Abstract
Aims: The long-term follow-up of the first-in-man ABSORB Cohort B trial showed that angiographic binary restenosis can occur early, late or very late after implantation of the Absorb everolimus-eluting bioresorbable vascular scaffold (Absorb BVS). Since the mechanical support of the scaffold decreases during bioresorption, the mechanism of in-segment restenosis (ISR) of the Absorb BVS might be different from that of metallic stents. The objective of the current analysis was to review the multimodality imaging of cases with binary restenosis to elucidate the mechanism of ISR after Absorb BVS implantation.
Methods and results: The ABSORB Cohort B trial enrolled 101 patients with a maximum of two de novo coronary lesions. At the three-year imaging and clinical follow-up, there were six cases of in-segment binary restenosis: two early ISR (<6 months), one late ISR (6-12 months) and three very late ISR (>12 months). Three of these ISR cases seemed to be induced by anatomical or procedural factors. In the other three cases, intravascular imaging (IVUS/OCT) demonstrated that the main mechanism of restenosis was significant intra-scaffold tissue growth, while the structural circularity and diameter of the scaffold were not affected.
Conclusions: Early and late restenosis after implantation of the Absorb bioresorbable scaffold could be related to anatomical or procedural factors. In this small cohort of patients late or very late restenosis seems to be attributed to pure intra-scaffold tissue growth without extrinsic encroachment of the scaffold.
Introduction
The Absorb everolimus-eluting bioresorbable vascular scaffold (Absorb BVS; Abbott Vascular, Santa Clara, CA, USA) shows unique potential in vascular repair, such as late lumen enlargement, restoration of vasomotion and plaque media reduction1-4. However, in-scaffold restenosis may occur as frequently as with metallic drug-eluting stents (DES). Since the Absorb BVS loses its mechanical strength during bioresorption, unlike metallic stents, the mechanism of in-scaffold restenosis might be unique and different from DES and bare metal stents (BMS).
The process of lumen loss (LL) is a “time limited phenomenon” due to negative remodelling of the vessel and neointimal hyperplasia inside the stent, which occurs three to six months after implantation5,6. Beyond this critical period, the mechanical support of the scaffold and the active pharmacological inhibition of the neointima are no longer necessary. According to this concept, mechanical support of the scaffold could decrease approximately six months after implantation. Actually, it has been established in humans that the mechanical integrity is maintained over a period of six months but subsides afterwards7. However, one could speculate that the plaque behind the struts of the scaffold might progress and narrow the lumen once the scaffold has lost its mechanical strength.
Timing of restenosis and its potential mechanism after implantation of the Absorb BVS have not been investigated. We report six cases of ISR (quantitative coronary angiography [QCA] diameter stenosis ≥50% within the scaffolded segment and 5 mm proximal and distal to the scaffolded segment) observed in the ABSORB Cohort B trial (101 patients, 102 lesions) according to the timing, and describe in detail the intravascular findings in an attempt to elucidate the mechanism of this complication. Two cases were early ISR (<6 months), one case was late ISR (6-12 months), and three cases were very late ISR (>12 months) (Table 1, Figure 1).
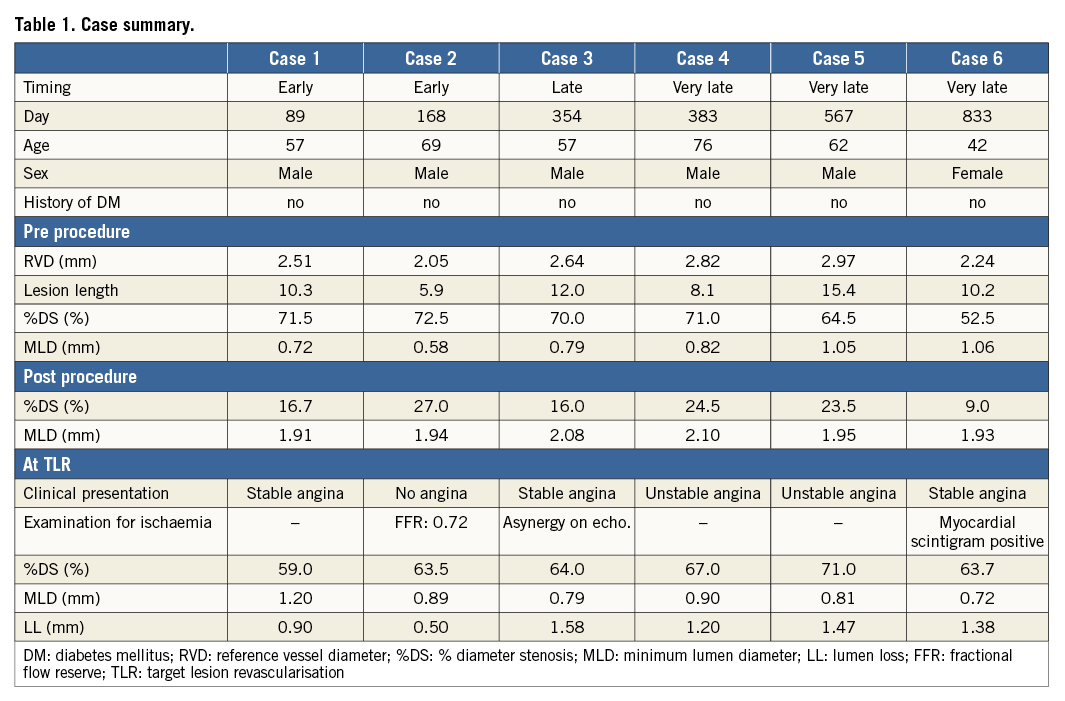
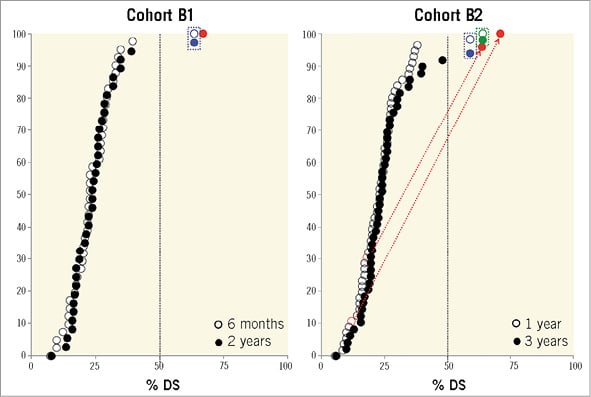
Figure 1. Cumulative frequency distribution curves of angiographic % diameter stenosis (%DS). A) %DS at 6 (circle) and 24 months (dot) of Cohort B1. B) %DS at 12 (circle) and 36 months (dot) of Cohort B2. If the patient had TLR before the planned angiography, %DS at the time of TLR (before repeat revascularisation) was used for the %DS at the later time point. Blue dots (●) and blue circles (○) represent %DS in TLR patients who experienced early ISR (<6 months). Green dots (●) and green circles (○) represent %DS in TLR patients who experienced late ISR (6-12 months). Red dots (●) and red circles (○) represent %DS in TLR patients who experienced very late ISR.
Case histories
CASE 1. EARLY ISR DUE TO MYOCARDIAL BRIDGE
A 57-year-old man with a history of hypertension, dyslipidaemia, smoking and COPD presented with stable angina. The coronary angiography showed a type B2 lesion in the mid left anterior descending (LAD) artery with severe stenosis in systole (percentage diameter stenosis [%DS]: 84%), but without significant stenosis in diastole (%DS: 26%), thus with typical evidence of a myocardial bridge (Figure 2A-Figure 2C)8. After predilatation with a 2.75 mm semi-compliant balloon, a 3.0×18 mm Absorb BVS was deployed and post-dilated with a 3.5 mm non-compliant balloon at 16 atm (Figure 2D). On day 89, the patient experienced recurrent angina and underwent re-catheterisation. The angiography revealed a focal ISR at the site of the myocardial bridge (QCA minimum lumen diameter [MLD]: 1.20 mm, %DS: 59.0%, LL: 0.90 mm) (Figure 2E). This ISR was treated by implantation of a 3.5×15 mm XIENCE V stent (Abbott Vascular) inside the Absorb BVS (Figure 2F). At three years, angiography revealed a significant re-ISR of this XIENCE V stent (QCA MLD: 1.20 mm, %DS: 59.0%, LL: 0.90 mm) (Figure 2G). Optical coherence tomography (OCT) revealed intra-scaffold tissue growth with homogeneous light reflectivity. Of interest, OCT and intravascular ultrasound (IVUS) showed that some struts of the previously implanted Absorb BVS were located inside the metal stent (Figure 2H, Figure 2I). Finally, this re-ISR lesion was treated by a coronary artery bypass graft (CABG).
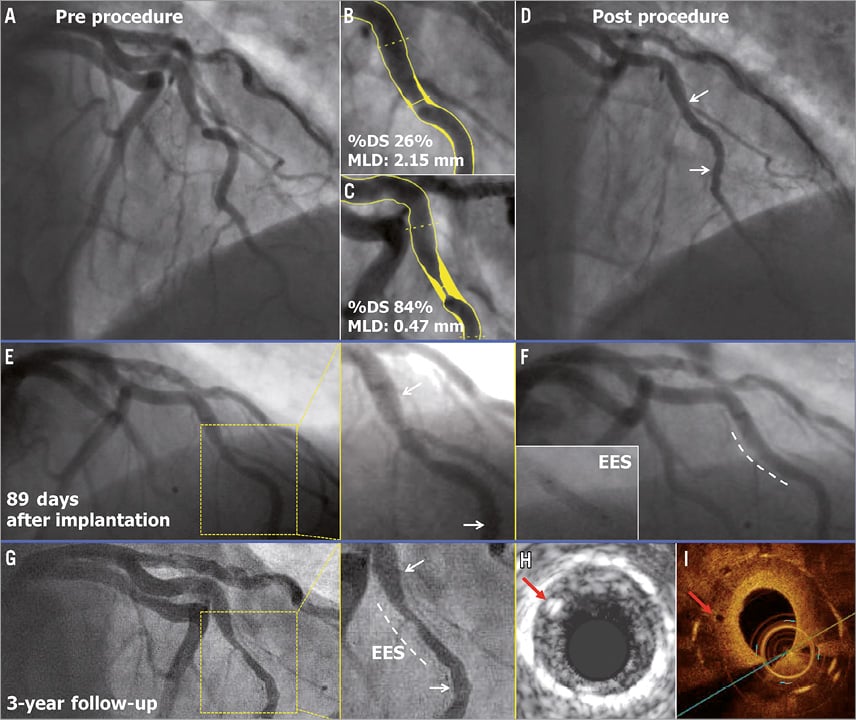
Figure 2. Early ISR due to myocardial bridge. The white arrows indicate the metallic markers of the implanted scaffold, while the white dotted line illustrates the implanted EES. The red arrows show the scaffold struts of the previously implanted Absorb BVS located inside the metal stent on IVUS (H) and OCT (I).
CASE 2. EARLY ISR AT PROXIMAL EDGE
A 69-year-old male with a history of dyslipidaemia and previous coronary intervention of the left circumflex (LCX) artery presented with stable angina. Coronary angiography showed a type B1 severe stenosis in the mid right coronary artery (RCA) (Figure 3A). After predilatation with a 2.5 mm semi-compliant balloon, a 3.0×18 mm Absorb BVS was deployed and post-dilated with a 3.0 mm balloon at 15 atm (expected diameter according to the manufacturer was 3.3 mm) (Figure 3B). As the IVUS catheter failed to pass through the implanted scaffold, the guiding catheter was changed from JR4 to AL1. After repeated attempts to cross the IVUS catheter through the scaffold by seating the guiding catheter deeply up to the proximal edge of the scaffold in order to perform the protocol-related imaging, the operator was unable to cross the scaffold and the procedure was ended (Figure 3C). On day 168, the patient underwent a planned repeat angiography, which showed a type 1B ISR at the proximal edge of the scaffold (QCA MLD: 0.89 mm, %DS: 63.5%, LL: 0.50 mm) without significant restenosis in the scaffold itself (Figure 3D)9. Greyscale IVUS (IVUS-GS) showed a high-echoic intra-scaffold tissue growth in the segment proximal to the scaffold, with maintained circularity of the scaffold (Figure 3E, Figure 3F). This ISR was treated by implantation of a 3×28 mm XIENCE V stent with full coverage of the previously implanted Absorb BVS.
Figure 3. Early ISR at proximal edge. The white and black arrows indicate the metallic markers of the implanted scaffold. In panels E and F, the red arrows show the polymeric strut on IVUS.
CASE 3. LATE ISR WITH HOMOGENEOUS INTRA-SCAFFOLD TISSUE ON OCT
In a 57-year-old man with a history of dyslipidaemia and smoking, and presenting with unstable angina, coronary angiography showed a severe type B1 stenosis in the proximal LAD with TIMI grade 2 flow (Figure 4A). After predilatation with a 2.5 mm semi-compliant balloon, a 3.0×18 mm Absorb BVS was deployed and post-dilated with a 3.0 mm non-compliant balloon at 20 atm (Figure 4B). On day 348, the patient had recurrence of angina pectoris. On day 354, the patient underwent a planned repeat angiography, which showed a significant type 1C ISR (QCA MLD: 0.79 mm, %DS: 64.0%, LL: 1.58 mm) in the body of the scaffold (Figure 4C)9. OCT showed that the circularity of the scaffold was maintained throughout the pullback (the mean scaffold area was 5.67 mm2 and the minimum scaffold area was 4.73 mm2) and all the struts were completely apposed and covered. At the site of the minimal lumen area (MLA), OCT revealed homogeneous signal-rich intra-scaffold tissue (Figure 4D-Figure 4G). On virtual histology IVUS (IVUS-VH), intra-scaffold tissue was documented as fibrous (Figure 4E). A XIENCE V stent was implanted to cover the previously implanted Absorb BVS.
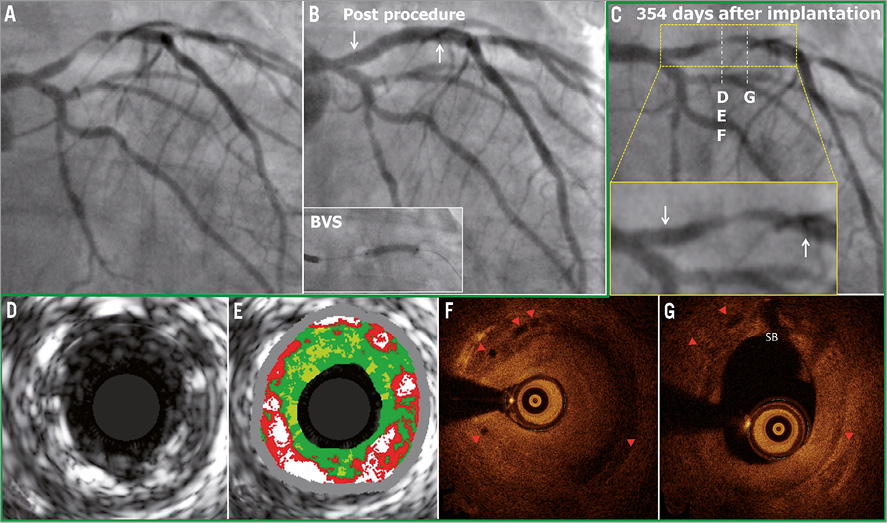
Figure 4. Late ISR with homogeneous intra-scaffold tissue on OCT. The white arrows show the metallic marker of the implanted scaffold. In panels F and G, the red triangles show the scaffold struts on OCT (case 3).
CASE 4. VERY LATE ISR AT THE PROXIMAL EDGE
A 76-year-old gentleman with a history of dyslipidaemia, chronic kidney failure and coronary intervention to the mid LAD (non-target lesion) presented with stable angina. The coronary angiography showed a type B1 moderate stenosis in the mid LAD (Figure 5A). After predilatation with a 2.5 mm semi-compliant balloon, a 3.0×18 mm Absorb BVS was deployed. However, the Absorb BVS did not cover the proximal part of the predilated segment, resulting in geographical miss (Figure 5B). After implantation of the scaffold, the jailed diagonal branch became occluded. Since this closure resulted in chest pain, bradycardia and hypotension, the side branch was dilated with a 1.5 mm balloon (Figure 5C). OCT post procedure revealed a small dissection in the proximal edge of the scaffold, presumably due to the geographical miss during the procedure (Figure 5D, Figure 5E). The patient refused the scheduled follow-up angiography at six months. One year later, the patient presented with recurrence of angina pectoris, and repeat angiography showed a type 1B ISR in the segment proximal to the scaffold (QCA MLD: 0.90 mm, %DS: 67.0%, LL: 1.20 mm) (Figure 5F), while the scaffolded segment was free from significant restenosis9. A 3.0×15 mm XIENCE V stent was implanted to overlap the proximal part of the previously implanted Absorb BVS.
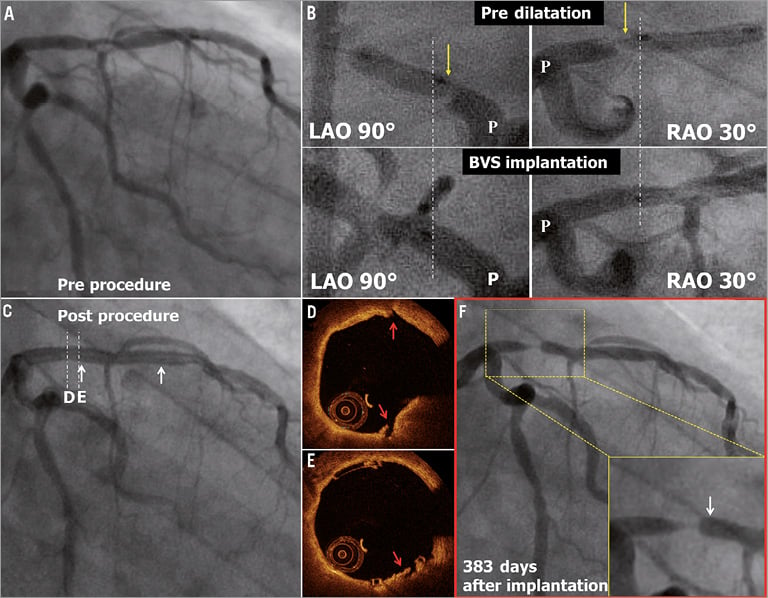
Figure 5. Very late ISR at the proximal edge. The white arrows show the metallic marker of the implanted scaffold. In panel B, the yellow arrows show the proximal edge of the predilatation balloon. The angiograms are matched anatomically between predilatation and scaffold implantation, and the white dotted lines show the proximal edge of the implanted scaffold. The red arrows show the small dissection observed post procedure on OCT in panels D and E.
CASE 5. VERY LATE ISR WITH HOMOGENEOUS INTRA-SCAFFOLD TISSUE ON OCT
A 62-year-old man with a history of hypertension, smoking and COPD presented with stable angina. Coronary angiography showed a type B1 severe stenosis in the proximal LAD (Figure 6A). Although the proximal maximum luminal diameter (Dmax) on QCA was 3.90 mm, a 3.0 mm Absorb BVS was planned (Figure 6B, Figure 6C). After predilatation with a 2.5 mm semi-compliant balloon, this 3.0×18 mm Absorb BVS was implanted and post-dilated with a 3.5 mm non-compliant balloon at 12 atm (Figure 6F). Post-procedural OCT showed large incomplete stent apposition (ISA)(max ISA area: 11.89 mm2, max ISA distance 1.63 mm, the number of cross-sections with ISA: 25 frames) at the proximal edge of the scaffold, but additional dilatation was not performed (Figure 6D, Figure 6E). At one year, the planned follow-up coronary angiography showed patency of the scaffold (QCA MLD: 1.99 mm, %DS: 10.0%, LL: -0.04 mm) (Figure 6G). OCT showed persistent numerous malapposed struts with intraluminal mass attached to or free from the vessel wall at the proximal edge of the previously implanted scaffold (Figure 7a). On the three-dimensional OCT image, the intraluminal masses were interlinked to the malapposed struts and connected proximally to the vessel wall (Figure 7a’). On day 564 the patient was hospitalised with unstable angina and underwent a repeat angiography on day 567, which showed a type 1C ISR in the body of the scaffold (QCA MLD: 0.81 mm, %DS: 71.0% and LL: 1.47 mm)9. OCT showed homogeneous signal-rich intra-scaffold tissue in the middle of the scaffold segment with persistent malapposed struts at the proximal edge (Figure 7B-E)10. After predilatation, repeat OCT revealed an extensive tissue protrusion with maintained circularity of the scaffold (Figure 7B’-E’). On OCT analysis, the mean scaffold area was 8.32 mm2, and the minimum scaffold area was 6.23 mm2 (Figure 7A). This ISR was treated by implantation of a 3.0×18 mm XIENCE V stent which overlapped the proximal part of the previously implanted Absorb BVS.
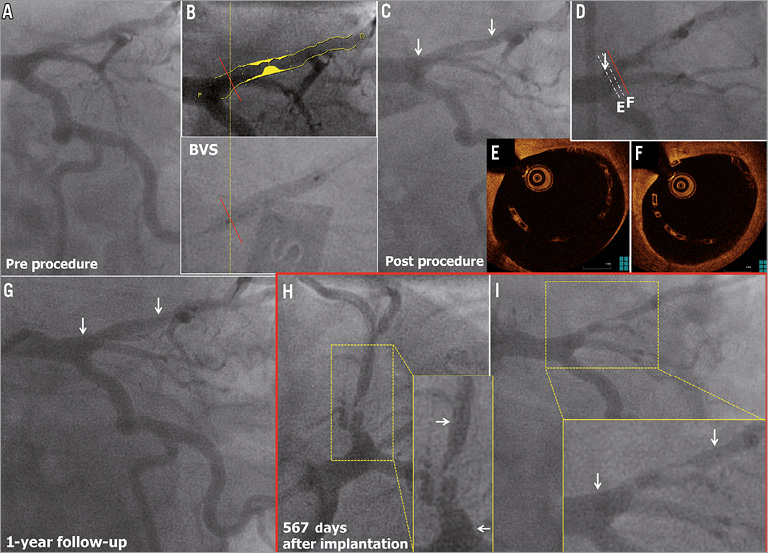
Figure 6. Very late ISR with homogeneous intra-scaffold tissue on OCT. The white arrows show the metallic marker of the implanted scaffold. The red line shows the point of Dmax on QCA, which is superimposed on the angiogram at scaffold implantation in panel B. The red line is superimposed on the angiogram at OCT study in panel D (case 5).
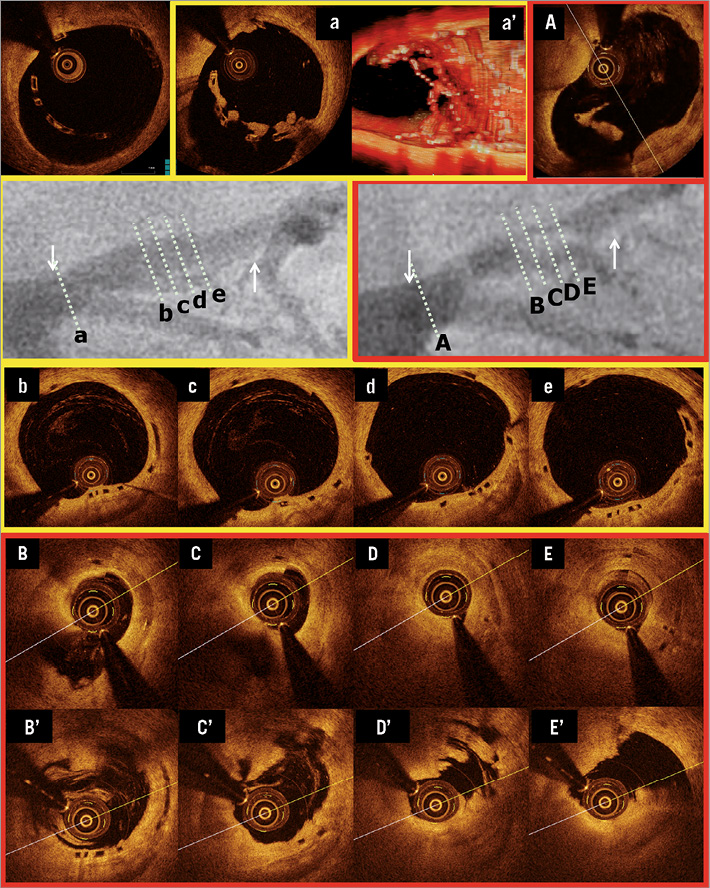
Figure 7. Very late ISR with homogeneous intra-scaffold tissue on OCT. The white arrows show the metallic marker of the implanted scaffold. OCT images pre TLR before ballooning are shown in panels B to E, and those after ballooning are shown in panels B’ to E’. In panels b to e, the corresponding cross-sections to ISR site are shown at 12-month follow-up.
CASE 6. VERY LATE ISR WITH ISO-ECHOIC INTRA-SCAFFOLD TISSUE ON IVUS-GS
In a 42-year-old female with a history of smoking presenting with stable angina, coronary angiography demonstrated a type B1 moderate stenosis in the mid LAD (Figure 8A). After predilatation with a 2.5 mm semi-compliant balloon, a 3.0×18 mm Absorb BVS was implanted. After post-dilatation with a 3.0 mm non-compliant balloon at 20 atm, post-procedural angiography showed a residual stenosis of 9.0% (Figure 8B). At one year, a planned repeat angiography showed patency of the previously implanted scaffold (QCA MLD was 1.73 mm, %DS was 18.0% and LL was 0.35 mm) (Figure 8C). On day 833, the patient underwent a repeat angiography because of stable angina with ischaemia on a myocardial scintigraphy: the angiogram showed a type 1B ISR at the distal margin of the scaffold segment (QCA MLD: 0.72 mm, %DS: 63.7% and LL: 1.38 mm) (Figure 8D)9. IVUS-GS showed that the scaffold circularity was maintained and that the eccentric intra-scaffold tissue was iso-echoic (Figure 8E-G). On IVUS-VH, small necrotic core and dense calcium were detected in the intra-scaffold tissue (Figure 8E’-G’). In this case, the ISR lesion was treated by the implantation of a 3.0×23 mm XIENCE V which overlapped the distal part of the previously implanted Absorb BVS.
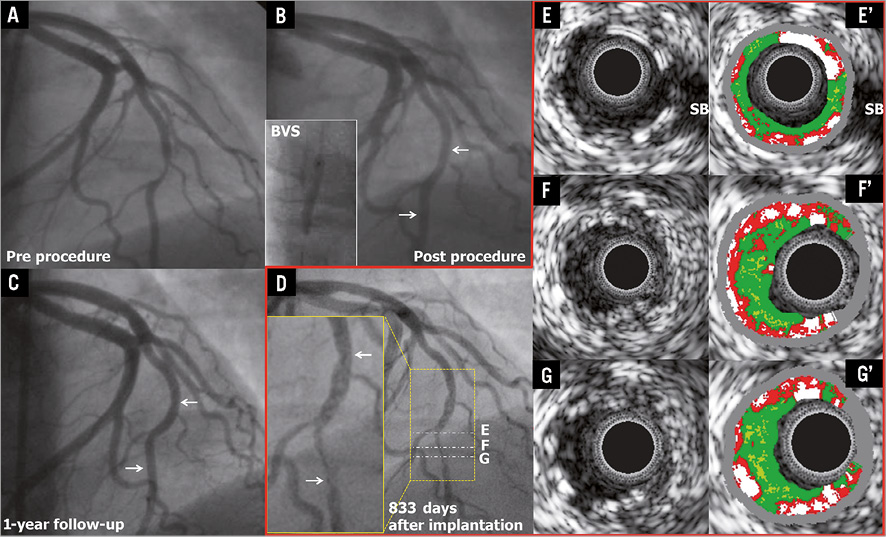
Figure 8. Very late ISR with iso-echoic intra-scaffold tissue on IVUS-GS. The white arrows show the metallic marker of the implanted scaffold.
Discussion
The main findings of the current report are the following: 1) out of 101 patients enrolled in the ABSORB trial, at three years early restenosis occurred in two cases (2.0%), late ISR in one case (1.0%), and very late ISR in three cases (3.0%); 2) the early ISR was associated with a myocardial bridge (case 1); 3) two of the three late ISR cases may have been caused by procedural edge injury at the index procedure (one case was due to the injury caused by deep insertion of the guiding catheter [case 2], while the other case could be attributed to proximal geographical miss [case 4]); 4) in three cases (one late ISR and two very late ISR), the mechanism of ISR could not be identified (case 3, case 5, and case 6) , but in all cases the circularity of the scaffold was maintained.
It is noteworthy that ISR occurred in patients without diabetes, with relatively large vessels (reference vessel diameter 3 or more mm) and short lesion length (less than 10 mm).
ISR ASSOCIATED WITH MYOCARDIAL BRIDGE
Myocardial bridging (MB) is a potential cause of myocardial ischaemia and in such cases medical therapy, with beta-blockers or calcium channel blockers, is recommended as a first-line strategy8,11. Although intracoronary stent implantation is another therapeutic approach to prevent external systolic compression and ischaemia caused by the MB, this therapy is still controversial because of concerns regarding high restenosis rates, plaque prolapse and stent fracture12,13. In the current series, one early TLR occurred in a myocardial bridge at three months after implantation of an Absorb BVS, followed by a second failure occurring after implantation of a metallic drug-eluting stent. This case suggests that scaffold implantation for a myocardial bridge treatment should be discouraged. Myocardial bridges generate compressive pressure (up to 300 mmHg) capable of fully closing the vessel in systole. Although the initial radial strength of an Absorb BVS is approximately 900 mmHg and superior to this compressive pressure, this systolic external compression occurs at least 100,000 times/day, and in the course of bioresorption a bioresorbable transient scaffold might yield to such a mechanical stress3,14.
In this case, at three years, OCT and IVUS detected one scaffold strut inside the area of the metal stent, while post procedure no disrupted strut had been detected on IVUS. The location of a polymeric strut inside the metallic stent may suggest that the discontinued strut could protrude between the metallic mesh of the stent after TLR at three months.
ISR PRESUMABLY TRIGGERED BY PROCEDURAL INJURY
It has been shown that procedural vessel injury might lead to late intra-scaffold tissue growth15. In one case in the current series, the deeply inserted AL1 guide catheter might have injured the edge of the scaffold at baseline and led to proximal edge ISR at six months.
Geographical miss is also a known cause of restenosis16,17. In one case, the predilatation might have caused “barotrauma” to the proximal plaque and the Absorb BVS did not cover the injured edge segment. Actually, a proximal edge dissection not scaffolded by the Absorb BVS was observed on OCT. The edge ISR could therefore be related to the proximal plaque injury associated with geographical miss18.
MAINTAINED CIRCULARITY OF SCAFFOLD AREA IN LATE/VERY LATE ISR
As previously described, the radial strength of the fully bioresorbable scaffold declines following polymer hydrolysis at six months, and this device becomes malleable without any supportive properties at 12 months. One could therefore speculate that the scaffold might become encroached by plaque growth behind the struts. In the balloon angioplasty era, Serruys et al demonstrated that the incidence of restenosis reaches a plateau at four months6. Ormiston et al demonstrated that the late loss in response to balloon injury reached a maximum before six months and then regressed, while some cases with an intermediate stenosis at six months showed very late lumen narrowing at five years19.
In this study, we observed three ISR cases without any possible causal relationship with procedural or anatomical factors: one case presented a late ISR and two cases a very late ISR. As shown in Figure 9, the circularity of the Absorb BVS, especially at MLA cross-section, was preserved post procedure in all cases. In addition, there were no changes in mean/minimum scaffold area.
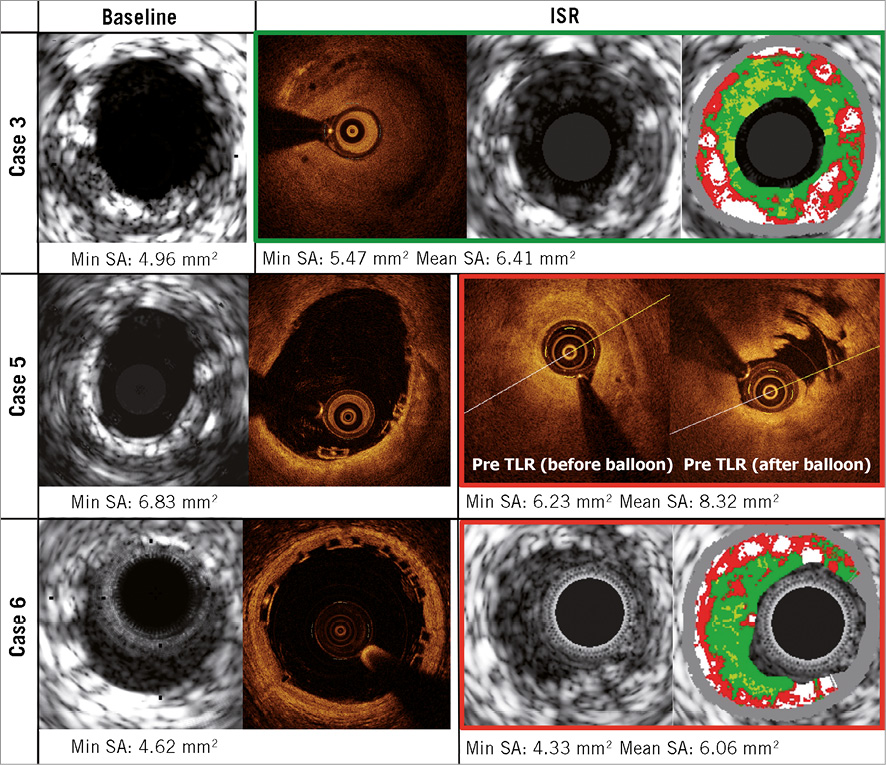
Figure 9. Intravascular imaging of the late and very late restenosis.
This report suggests that the perceived relative weakness of the scaffold related to its polymeric nature does not seem to have any direct impact on the restenosis phenomenon, and late lumen loss seems to be the result of intra-scaffold tissue growth rather than late scaffold recoil.
Late and very late restenosis have also been reported with metallic drug-eluting stents. In the SPIRIT II trial, the rate of ischaemia-driven TLR steadily increased from 1.8% at one year to 3.8% at two years and 4.6% at three years20-22. In the ABSORB Cohort B trial, the rate of ischaemia-driven TLR beyond one year increased similarly (4.0% at one year, 5.0% at two years, and 5.9% at three years). These data suggest that ISR with the Absorb BVS occurs as frequently as with metallic DES, and a reduced mechanical strength of the scaffold cannot be implicated in the occurrence of ISR.
MORPHOLOGICAL CHARACTERISTICS OF INTRA-SCAFFOLD TISSUE IN LATE/VERY LATE ISR
On IVUS-VH, a typical neointimal restenosis within one year after BMS implantation appears as fibrous or fibro-fatty tissue (green or green-yellow)23. Neointima after DES implantation or beyond one year after BMS implantation is sometimes detected as necrotic core tissue (red and red-white) on IVUS-VH23. It should be noted that intramural thrombus also has the appearance of fibrous or fibro-fatty tissue24. In the current series, two IVUS-VH images were available: one was a late ISR and the other was a very late ISR. In the late ISR case, the intra-scaffold tissue appeared as fibrous tissue, while, in the very late ISR case, the small necrotic core and dense calcium were interspersed in fibrous tissue (Figure 9).
OCT has been used to evaluate the efficacy of stenting by analysing tissue characteristics of in-stent intima25. Goto et al demonstrated that homogeneous high-signal band is a typical in-stent intima within one year after BMS implantation, while heterogeneous mixed-signal band is observed at all timing points after DES implantation24. In the current series, two OCT images were available: both of them were late ISR. In these cases, the intra-scaffold tissue appeared as a homogeneous tissue (Figure 9).
Limitations
The sample size was small and the first-in-man trial had no control arm. The current findings therefore need to be confirmed in a large randomised trial.
Conclusion
The current analysis suggests that early and late restenosis after implantation of the Absorb bioresorbable scaffold could be related to anatomical or procedural factors. Late or very late restenosis could be attributed to intra-scaffold tissue growth but not to the encroachment of the scaffold.
| Impact on daily practice Since the Absorb everolimus-eluting bioresorbable vascular scaffold (BVS) loses its mechanical strength during bioresorption, the mechanism of in-scaffold restenosis (ISR) could be different from metallic stents. In the ABSORB Cohort B trial, at three years, ISR with the BVS occurs as frequently as with drug-eluting metallic stents. By investigating the ISR according to the time line of the mechanical change of the BVS, the perceived relative weakness of the scaffold does not seem to have any direct impact on the restenosis phenomenon, and seems to be the result of intra-scaffold tissue growth rather than late scaffold recoil and extrinsic encroachment of the scaffold. The disappearance of the device was not found to be a disadvantage in relation to ISR in the current study. |
Guest Editor
This paper was guest edited by Rafael Beyar, MD, DSc, MPH, Director, Rambam Health Care Campus, Women’s Division/Dr Phillip and Sara Gotlieb Chair, Department of Medicine and Biomedical Engineering, Technion, Israel.
Funding
The ABSORB trial was sponsored by Abbott Vascular.
Conflict of interest statement
The authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

