GUEST EDITOR: Giulio Guagliumi, MD; Division of Cardiology, Cardiovascular Department, Ospedali Riuniti di Bergamo, Bergamo, Italy
Abstract
Aims: To assess serially the edge vascular response (EVR) of a bioresorbable vascular scaffold (BVS) compared to a metallic everolimus-eluting stent (EES).
Methods and results: Non-serial evaluations of the Absorb BVS at one year have previously demonstrated proximal edge constrictive remodelling and distal edge changes in plaque composition with increase of the percent fibro-fatty (FF) tissue component. The 5 mm proximal and distal segments adjacent to the implanted devices were investigated serially with intravascular ultrasound (IVUS), post procedure, at six months and at two years, from the ABSORB Cohort B1 (n=45) and the SPIRIT II (n=113) trials. Twenty-two proximal and twenty-four distal edge segments were available for analysis in the ABSORB Cohort B1 trial. In the SPIRIT II trial, thirty-three proximal and forty-six distal edge segments were analysed. At the 5-mm proximal edge, the vessels treated with an Absorb BVS from post procedure to two years demonstrated a lumen loss (LL) of 6.68% (–17.33; 2.08) (p=0.027) with a trend toward plaque area increase of 7.55% (–4.68; 27.11) (p=0.06). At the 5-mm distal edge no major changes were evident at either time point. At the 5-mm proximal edge the vessels treated with a XIENCE V EES from post procedure to two years did not show any signs of LL, only plaque area decrease of 6.90% (-17.86; 4.23) (p=0.035). At the distal edge no major changes were evident with regard to either lumen area or vessel remodelling at the same time point.
Conclusions: The IVUS-based serial evaluation of the EVR up to two years following implantation of a bioresorbable everolimus-eluting scaffold shows a statistically significant proximal edge LL; however, this finding did not seem to have any clinical implications in the serial assessment. The upcoming imaging follow-up of the Absorb BVS at three years is anticipated to provide further information regarding the vessel wall behaviour at the edges.
Abbreviations
BVS: bioresorbable vascular scaffold
DES: drug-eluting stent
EEM: external elastic membrane
EES: everolimus-eluting stent
EVR: edge vascular response
FU: follow-up
GM: geographic miss
IVUS: intravascular ultrasound
LL: lumen loss
PCI: percutaneous coronary intervention
PES: paclitaxel-eluting stent
SES: sirolimus-eluting stent
VBT: vascular brachytherapy
Introduction
Although the initial use of radioactive devices in the era of vascular brachytherapy (VBT) and later the utilisation of first-generation drug-eluting stents (DES) partially eliminated the iatrogenic entity of neointimal hyperplasia1,2, the vascular response at the stent-to-artery transitions, presenting as a late lumen loss (LL) at the margins of the treated segments, remains one of the pitfalls of percutaneous coronary intervention (PCI)3. The term “edge effect” –defining a flow-limiting lesion at the stent edges– is meant to describe one of the major drawbacks of VBT induced by a combination of factors: the radioactive dose fall-off at the transition zones in association with either the axial geographic miss (GM) (injured or diseased segment not covered by the device), or longitudinal GM phenomena (balloon-artery ratio <0.9 or >1.3)4-7. DES failure as a consequence of LL has been shown to present with a focal pattern, affecting particularly the proximal stent edge, as was demonstrated in >60% of in-stent restenosis cases with either paclitaxel-eluting stents (PES) or sirolimus-eluting stents (SES)8.
In the SIRIUS trial (a multicentre study of the SIRolImUS-eluting Bx-Velocity stent in the treatment of patients with de novo coronary artery lesions) a significant proximal edge LL was observed and was attributed to the vascular “trauma” at the stent margins caused by pre/post balloon dilatation (100%/70%, respectively). Therefore, less traumatic stent implantation (e.g., direct stenting without high-pressure post-dilatation) was proposed which partially eliminated the procedure-related complication of proximal restenosis as shown in the intravascular ultrasound (IVUS) substudy of the E-SIRIUS trial9.
In the TAXUS II trial, the slow release and moderate release polymer formulations of the PES resulted in proximal LL of 0.54±2.1 mm2 and 0.88±1.9 mm2, respectively, while in the BETAX trial, utilising the Taxus™ Express™ DES (Boston Scientific, Natick, MA, USA), significant plaque changes in tissue composition were observed, mainly due to an increase in the fibro-fatty (FF) tissue component causing adaptive expansive remodelling at both stent edges10.
In the ABSORB Cohort B trial the second-generation Absorb™ BVS (Abbott Vascular, Santa Clara, CA, USA) has recently been evaluated non-serially at six-month and one-year follow-up (FU) demonstrating some degree of proximal edge constrictive remodelling of: Δ vessel area: –1.80% (–3.18; 1.30) (p<0.05), at six months that tended numerically to regress at one year and distal edge changes in plaque phenotype with an absolute increase of the FF tissue component from 0.06 mm2 (0.01; 0.17) to 0.10 mm2 (0.03; 0.28) translated to a relative increase of: ΔFF +43.32% (–19.90; 244.28) (p<0.05) that caused non-significant plaque progression with signs of adaptive expansion at this segment11.
The advent of next-generation devices with either metallic or bioresorbable platforms has prompted the in vivo re-evaluation of the edge vascular response (EVR) with state-of-the-art sound-based imaging modalities like intravascular ultrasound (IVUS). The purpose of this study is to investigate the EVR following implantation of two different platforms which have the same everolimus drug elution: the second-generation bioresorbable Absorb BVS and the second-generation metallic XIENCE V® EES (Abbott Vascular, Santa Clara, CA, USA). We analysed greyscale IVUS data acquired serially post procedure, at six months and at two years, from patients who were included in the ABSORB Cohort B and the SPIRIT II trials to report for the first time the early and late vascular responses at the edges.
Methodology
ABSORB COHORT B TRIAL
STUDY DESIGN AND POPULATION
The ABSORB Cohort B trial (NCT00856856) is an ongoing multicentre single-arm prospective, open-label trial assessing the safety and performance of the second-generation Absorb BVS in the treatment of patients with a maximum of two de novo native coronary artery lesions. In total, 101 patients were enrolled, divided into two subgroups –Cohort B1 (n=45) and Cohort B2 (n=56)– according to the predefined study design. Both groups underwent invasive FU at different time points: Cohort B1 at six months and two years, and Cohort B2 at one year. Additionally, a three-year invasive imaging evaluation of Cohort B2 is expected.
TREATMENT DEVICE
The Absorb BVS (Abbott Vascular) is a balloon-expandable scaffold consisting of a polymer backbone of Poly-L-lactide (PLLA) coated with a thin layer of a 1:1 mixture of Poly-D, L-lactide (PDLLA). The polymer controls the release of the antiproliferative drug everolimus, and forms an amorphous drug-eluting coating matrix that contains 100 micrograms of everolimus/cm2 of scaffold. According to non-human studies, the Absorb BVS has shown a dynamic biologic behaviour at six months, one year and two years, beyond which almost complete bioresorption of the polymeric backbone is expected12,13.
TREATMENT PROCEDURE
Lesions were treated with routine interventional techniques that included mandatory predilatation with a balloon shorter, and 0.5 mm smaller in diameter, than the study device. The Absorb BVS was implanted at a pressure not exceeding the rated burst pressure (16 atm) (avoidance of axial GM). Post-dilation with a balloon shorter than the implanted device (avoidance of longitudinal GM) was allowed at the discretion of the operator to optimise device expansion.
SPIRIT II TRIAL
STUDY DESIGN AND POPULATION
The SPIRIT II study (NCT00180310) was a prospective, two-arm trial that randomised 300 patients in a 3:1 ratio to either a XIENCE V EES (n=223) or a TAXUS PES (n=77) in the treatment of coronary artery disease. Serial intravascular imaging was performed in a subset of 152 patients (EES: n=113, and PES: n=39). Thirty-two patients were included in the serial evaluation of the proximal edge and 14 (42%) received at least one 3.0×18 mm device. At the distal edge 41 patients were included with 20 (43%) having undergone implantation of a 3.0×18 mm device.
TREATMENT DEVICE
The XIENCE V everolimus-eluting stent system (Abbott Vascular) is a balloon-expandable device which consists of serpentine rings connected by links fabricated from a single piece of medical grade L-605 cobalt-chromium alloy. Everolimus is blended in a non-erodable polymer coated over another non-erodable polymer primer layer. The coating consists of acrylic and fluoro polymers, both approved for use in blood-contacting applications. This layer of everolimus-polymer matrix with a thickness of 5-6 microns is applied to the surface of the stent and is loaded with 100 micrograms of everolimus/cm2 of stent surface area with no topcoat polymer layer. The stent is designed to release approximately 80% of the drug within 30 days after implantation.
TREATMENT PROCEDURE
Lesions were treated using standard interventional techniques that included mandatory predilatation and stent implantation at a pressure not exceeding the burst pressure. Post-dilatation was left to the discretion of the physician; however, if performed, it was only to be done with balloons sized to fit within the boundaries of the stent.
QUANTITATIVE IVUS ANALYSIS
ABSORB COHORT B AND SPIRIT II TRIALS
Scaffolded segments, including the 5-mm proximal and distal parts, underwent imaging evaluation post procedure, at six-month and two-year FU with a phased array 20 MHz IVUS catheter (Eagle Eye®; Volcano Corporation, Rancho Cordova, CA, USA, and Atlantis™; Boston Scientific, Natick, MA, USA) after intracoronary administration of 100-200 µg nitroglycerine, using automated pullback at 0.5 mm/sec (30 frames/sec). Geometrical parameters in the 5-mm proximal and distal edge segments derived from the greyscale IVUS acquisition14,15 were analysed in each separate frame, i.e., vessel area, lumen area, plaque area as absolute values and percentages by an independent clinical research organisation (Cardialysis BV, Rotterdam, The Netherlands).
STATISTICAL ANALYSIS
Continuous variables are presented as medians and interquartile ranges. Discrete variables are presented as counts and percentages. Paired comparisons between continuous variables within groups at different time points were estimated with the Wilcoxon signed-rank test, while the Mann-Whitney U test was used for independent two-sample comparisons. Changes (differences) for each measurement were calculated as: follow-up minus post procedure values. Percent changes (differences) for each variable were calculated as: follow-up – post procedure/post procedure ×100%. A p-value <0.05 was considered statistically significant. Data analyses were performed with SAS version 9.1 software (SAS Institute Inc., Cary, NC, USA).
RESULTS
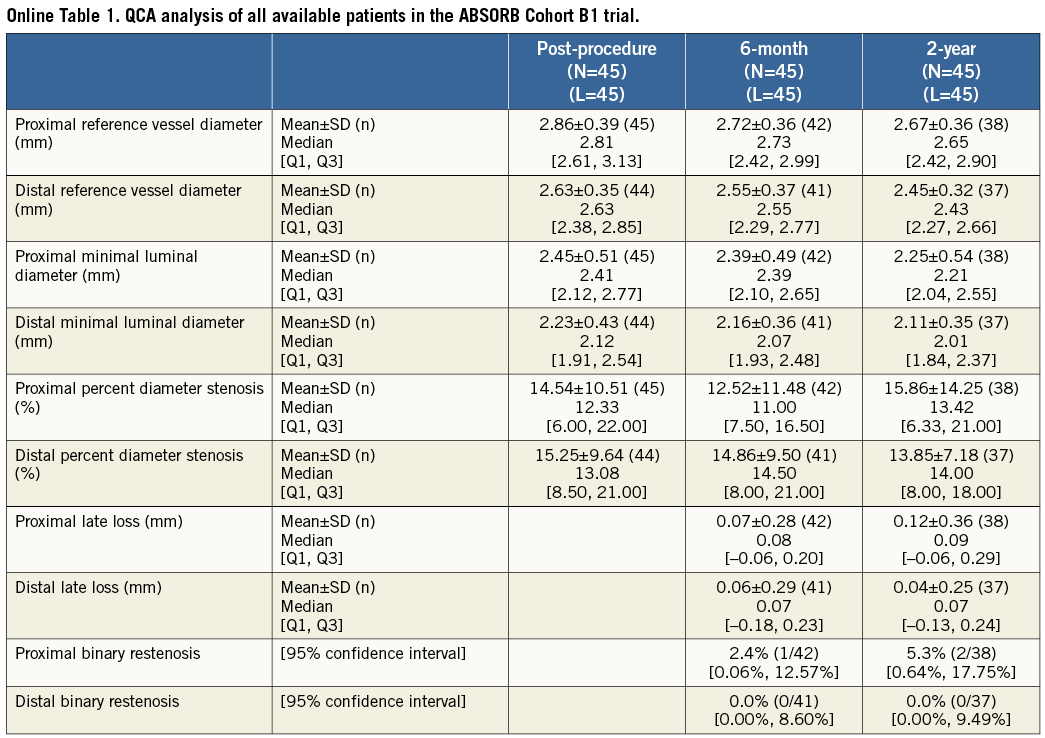
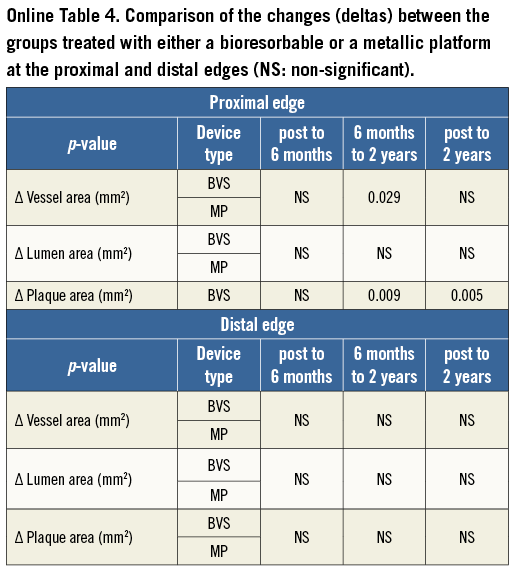
Twenty-two lesions from the ABSORB Cohort B1 and 33 from the SPIRIT II trial had IVUS imaging at the proximal edge in all time points and were included in the proximal edge analysis, while 24 from the ABSORB Cohort B1 and 46 from the SPIRIT II trial had serial IVUS assessment of the distal edge. None of the patients included in the serial analysis of the ABSORB Cohort B1 underwent TLR due to restenosis at the scaffold edges. However, two patients from the complete (non-serial) ABSORB Cohort B1 had a target lesion revascularisation (TLR), due to proximal edge restenosis16. The first patient returned on day 358 with progressive angina, and coronary angiography revealed proximal edge restenosis adjacent to the implanted Absorb BVS. The patient was revascularised with a XIENCE V EES. The second patient returned on day 168 with progressive angina, and repeat angiography also revealed proximal edge restenosis associated with operator-related mechanical trauma. These two patients were excluded from the final analysis as they did not have truly serial IVUS acquisitions at all time points. With regard to the SPIRIT II trial, two patients who had distal edge restenoses at days 175 and 731 were also excluded from the final analysis (similar to the ABSORB Cohort B) as they lacked truly serial IVUS imaging (Figure 1). However, the calculations including the IVUS results of the patients who underwent IVUS prior to the TLR were imputed at the two-year results stage (Table 2, Table 3, Table 4, and Online Appendix).
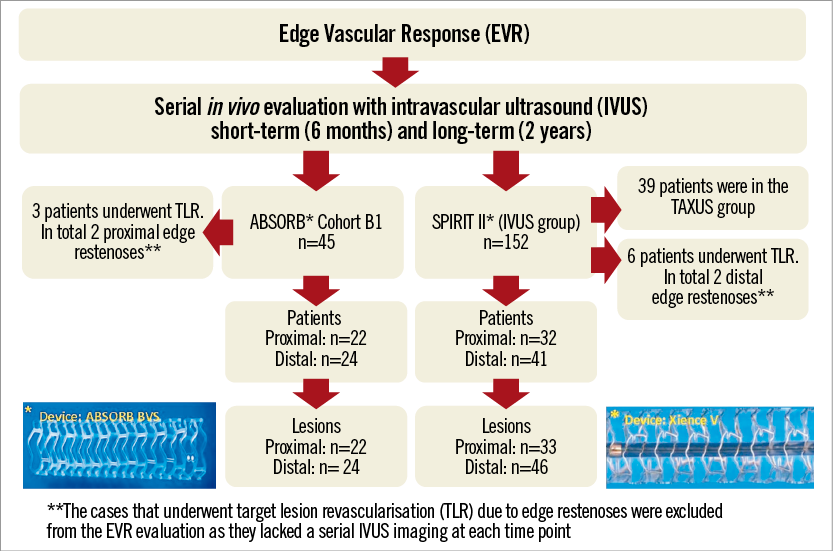
Figure 1. Flow chart of patient/lesion selection for the assessment of the edge vascular response after implantation of either an Absorb bioresorbable vascular scaffold or a metallic XIENCE V everolimus-eluting stent.
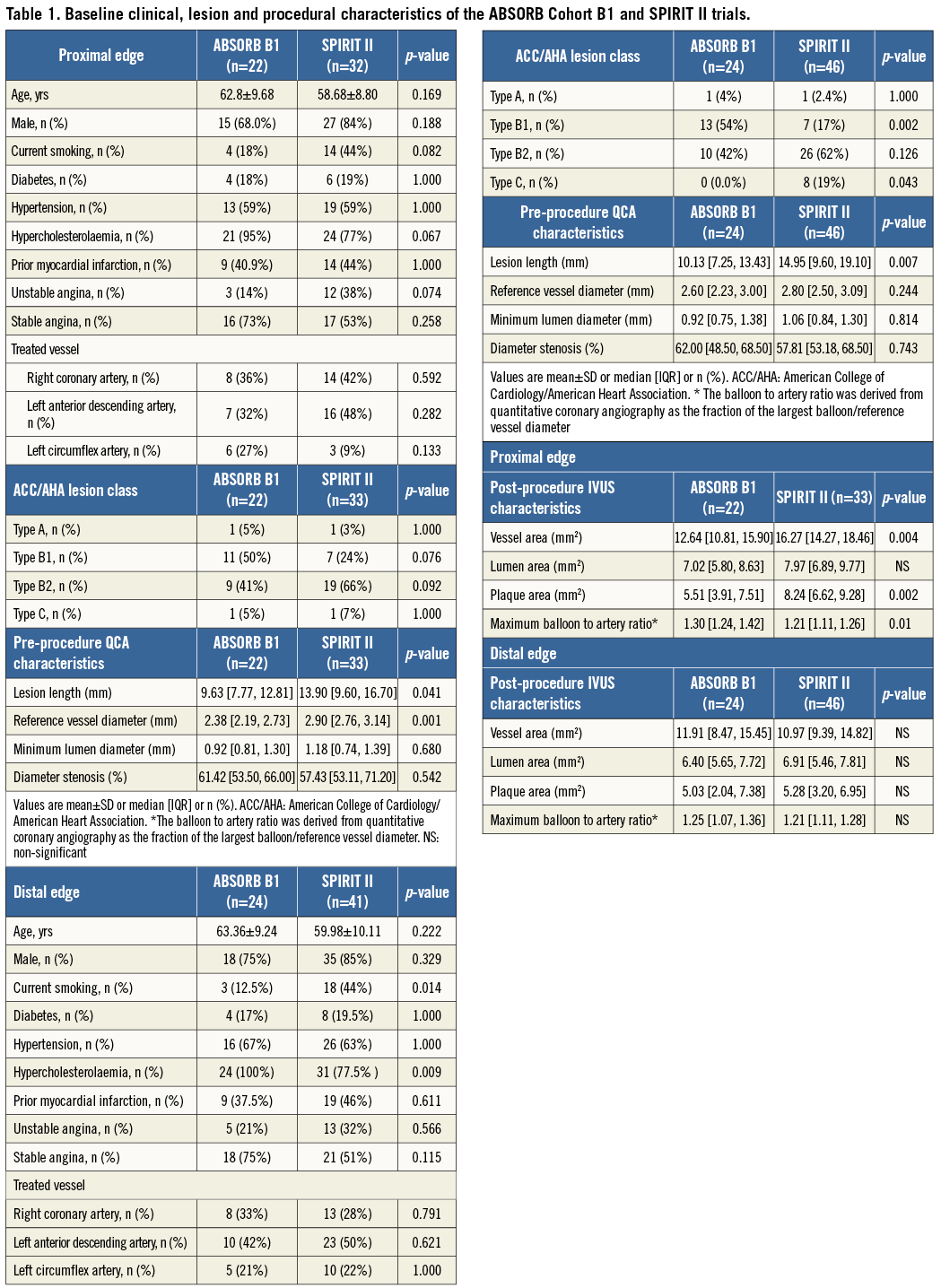
The baseline clinical, lesion and procedural characteristics of the studied populations are reported (Table 1A and Table 1B). There were no significant statistical differences between the patients treated with an Absorb BVS and a XIENCE V EES in the proximal edge analysis. At this segment, a higher lesion length and reference vessel diameter in the XIENCE V group was noted. The baseline characteristics between the two subgroups included in the distal edge analysis were similar; however, the Absorb BVS patients were more likely to suffer from hypercholesterolaemia and less likely to smoke. At this segment, a higher incidence of Type C lesions and a higher lesion length in the XIENCE V vs. the Absorb BVS treated vessels became evident (Table 1). With regard to the post-procedure IVUS measurements there were no significant statistical differences of the lumen areas between the two groups; however, in the SPIRIT II population an increased plaque burden and vessel area were noted at the proximal edge.
ABSORB BVS
PROXIMAL EDGE
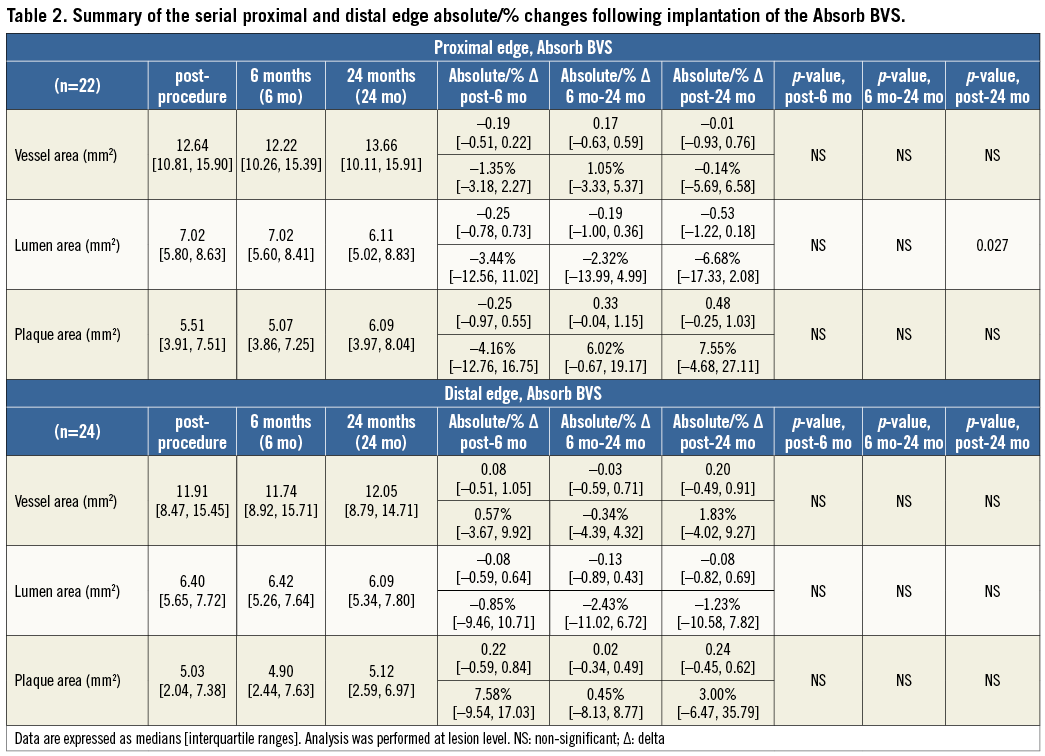
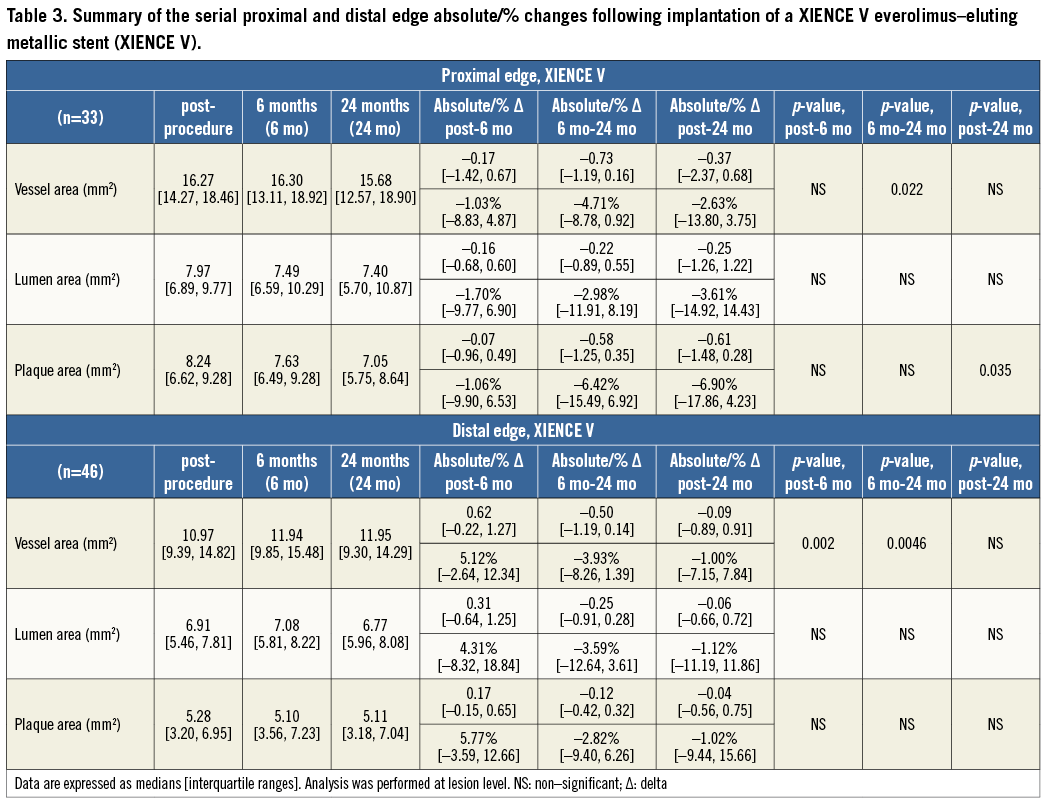
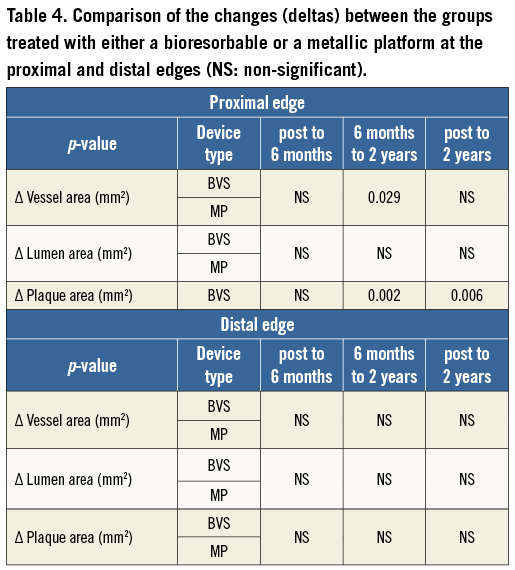
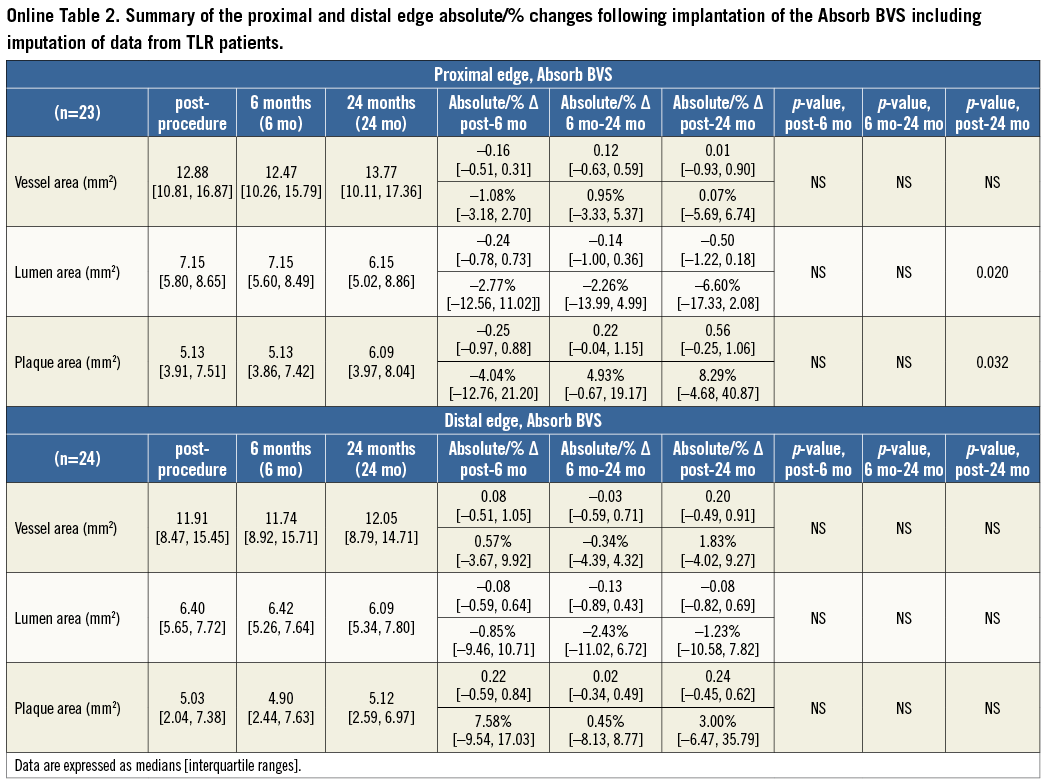
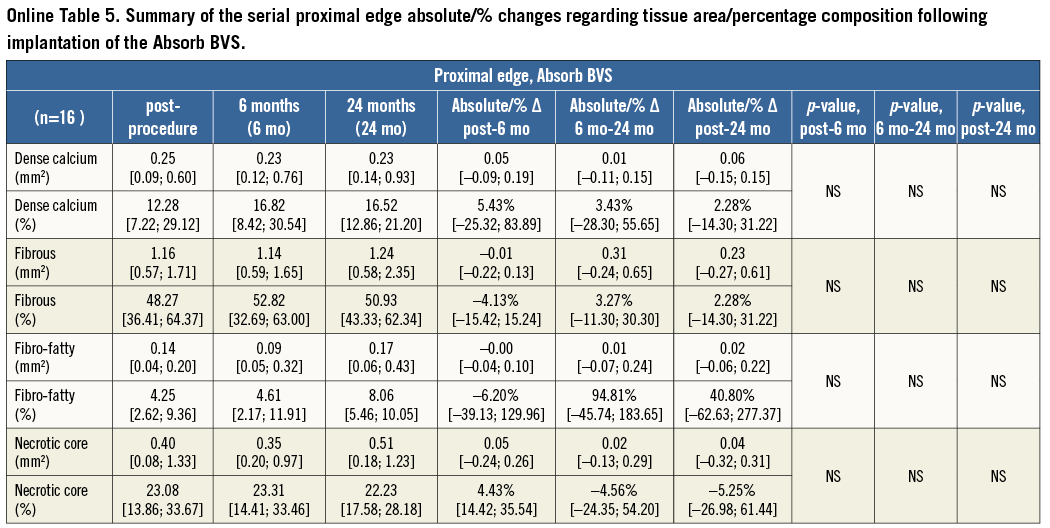
At the 5-mm segment, no major absolute or relative changes were shown in terms of vessel, lumen and plaque areas in the short term (post procedure to six months). In the long term (post procedure to two years), a LL of 6.68% (–17.33; 2.08) (p=0.027) was observed with a trend towards a plaque area increase of 7.55% (–4.68; 27.11) (p=0.06) (Figure 2, Figure 4 and Table 2).
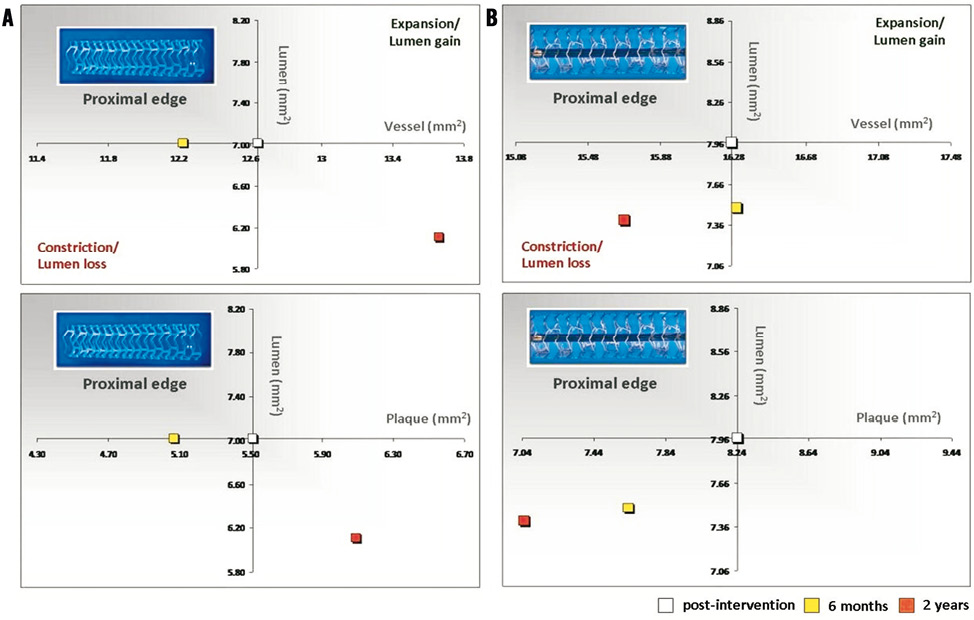
Figure 2. Absolute values of the vessel and plaque areas related to lumen area at the proximal edges of the Absorb BVS (A) and XIENCE V (B) devices.
DISTAL EDGE
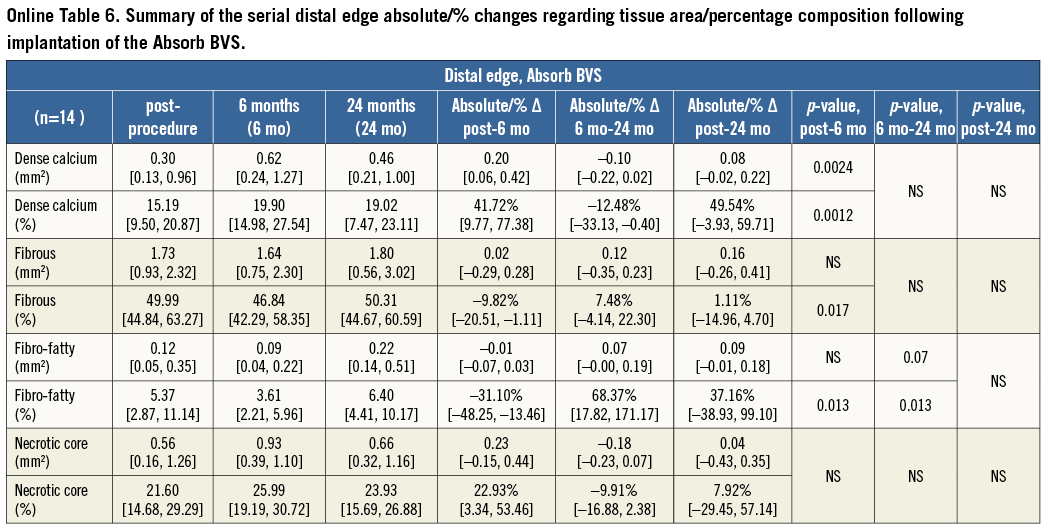
At the distal edge no major changes were demonstrated either in the short or in the long term (Figure 3, Figure 4 and Table 2).
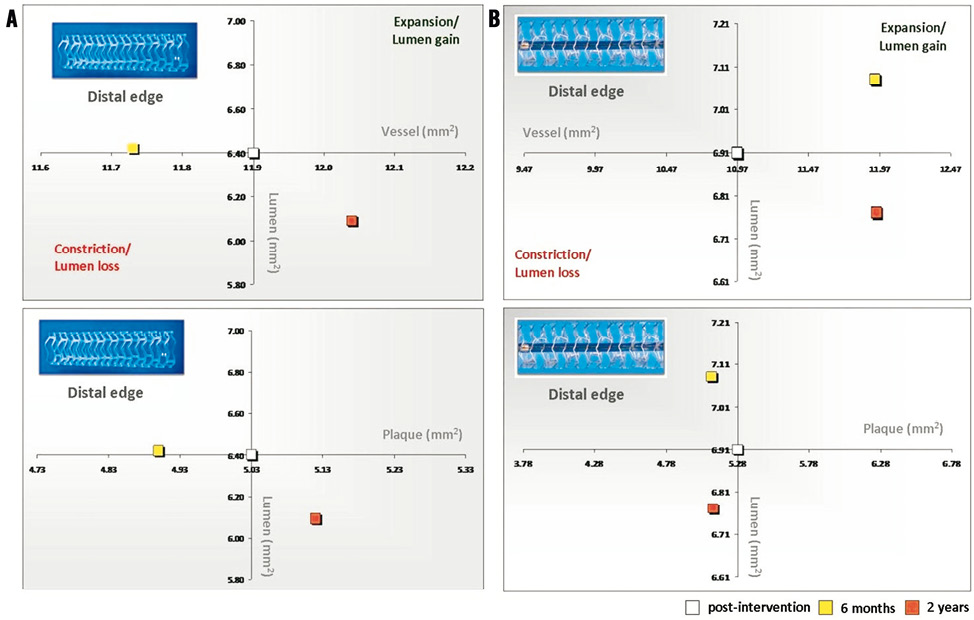
Figure 3. Absolute values of the vessel and plaque areas related to lumen area at the distal edges of the Absorb BVS (A) and XIENCE V (B)devices.
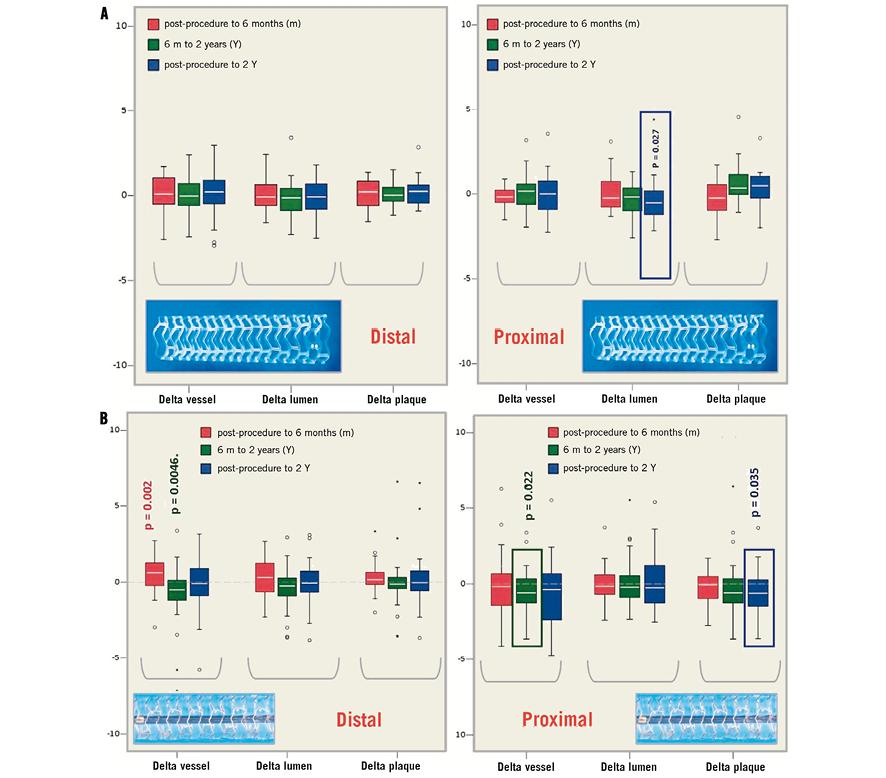
Figure 4. A) The changes (deltas) in vessel, lumen and plaque areas at the proximal and distal edges following implantation of the Absorb BVS post procedure, at 6 months and 2 years (significant changes are demonstrated with the p-value). B) The changes (deltas) in vessel, lumen and plaque areas at the proximal and distal edges following implantation of the XIENCE V EES, post procedure, at 6 months and 2 years (significant changes are demonstrated with the p-value).
XIENCE V
PROXIMAL EDGE
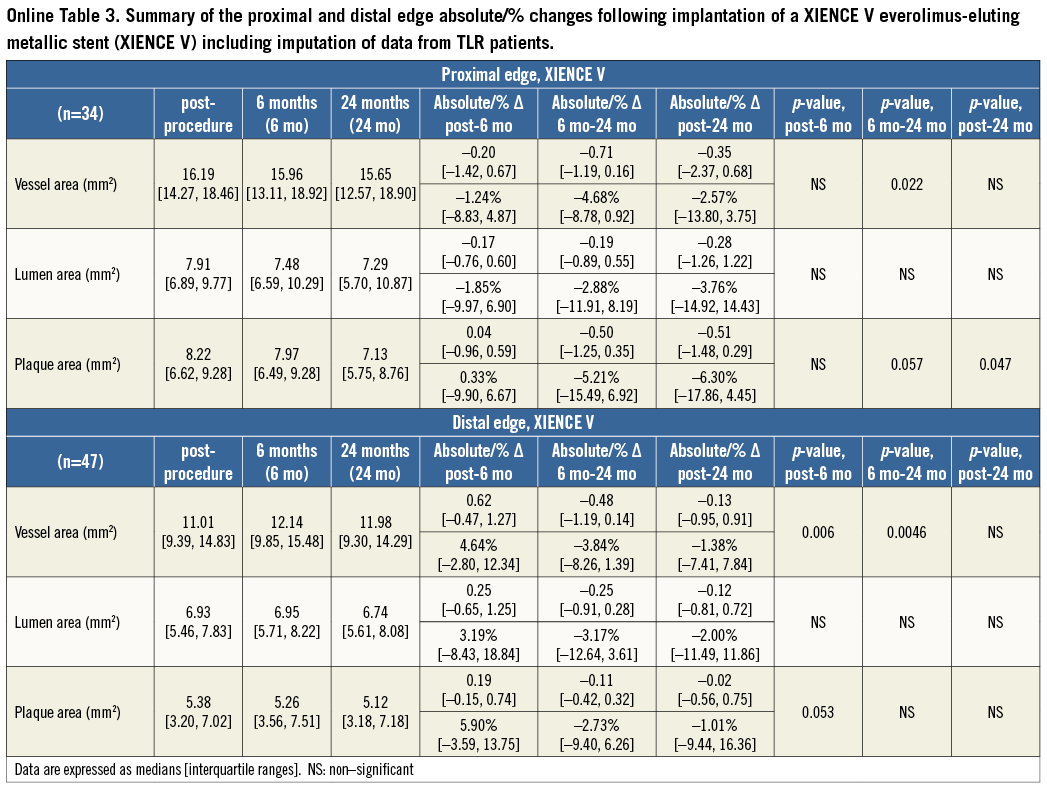
At the 5-mm segment no major absolute or relative changes were shown in terms of vessel, lumen and plaque areas in the short term. In the long term the plaque area decreased by 6.90% (–17.86; 4.23) (p=0.035) while the lumen area remained unchanged indicating adaptive constrictive remodelling (Figure 2, Figure 5 and Table 3).
DISTAL EDGE
At the distal edge, a dynamic vascular response was evident: a compensative expansive remodelling of Δ vessel area: 5.12% (–2.64; 12.34) (p=0.002) at six months that tended to regress at two years losing its statistical significance (Figure 3, Figure 5 and Table 3).
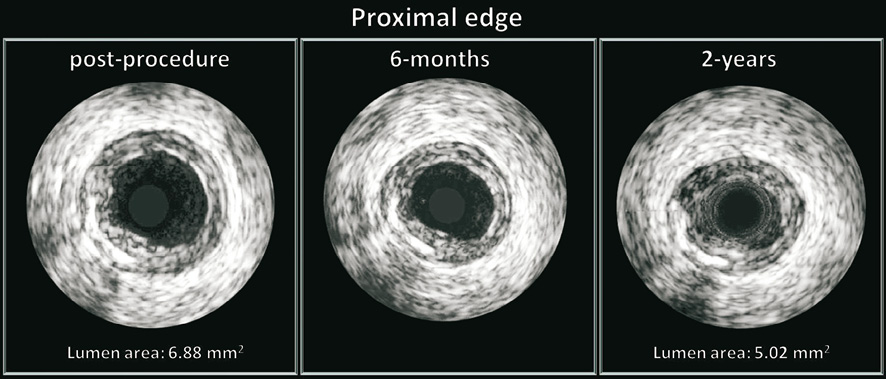
Figure 5. The vascular response at the proximal edge following implantation of a bioresorbable vascular scaffold. Greyscale IVUS cross-sections post procedure, at 6 months and 2 years demonstrating the gradual lumen loss. At the 5-mm proximal segment of this patient the mean lumen area decreased from 6.88 mm2 (post procedure) to 5.02 mm2 (2 years).
Discussion
The main findings of this study are:
– Short term: i) the vessels treated with an Absorb BVS did not show any signs of remodelling or LL either at the proximal or at the distal edges; ii) the vessels treated with a XIENCE V did not show any signs of remodelling or LL at the proximal edge; however, compensative expansive remodelling at the distal edge was observed.
– Long term: i) the vessels treated with the Absorb BVS showed evidence of proximal edge LL (this finding is consistent with the previously reported reduction in the minimum lumen diameter assessed angiographically at the proximal edge - from post procedure: 2.39±0.50 mm, to two years: 2.31±0.42 [p=0.04])17; ii) the vessels treated with a XIENCE V did not show any signs of LL at either the proximal or the distal stent edges. At the proximal edge, a significant plaque decrease became evident with a trend towards adaptive constrictive remodelling.
The current analysis used serial IVUS data to evaluate for the first time the short-term and long-term EVR after Absorb BVS and XIENCE V EES implantation. Although both devices share the same release kinetics and dose density of everolimus, we found a different vascular response at the proximal and distal edges, potentially attributed to the different mechanical properties of the investigated platforms.
The implantation of a device either metallic or polymeric induces local arterial stiffness of the stented/scaffolded segment, abrogating the physiologic cyclic strain and its vascular compliance and further creating compliance mismatch with the adjacent proximal and distal edges18. It has recently been confirmed that bioresorbable scaffolds can also transiently reduce the arterial compliance resulting in compliance mismatch between the scaffolded and the adjacent segments, an observation that tended to disappear at short and mid term (one year) following implantation of the Absorb BVS19. Additionally, the changes in three-dimensional vessel geometry and vessel curvature following implantation of either a polymeric or a metallic device alter the flow velocities at the transition zones (proximal and distal edges) creating regions of disturbed laminar flow, flow separation with retrograde axial velocities (low endothelial shear stress [ESS] regions) known to be proatherogenic and to alter cell mechanotransduction19,20-22. These changes may potentially cause adaptive remodelling of the extracellular matrix through alterations of the physiological local mechanical loading conditions with various patterns of compensation (from poor to overcompensation)23.
This concept became evident with the XIENCE V metallic EES in regard to vascular remodelling and matrix production that appeared to have a dynamic response at the distal edge. In particular, in the short term, a compensatory expansive remodelling of 5.12% (–2.64; 12.34) (p=0.002) was evident to counterbalance a trend towards plaque increase of 5.77% (–3.59; 12.66) (p=0.083) (low ESS region) that was further converted into constrictive remodelling between six months and two years resulting in a neutral net effect from post procedure to two years.
PROXIMAL EDGE
At this segment, the vessels treated with an Absorb BVS did not show any major changes at short-term FU; however, at long-term FU a slight but statistically significant LL was observed, attributed to plaque area increase. LL at the proximal edge has previously been reported with bare metal stents and DES; however, the reduction in lumen area with the Absorb BVS commenced at a later time point (after six months), suggesting that different mechanisms are involved in this process. The recently reported plaque/media and neointima increase from six months to two years in the scaffolded segment has been attributed to the vessel wall/scaffold interaction during the bioresorption process that affects the vessel wall physiology and alters the plaque’s components18,24,25. We surmise that the LL and plaque increase noted in the proximal edge are due to the potentially insufficient suppression of the EVR by the antiproliferative drug. On the contrary, the vessels treated with a XIENCE V stent did not show any signs of LL at both FU points. At late FU, a reduction in the plaque and vessel area became evident indicating an adaptive constrictive remodelling. This effect could be attributed to the mechanical injury following stent implantation which triggers a pathophysiological process that leads to constrictive remodelling26. This process appears to be initiated immediately after device implantation; however, the changes in plaque and vessel wall dimensions become significant at two years. In contrast to the bare metal stents and SES, the vessel response at the proximal edge of a XIENCE V EES did not affect the lumen area9. This observation could be attributed to the smaller strut thickness of the XIENCE V (89 μm vs. 152 μm in the first-generation SES) and the presence of the antiproliferative drug everolimus that can potentially delay and attenuate the vascular tissue response27,28.
DISTAL EDGE
In contrast to the proximal edge, at the distal edge the vessels treated with an Absorb BVS did not show any major changes either at short-term or at long-term FU. This difference in the EVR can potentially be explained by the sufficient concentration of the everolimus elution at the downstream vessel which can inhibit atherosclerosis and reduce local inflammation. Everolimus has been shown to inhibit strongly the development of progressive atherosclerotic lesions in animal models: 1) by delaying the transition from early macrophage-enriched lesions to advanced atherosclerotic plaques in LDL receptor –/– (knockout) mice; and 2) by selective clearance of macrophages through autophagy in atherosclerotic plaques29-31.
Our findings in the XIENCE V EES are in agreement with those previously reported, as there were no significant differences in the lumen and plaque area at short-term FU32-34. An increase in the vessel area was noted at six months which, however, appeared to be temporal as it decreased at two years. Of note, these observations were not accompanied by statistically significant changes in the lumen and plaque.
Expansive remodelling has previously been reported at the distal edge of other metallic DES and has been attributed to endothelial dysfunction. A dysfunctional endothelium can promote expansive remodelling and plaque progression especially in an unfavourable haemodynamic environment created by the modified vessel geometry and the compliance mismatch demonstrated in an experimental setting at the distal edge35-37. Endothelial dysfunction at the edges of a DES can be present up to one year following device implantation38,39. Unfortunately, long-term FU results which would allow us to estimate the duration of the endothelial dysfunction and the effect of a functional/dysfunctional endothelium on the progression/regression of atherosclerosis at the edges of these stents are not available.
Conclusion
The fully bioresorbable device (Absorb BVS) and the metallic platform (XIENCE V) demonstrated a different EVR at six months and two years which is likely to be associated with the distinct properties of each device. The serial assessment of the XIENCE V EES did not show any LL at both FU points, while the observed significant proximal edge LL induced by the Absorb BVS at two years did not have any clinical implications. The upcoming imaging FU of the ABSORB Cohort B2 trial at three years is anticipated to provide additional information about the EVR after Absorb BVS implantation.
Limitations
The major limitation of the present analysis is the small number of investigated proximal and distal edges (patient/lesion level). Thus the present study may be underpowered to evaluate the exact changes at the stent/scaffold edges during follow-up and the p-values should be considered exploratory and interpreted with caution. However, these cohorts of patients represent the only available data on the serial assessment of the edge vascular response utilising a metallic and a bioresorbable device at three different imaging time points. The approximate final tested samples of the Cohort B1 and SPIRIT II studies were <50%. The reasons for this were: 1) the dropout of patients at follow-up and exclusion of the unpaired samples from our final analysis; 2) the exclusion of cases according to the standard operational procedure of the independent core laboratory (Cardialysis, Rotterdam, The Netherlands) with: i) side-branch outgrowth of >90 degrees at the side of the scaffold edge that did not allow the analysis of the complete 5-mm segment; ii) vessel wall out of the field of the vessel; and 3) the exclusion of cases adjudicated as target lesion revascularisation –some of them attributed to edge restenosis– where serial assessment was not possible.
Although the SPIRIT II trial had more complex lesions compared to the ABSORB Cohort B, this was an exploratory study that investigated for the first time a second generation of devices with different platforms (metal vs. polymer) in a follow-up spanning two years. Future studies are expected to validate these preliminary results.
Guest Editor
This paper was Guest Edited by Giulio Guagliumi, MD; Division of Cardiology, Cardiovascular Department, Ospedali Riuniti di Bergamo, Bergamo, Italy.
Funding
Abbott Vascular was the sponsor of the ABSORB Cohort B trial.
Conflict of interest statement
K. Miquel-Hebert, C. Dorange, R. Rapoza and S. Veldhof are employees of Abbott Vascular. H.M. Garcia-Garcia is an employee of Cardialysis. The other authors have no conflicts of interest to declare. The Guest Editor, G. Guagliumi, is a consultant for Boston Scientific, St. Jude Medical, Volcano Corporation, and Cordis; and has received grant support from Abbott Vascular, Medtronic, Boston Scientific, and LightLab.
Online Appendix
The edge vascular response following implantation of the Absorb everolimus-eluting bioresorbable vascular scaffold and the XIENCE V metallic everolimus-eluting stent. First serial follow-up assessment at 6 months and 2 years.
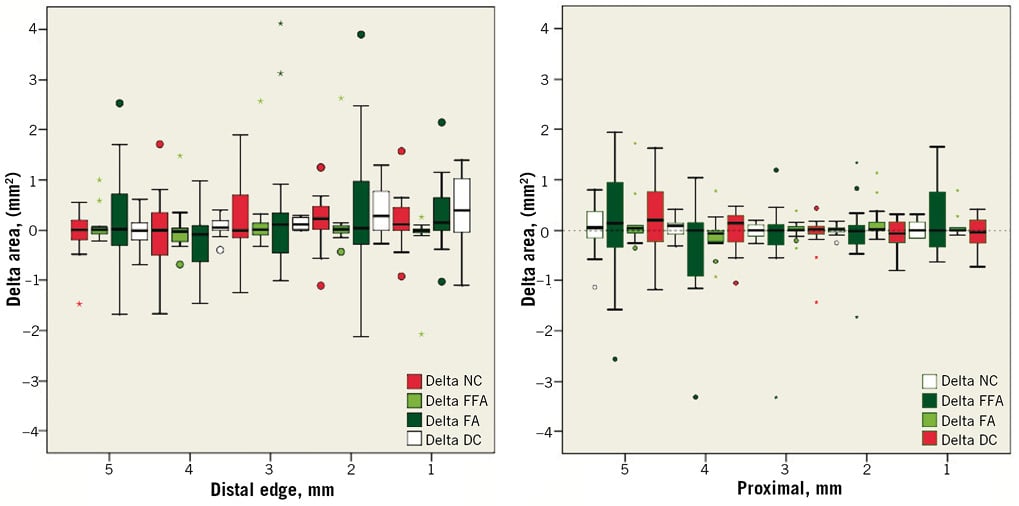
Online Figure 1. Serial proximal and distal edge per mm absolute changes regarding tissue composition from post intervention to 6 months following implantation of the Absorb BVS. The dense calcium (DC) tissue component increased significantly at the 2-mm distal subsegment –Δ DC: +0.29 mm2 [0.00, 0.78] (p<0.05) and the 3-mm distal subsegment –Δ DC: +0.12 mm2 [0.02, 0.26] (p<0.001).
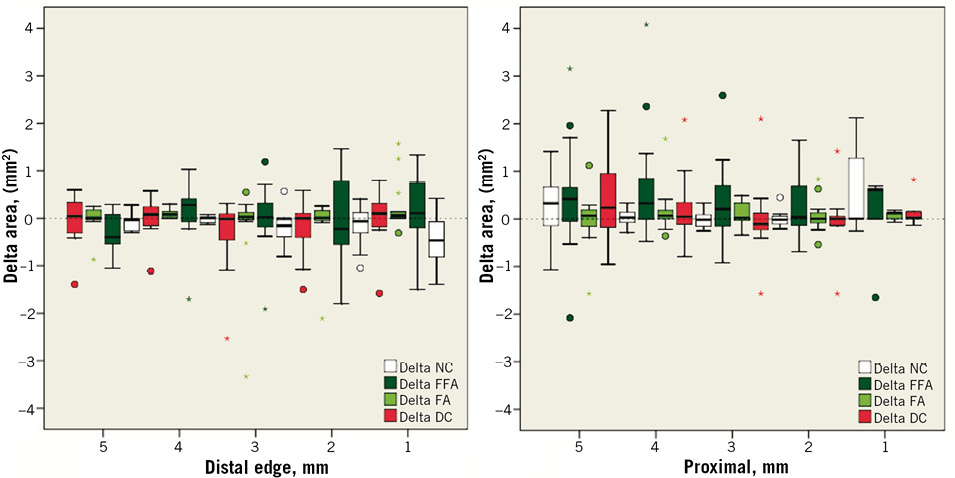
Online Figure 2. Serial proximal and distal edge per mm changes regarding tissue composition from 6 months to 2 years following implantation of the Absorb BVS. The dense calcium (DC) tissue component decreased significantly at the 1-mm distal subsegment –Δ DC: –0.47 mm2 [–0.81, –0.07] (p<0.01) and the 3-mm distal subsegment –Δ DC: –0.16 mm2 [–0.39, –0.03] (p=0.02)
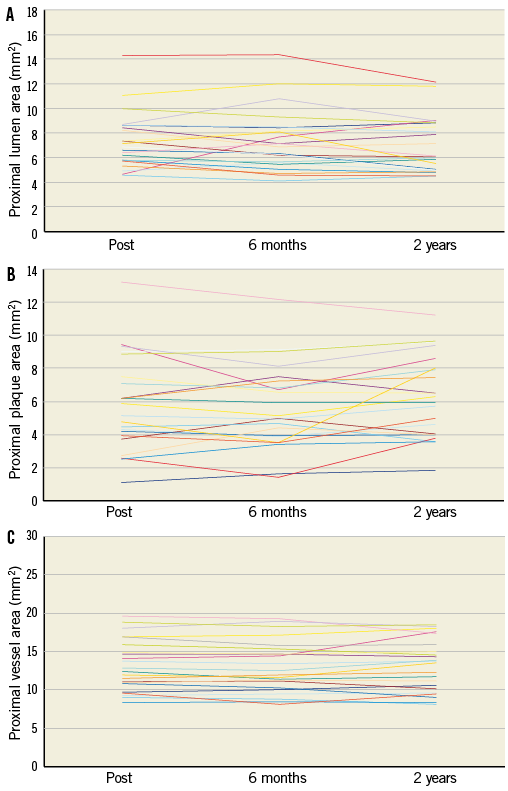
Online Figure 3. Serial changes of the lumen (A), plaque (B) and vessel (C) areas at the proximal edge of the Absorb BVS on a per lesion basis.
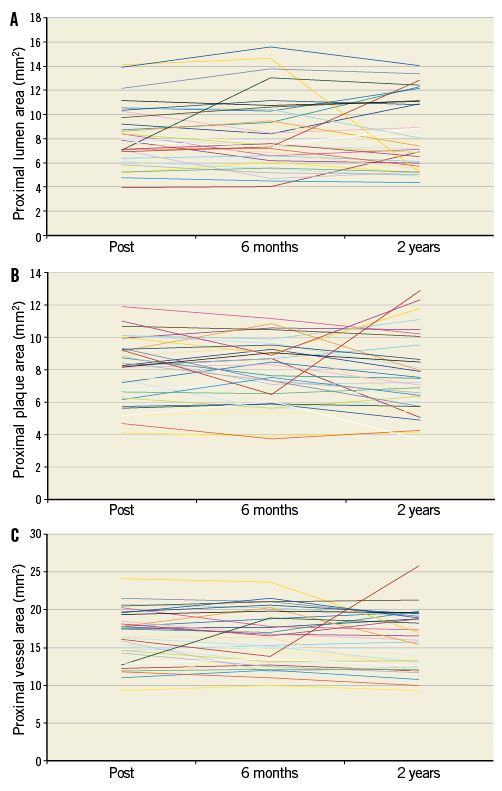
Online Figure 4. Serial changes of the lumen (A), plaque (B) and vessel (C) areas on a per lesion basis at the proximal edge of the XIENCE V stent.
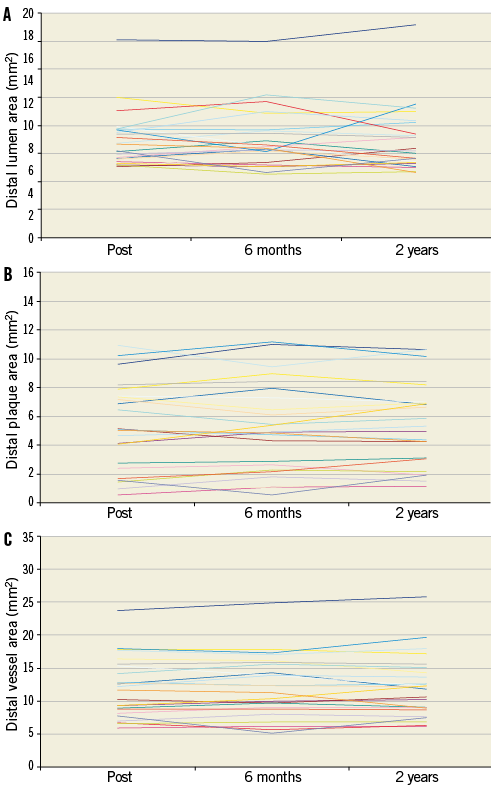
Online Figure 5. Serial changes of the lumen (A), plaque (B) and vessel (C) areas on a per lesion basis at the distal edge of the Absorb BVS.
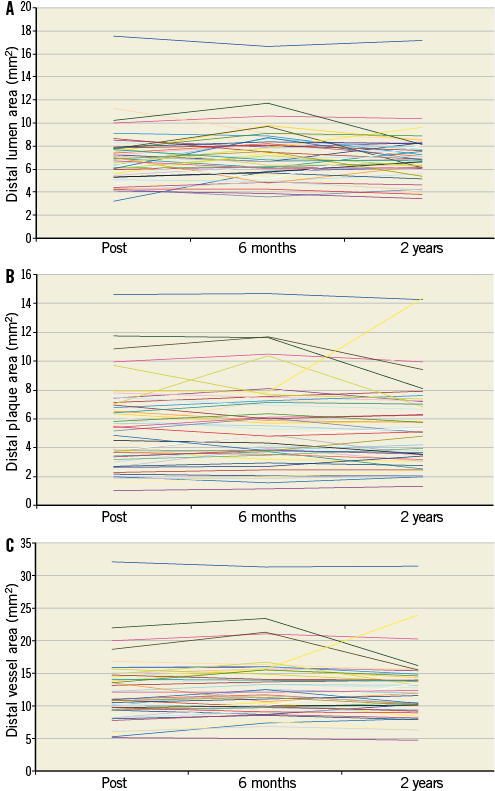
Online Figure 6. Serial changes of the lumen (A), plaque (B) and vessel (C) areas on a per lesion basis at the distal edge of the XIENCE V stent.

