did not differ between the Absorb BVS (0.7%) and the XIENCE EES (0.8%) arms (HR 0.83, 95% CI: 0.25-2.73; p=0.763). Also, the incidence of TLR did not differ between the Absorb BVS and the XIENCE EES arms, 0.8% versus 1.1%, respectively (HR 0.75, 95% CI: 0.26-9.02; p=0.602).
Device thrombosis
DT rates are shown in Table 3. At five years, 38 Absorb BVS-treated patients suffered from definite DT compared to 9 XIENCE EES-treated patients (HR 4.24, 95% CI: 2.05-8.77; p<0.001). Descriptive characteristics of the definite DT cases throughout the five-year follow-up period are presented in Supplementary Table 4 and Supplementary Table 5. The rate of definite/probable DT was significantly increased in the Absorb BVS arm compared with the XIENCE EES arm, with a five-year rate of 4.8% (43 cases) versus 1.5% (13 cases), respectively (HR 3.32, 95% CI: 1.78-6.17; p<0.001) (Figure 1).
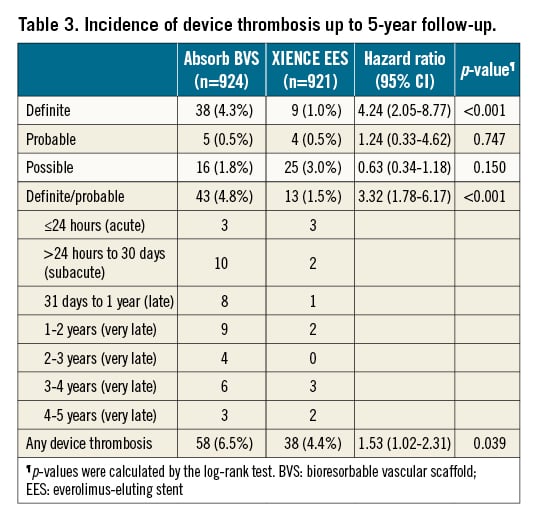
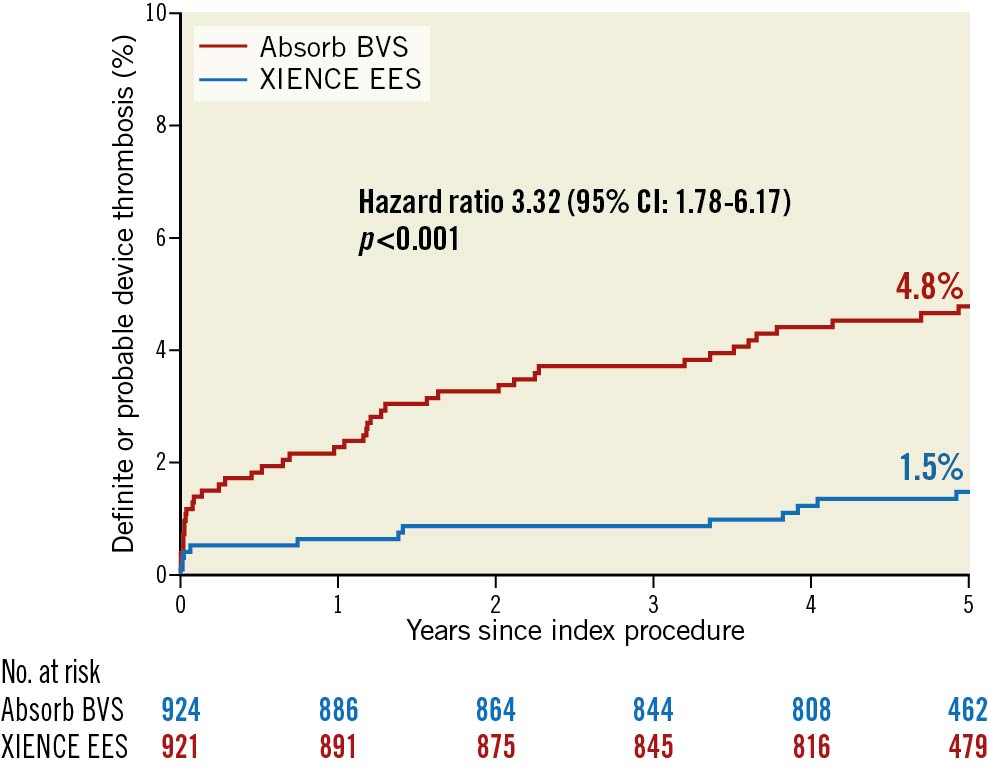
Figure 1. Kaplan-Meier curves for definite/probable device thrombosis up to five-year follow-up per study arm.
Between 3 and 4 years, 5 definite DT and 1 probable DT were noted in the Absorb BVS arm compared to 3 definite DT in the XIENCE EES arm. Of the 5 definite ScT cases, 1 case was treated with a two-stent technique in a bifurcation lesion and the DT occurred at 1,277 days post index PCI. The second very late scaffold thrombosis (VLST) was described as thrombosis on severe restenosis by the clinical events committee. The other 3 VLST cases had target lesion revascularisation with a drug-eluting stent (DES) prior to the occurrence of DT. Between 4 and 5 years, 3 definite DT in the Absorb arm versus two in the XIENCE arm were noted. Two of these 3 DT cases were randomised at baseline to the Absorb BVS arm but were treated with the XIENCE EES during the index procedure. Temporal patterns of DT are shown in Figure 2.
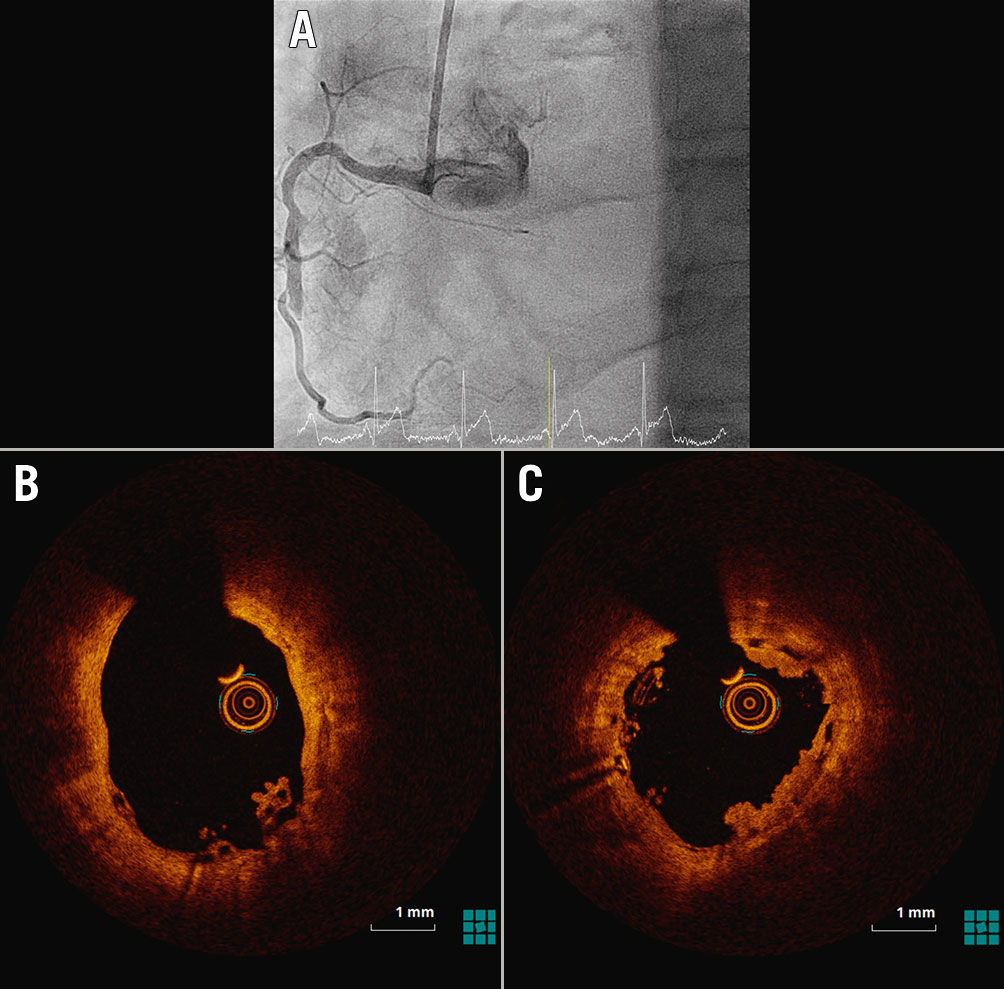
Figure 2. Temporal patterns of device failure in BVS treated lesions. The upper panel depicts a pattern of early scaffold thrombosis, procedure-related edge dissection (A). Malapposition and DAPT cessation patterns are not visualised. The lower panel depicts patterns observed in very late scaffold thrombosis in the same patient, scaffold discontinuation (B) and acquired malapposition (C).
Effect of DAPT on scaffold thrombosis
During five-year follow-up, 21 very late definite scaffold thrombosis occurred in the Absorb arm. Only 1 of these 21 VLST (4.8%) was on DAPT at the time of the event. This is in stark contrast to early DT, where 12 of the 17 patients (70.6%) used DAPT at the time of the event (Figure 3). Patients were advised to prolong DAPT up to three years. Supplementary Figure 2 shows data on aspirin, P2Y12 inhibitors, direct oral anticoagulant (DOAC) and DAPT use at all follow-up points. All VLST between 3 and 4 years occurred in patients without use of DAPT regimens. These patients discontinued DAPT 331 days (range 119-632) prior to the event. Detailed information on DAPT status at the time of ScT can be found in Supplementary Table 4.
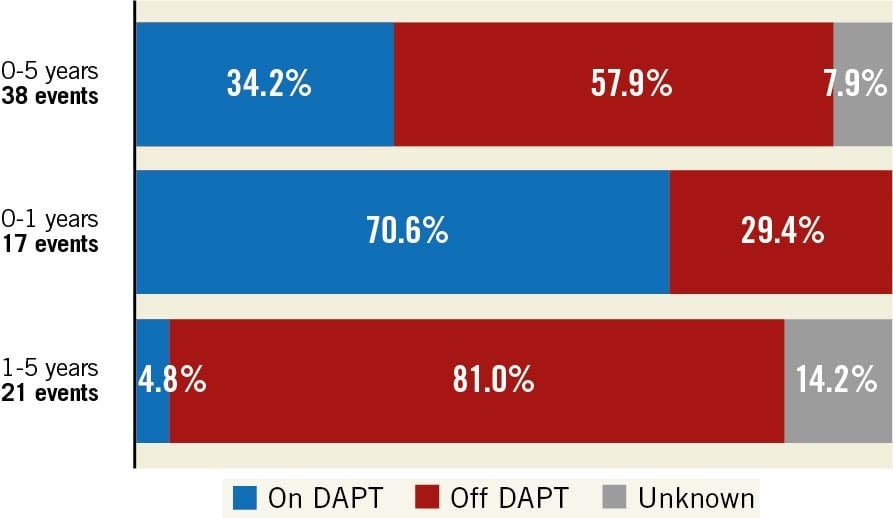
Figure 3. Relationship between definite device thrombosis and dual antiplatelet therapy (DAPT) status at the time of the event during five-year follow-up.
To make the effect of DAPT more transparent, the definite ScT cases were matched with control cases. Four of 38 ScT cases were not eligible; in 2 ScT cases the SYNTAX score was not available and DAPT status was unknown in another 2 ScT cases. DAPT status was also missing in 3 matched controls. Therefore, 34 ScT cases with 65 matched controls were included for analysis. Of those who suffered ScT, 13 patients were on DAPT and 21 patients off DAPT. Of those who did not develop ScT, 41 used DAPT and 24 did not. The odds ratio of ScT with the use of DAPT throughout five-year follow-up was 0.36 (95% CI: 0.15-0.86). Within the first year, the OR of ScT was 0.14 (95% CI: 0.02-0.85) and between 1- and 5-year follow-up the OR was 0.17 (95% CI: 0.02-1.63) (Figure 4).
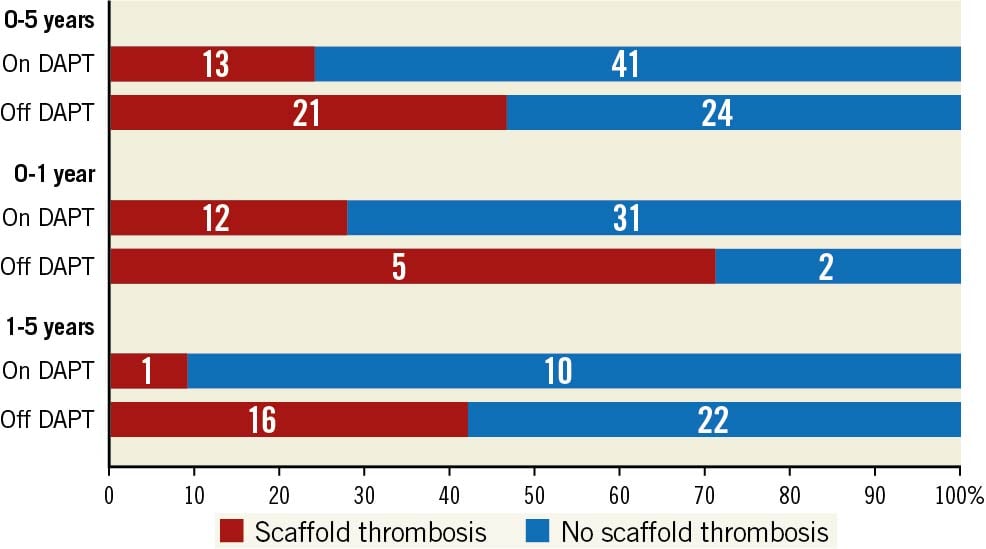
Figure 4. Effect of DAPT on occurrence of scaffold thrombosis.
Discussion
The main findings of this final five-year report on clinical outcomes of the Absorb BVS in comparison with the XIENCE EES from the AIDA trial are as follows: 1) the Absorb BVS was associated with a significantly increased risk of target vessel myocardial infarction and DT compared to the XIENCE EES as tested in daily clinical practice; 2) landmark analysis has shown a plateauing of this excess risk with the Absorb BVS starting at four years; and 3) retrospective analysis indicates a reduced odds ratio of ScT in patients using a DAPT regimen.
The excess risk of Absorb BVS thrombosis
Randomised clinical trials comparing the Absorb BVS with the XIENCE EES have identified an increased risk with the Absorb BVS on TV-MI and DT up to three years after implantation. In a pooled analysis of the Absorb trials, Stone et al12 demonstrated that this excess risk with the Absorb BVS was no longer apparent beyond three years. Compared to the first three years, the hazard ratios of target lesion failure dropped from 1.42 to 0.92, and the hazard ratio of DT dropped from 3.86 to 0.44 between 3 and 5 years12. Our results, however, show a continued excess risk up to four years. Between 3 and 4 years, the hazard ratio of target lesion failure increased from 1.133 to 1.22 at four years, and the increased risk of DT diminished but did not disappear (HR dropped from 6.023 to 2.52). It was only after four years that the excess risk with the Absorb BVS was no longer apparent. The hazard ratio of target lesion failure dropped to 0.56, and for DT it dropped to 1.51. However, 2 of the 3 DT cases between 4 and 5 years occurred in XIENCE EES-treated lesions instead of the randomised device scaffold. Therefore, the hazard ratio is overestimated.
The difference in outcomes between the ABSORB trials and AIDA might be partly explained by the difference in study populations. The study populations of the ABSORB trials mainly consisted of patients with simple lesions and low risk of restenosis. In comparison, the AIDA trial represented daily clinical practice and included patients with complex lesions and patients who had presented with acute coronary syndrome, including ST-segment elevation myocardial infarction. It might be that the resorption of the Absorb BVS is prolonged in these complex and severely diseased lesions, thereby creating a longer lasting risk of device-related events13. A better understanding of this resorption process and the factors that influence it could help us to improve next-generation bioresorbable scaffold (BRS) devices.
Effect of DAPT
Device thrombosis is a serious complication with high morbidity and mortality14. DAPT significantly reduces the risk of stent thrombosis in DES1516. The introduction of bioresorbable scaffolds led to the question on whether current DAPT recommendations after DES implantation are also applicable to this different technology. A prolonged ischaemic risk period could be expected due to its larger footprint (strut thickness 157 µm) compared with contemporary second-generation DES (60 to 90 µm), which may lead to greater platelet activation and delayed endothelialisation1718. In addition, intraluminal dismantling of the Absorb BVS at sites without complete endothelialisation during the resorption process has been suggested as a new mechanism of DT19. Well-apposed and embedded struts at baseline can still protrude into the lumen later on during the reabsorption process20. It is also plausible that good apposition at baseline would not prevent the occurrence of acquired malapposition, as large plaque burden continues to exert an inner force on the progressively weaker resorbing device. Therefore, a cause of ScT may occur at any time during the reabsorption process, rather than being present continuously and cause thrombosis after DAPT discontinuation.
Our results demonstrated an increased ischaemic risk period of four years with the Absorb BVS compared to the XIENCE EES. In particular, highly complex PCI, such as bifurcation stenting, long lesions or double layer stents, led to ischaemic events long after the index procedure. Our retrospective analysis generates a hypothesis of a potentially positive effect of DAPT on the odds ratio of ScT. In addition, there was no temporal relationship between DAPT discontinuation and VLST. For example, all ScT between 3- and 4-year follow-up occurred on average 331 days after DAPT cessation. To test whether prolonged DAPT truly outweighs the drawbacks in all patients treated with BVS or only in patients with highly complex PCI using BVS, prospective randomised studies using future-generation BVS should be carried out.
Causes of scaffold thrombosis
As previously reported, early ScT seems to be related to DAPT adherence and to procedural factors because optimisation in implantation techniques (predilatation, sizing and post-dilatation [PSP]) reduces the early stent thrombosis (ST) rate2122. In contrast, the causes of VLST are not yet fully understood and are thought to be multifactorial. Figure 2 depicts temporal patterns of ScT. Delayed scaffold resorption causing scaffold discontinuation may lead to acquired malappostion, scaffold dismantling and neoatherosclerosis. These seem to be the leading mechanisms for VLST2324. Also, the current data uncovered another possible mechanism of ScT. Three VLST occurred in lesions previously treated for restenosis with the XIENCE EES. Lack of optical coherence tomography (OCT) images precludes us from making a more definitive conclusion about the mechanisms of these particular cases and allows us only to speculate. It is possible that the DES itself caused DT. However, it cannot be excluded that resorption of the underlying BVS caused DT due to protrusion of the thrombogenic material or that it caused acquired malapposition of the DES. As new generations of scaffolds are being developed, it is important to investigate further whether it is safe to implant a metallic stent over the scaffold.
Overcoming very late device-related events
Bioresorbable scaffolds were designed to overcome very late device-related events often caused by neoatherosclerosis25. However, neoatherosclerosis did also appear in Absorb BVS-treated lesions26 and led to at least one ScT. Neoatherosclerosis will eventually occur within any device if sufficiently potent risk factors remain active and the Absorb BVS is not immune to the progression of neoatherosclerosis. In addition, the incidence of patient-oriented and device-related adverse events in the XIENCE EES group reported in the current article are not negligible. The rate of target lesion failure within the first year was 5.2%, with an annual change of ±2.2% thereafter, and a total target lesion failure rate of 16.1% at five-year follow-up. The patient-oriented composite endpoint in the XIENCE arm within first year was 10.6%. Afterwards, there was an annual change of ±4.0%, reaching 26.6% at five-year follow-up. Therefore, regardless of the stent platform, more effort on secondary prevention is needed.
Limitations
The present study has several limitations. First, the AIDA trial was powered for the primary endpoint of TVF at two years. All secondary analyses on individual components of the primary endpoint, such as ScT, should be considered as hypothesis-generating. Second, the lack of systematic intravascular imaging in patients with clinical events, precludes more definite conclusions about the mechanisms related to BVS failure at different time points. Third, restarting or prolonging DAPT to three years after scaffold implantation was recommended at the request of the DSMB. This recommendation might have influenced the occurrence of thrombosis-related outcomes in patients on prolonged or restarted DAPT compared to patients who were treated according to the applicable guidelines and instructions for use. In addition, due to this change in recommendation and the retrospective character of the DAPT analysis, selection bias cannot be ruled out. Fourth, patients and clinicians were unblinded to treatment assignment after the report of concerns about the safety of the Absorb BVS upon the recommendation of the DSMB. Fifth, bleeding events were not monitored or adjudicated by a clinical events committee which precludes us from assessing the net benefit of prolonged DAPT.
Conclusions
In addition to previous reports, the increased risk of device-related myocardial infarction and revascularisation in patients treated with the Absorb bioresorbable vascular scaffold continues up to four years after index PCI and seems to plateau afterwards. Retrospective analyses implicate a reduction of the odds for scaffold thrombosis with the use of prolonged DAPT. The latter, however, is only hypothesis-generating and should be investigated further.
Impact on daily clinical practice
The current DAPT recommendations after DES implantation cannot simply be applied to scaffolds since the Absorb BVS is known to have a prolonged ischaemic risk: during the reabsorption process, it is associated with higher rates of device thrombosis. Our analysis hypothesises a reduction of this excessive risk by prolonging DAPT after BVS implantation. However, further research on DAPT duration after future-generation BVS implantation is warranted.
Acknowledgements
The authors greatly acknowledge all the patients for their participation.
Funding
The AIDA trial was supported by an unrestricted educational grant from Abbott Vascular. The Amsterdam UMC Heart Center received an unrestricted educational research grant from Abbott Vascular for the AIDA trial. The Research Department of the Cardiology Division of the Medical Center Leeuwarden received non-study-related unrestricted educational research grants from Abbott Vascular.
Conflict of interest statement
H.M. Garcia-Garcia was a member of the clinical events committee of the trial. J. Piek is a member of the Medical Advisory Board of Abbott Vascular. MedStar Washington Hospital Center receives research grants for clinical research from Abbott Vascular. J. Tijssen has served on the DSMB of the early ABSORB trials, including ABSORB II. J. Henriques has received research grants from Abbott Vascular. J. Wykrzykowska had received consultancy fees and research grants from Abbott Vascular. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

