Abstract
Aims: To investigate the extent and the circumferential distribution of the neointima tissue developed following an Absorb bioresorbable vascular scaffold (BVS) implantation.
Methods and results: Twenty-three patients who were treated with the Absorb BVS and had optical coherence tomographic examination after scaffold implantation, at six-month and at two-year follow-up, were included in the current analysis. The lumen and the scaffold borders were detected and the circumferential thickness of the neointima was measured at one degree intervals. The symmetry of the neointima was defined as: minimum/maximum thickness. The lumen area was decreased at six months compared to baseline but it did not change between six-month and two-year follow-up (baseline: 7.49 [6.13-8.00] mm2, six months: 6.31 (4.75-7.06) mm2, two years: 6.01 [4.67-7.11] mm2, p=0.373). However, the mean neointima thickness (six months: 189 [173-229] μm, two years: 258 [222-283] μm, p<0.0001) and the symmetry index of the neointima (six months: 0.06 [0.02-0.09], two years: 0.27 [0.24-0.36], p<0.0001) were increased at two years. Full circumferential coverage of the vessel wall by neointima tissue was seen in 91% of the studied frames at two years.
Conclusions: This study demonstrates that after an Absorb BVS implantation neointima tissue develops that covers almost the whole circumference of the vessel wall. In contrast to the metallic stents, the neointima tissue does not compromise the luminal dimensions. Further research is required to evaluate the neointimal characteristics and assess the potential value of the device in passivating high-risk plaques.
Introduction
Conventional metallic stents have considerable drawbacks as they may preclude surgical revascularisation, they do not permit accurate non-invasive evaluation of the treated segment by computed tomography, predispose to late stent thrombosis and entrap the vessel in a permanent metallic cage, thereby hindering the restoration of vessel wall physiology1. To address these drawbacks, bioresorbable vascular scaffolds (BVS) were introduced that constitute a novel approach to coronary revascularisation as they provide transient scaffolding which initially secures the patency of the vessel and then are resorbed allowing the vessel to return to its natural state.
Upon implantation, water in blood and tissues will penetrate the scaffold and begin the process of hydrolytic degradation. Once the molecular weight has decreased enough to diffuse and dissolve in tissue, low molecular weight oligomers can migrate and cause measurable mass loss. The final outcome of this process is the full resorption of the scaffold and the concurrent formation of neointimal tissue which covers the underlying plaque2,3.
The healing process following the implantation of an Absorb BVS device has been extensively studied in experimental models but there is only one study which describes the course of neointimal proliferation in humans2-4. In that report, Brugaletta et al used optical coherence tomography (OCT) to assess the thickness and the symmetry of the neointima tissue developed at six- and at twelve-month follow-up. However, the data acquired at these two time points were from two different populations and thus it remains unclear how neointimal proliferation evolves.
In this study we used serial OCT examinations in order to assess and quantify neointimal formation and its circumferential distribution at six-month and at two-year follow-up following an Absorb BVS implantation.
Methods
INCLUDED PATIENTS AND STUDY DESIGN
The ABSORB Cohort B Trial (A Clinical Evaluation of the Bioabsorbable Everolimus Eluting Coronary Stent System [BVS EECSS] in the Treatment of Patients with deNovo Native Coronary Artery Lesions) (NCT00856856) was a prospective multicentre single-arm study which included 101 patients treated with an Absorb BVS. The design of the study has previously been described in detail5,6. In brief, the studied population was divided into two groups (B1 and B2), of which the first underwent repeat coronary angiography, greyscale intravascular ultrasound (IVUS), IVUS virtual histology and OCT evaluation at six-month and at two-year follow-up; while the second group had the same invasive tests at one-year and at three-year follow-up. In the ABSORB Cohort B Trial OCT examination was regarded as an optional investigation. The current analysis included only the patients from the ABSORB Cohort B1 who underwent OCT evaluation at all time points (baseline, six months and two years). The trial was approved by the ethics committee of each participating institution and all the included patients gave written informed consent. The sponsor of this study was Abbott Vascular.
BIORESORBABLE VASCULAR SCAFFOLD
The Absorb BVS device (Abbott Vascular, Santa Clara, CA, USA) used in the ABSORB Cohort B Trial is a fully bioresorbable device with dimensions of 3.0×18 mm. The scaffold consists of poly-L-lactide (PLLA), covered by a thin layer of an amorphous matrix of poly-D,L-lactide (PDLLA) that contains and controls the release of the antiproliferative drug everolimus (concentration: 100 μg/cm2). Both PLLA and PDLLA are hydrolysed in the presence of water and are fully resorbable.
QUANTITATIVE CORONARY ANGIOGRAPHY
Quantitative coronary angiography (QCA) was performed in corresponding end-diastolic angiographic images acquired post Absorb BVS deployment, at six-month and at two-year follow-up using dedicated software (CASS II; Pie Medical BV, Maastricht, The Netherlands). For each treated lesion the scaffolded segment as well as the proximal (5 mm before the proximal end) and distal (5 mm after the distal end) periscaffold segments were analysed. In particular the following metrics were obtained: reference vessel diameter (RVD) estimated using an interpolated approach, minimum lumen diameter (MLD), diameter stenosis (DS) and late lumen loss (LLL) defined as the difference between the MLD post procedure and the MLD at six-month and at two-year follow-up.
OPTICAL COHERENCE TOMOGRAPHY
OCT image acquisition was performed at baseline (immediately after scaffold implantation), at six-month and at two-year follow-up using either a M-3 Time Domain system or a C7XR Fourier domain system (LightLab Imaging, Westford, MA, USA). Data analysis was undertaken by an independent core laboratory (Cardialysis BV, Rotterdam, The Netherlands) blinded to the examination time and the patients’ procedural and clinical characteristics, using dedicated off-line software (St. Jude Medical Inc., St. Paul, MN, USA). Prior to data analysis the Z-offset adjustment was performed manually by an experienced operator. In the data acquired with an M-3 system this correction was repeated whenever it was necessary. In each pullback, an experienced operator identified the frames portraying the scaffolded segment and analysed frames at 1 mm intervals. Border detection was performed using the methodology described by Serruys et al6. At baseline, the lumen area was delineated by the endoluminal border of the vessel wall and by the back side of the scaffold struts if these were embedded or well apposed. In case of malapposed struts, the lumen border was defined only by the endoluminal contour of the vessel wall. At follow-up the lumen was delineated by the endoluminal border of the neointima. At all time points the scaffold area was defined by the abluminal side of the scaffold’s struts.
To calculate the neointima area we implemented the methodology introduced by Brugaletta et al4. This approach assumes that the struts are part of the neointima, allowing circumferential measurements of its thickness. Hence, in each frame the neointima area was estimated as: scaffold - lumen area. The neointimal thickness was measured at one degree intervals with the use of dedicated software (Figure 1). A limitation of this approach is that it provides negative values in malapposed struts which would affect the mean neointimal thickness. Hence, it was decided to exclude follow-up frames exhibiting malapposed struts from the analysis. In each frame, two additional metrics were computed: i) the scaffold area obstruction: 100×neointima/scaffold area; and ii) the symmetry index of the neointima, estimated as the ratio minimum/maximum thickness4,7,8.
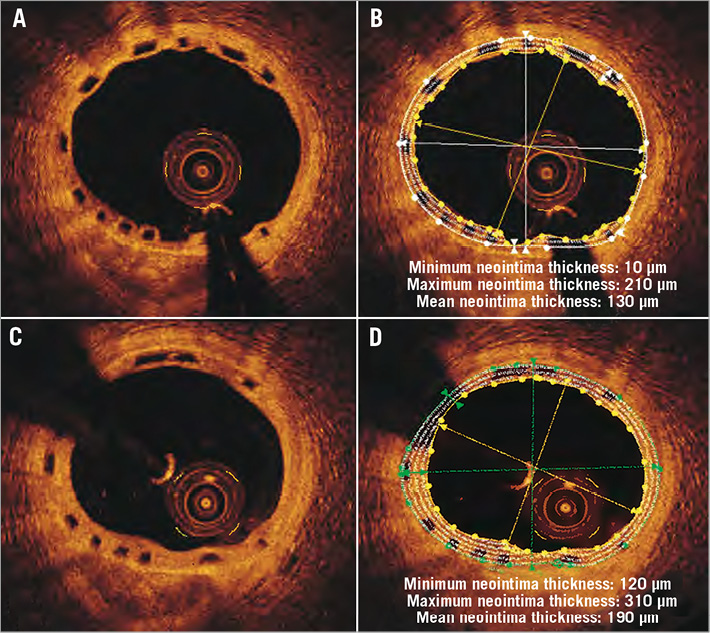
Figure 1. Corresponding optical coherence tomographic frames acquired at six-month (A) and at two-year follow-up (C). After the detection of the luminal and the scaffold borders, the thickness of the neointima was measured at one degree intervals and the minimum, maximum and mean thickness were determined (B, D). Compared to six months, at two years the neointima has a more uniform distribution and covers the whole circumference of the vessel wall.
The correspondence between the OCT frames, acquired at baseline and at follow-up examinations, was determined based on the distance of the visualised segment from the distal end of the scaffold.
Statistical analysis
The Kolmogorov-Smirnov test was applied to examine the distribution of the continuous variables. A non-normal distribution was found for most of the variables and the results are therefore presented as medians with 25th and 75th centiles. Categorical variables are given as absolute values and percentages. The Wilcoxon signed rank test was used for two related sample comparisons, while the Pearson correlation coefficient was applied to investigate the association between continuous variables. A p-value <0.05 was considered statistically significant. Data analysis was performed using the SPSS 18.0 statistical computer package (SPSS, Inc., Chicago, IL, USA).
Results
PATIENTS’ CHARACTERISTICS
Twenty-three patients (N=23 lesions) from the ABSORB Cohort B1 group had OCT examination at baseline, six-month and two-year follow-up and were included in the current analysis. The majority of the patients had stable angina (74%) and 43% had a history of myocardial infarction. The baseline clinical and angiographic characteristics of the studied population are shown in Table 1.
QUANTITATIVE CORONARY ANGIOGRAPHY ANALYSIS
Table 2 summarises the results of the QCA analysis at baseline, six-month and at two-year follow-up. At six months the RVD and MLD were mildly but significantly reduced compared to baseline, while the LLL was 0.18 (0.08-0.30) mm. The LLL of 0.22 (0.10-0.37) mm measured at two years did not differ significantly from that measured at six-month follow-up (p=0.168).
OPTICAL COHERENCE TOMOGRAPHIC ANALYSIS
A total of 1,277 OCT frames were analysed. From these, 68 were excluded as there were no corresponding frames in other examinations. The rest (1,209 frames) defined 403 sets of corresponding frames from which 54 sets were also excluded from the analysis: three sets were excluded because of poor image quality, 37 sets due to the fact that the struts portrayed at follow-up frames were too few in number to delineate accurately the scaffold border (there was one case at six-month and the rest at two-year follow-up) and 14 sets because of strut malapposition at follow-up (strut malapposition was detected in 13 frames at six months and in one frame at two years). The remaining 349 sets (1,047 frames) were included in the final analysis.
With respect to baseline, a statistically significant reduction in the lumen area was noted at six-month follow-up which should be attributed to the neointimal formation but also to the fact that the scaffold’s struts were considered as part of the lumen area at baseline and as part of the neointima area at follow-up. There was a trend towards an increase in the scaffold area between baseline and six-month follow-up. From six months to two years the lumen area remained unchanged although the neointimal area increased. There was a trend towards an increase in the scaffold area allowing the Absorb BVS to accommodate the developing neointimal tissue (Table 3). The scaffold area at two-year follow-up was significantly increased compared to baseline (p=0.027).
The symmetry index and the minimum neointima thickness were higher at two years compared to six months while the maximum thickness did not change. Thus, neointimal tissue formation at two years was primarily seen in areas with thin or no neointima at six months. Neointimal tissue covered the entire circumference of the vessel wall in 115 (33%) frames at six months and 318 (91%) frames were covered at two years. The minimum neointima thickness at two years was >65 μm in 76% (264 frames) of the studied frames.
Figure 2 illustrates the histogram distribution of the mean neointimal thickness at six-month and two-year follow-up. It is apparent that the distribution of the neointima does not follow a Gaussian pattern and that it is moderately skewed to the right at both follow-up points. A shift of the thickness towards higher values was noted at two years. In contrast to our previous report there was no correlation between the mean thickness at the frame with the minimum lumen area and the reported LLL at six-month (r=0.23, p=0.300) and at two-year follow-up (r=0.25, p=0.257)4.
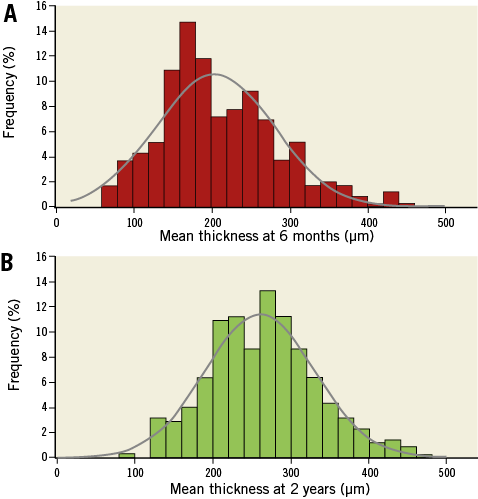
Figure 2. Histogram distribution of the mean neointima thickness at six-month and at two-year follow-up (N=349 frames for each time point).
Discussion
In this study we used serial OCT examination to investigate, for the first time, the evolution and the circumferential distribution of the neointimal tissue developed after an Absorb BVS implantation. We found that neointimal proliferation continues beyond the first six months of follow-up. Prolonged or delayed neointimal proliferation has also been noted in drug-eluting stents –the so-called “late catch-up phenomenon”– and has raised concerns about their efficacy, as it compromises the luminal dimensions9,10. In contrast to metallic drug-eluting stents, in the Absorb BVS the prolonged neointimal proliferation did not affect the luminal dimensions. This may be related to the expected loss of the scaffold’s structural integrity which allows the device to expand and accommodate the developed tissue3,11. The fact that the luminal dimensions in Absorb BVS depended not only on the neointimal formation but also on the changes in the scaffold’s dimensions may explain the lack of correlation between the mean neointima thickness at the frame with the minimum lumen area and the LLL on QCA.
Another finding of this analysis is that the circumferential distribution of the neointima changes with time and becomes more symmetric at two years. However, although the symmetry index improves with time, it remains low (0.27 [0.24-0.36]), suggesting a non-uniform neointima distribution. Asymmetric neointima has also been found in traditional metallic stents. In these devices, eccentric and focal allocation has been attributed to the vessel wall trauma, the local haemodynamic patterns, the plaque burden, as well as to the composition of the underlying plaque12-17. Some of these mechanisms are likely to affect neointimal proliferation in the Absorb BVS as well and further research is warranted to elucidate their role in this process.
In a previous report Brugaletta et al compared the neointimal distribution in two different populations that had OCT examination at six-month and at one-year follow-up after an Absorb BVS implantation. The neointima tissue was increased at one-year follow-up and had a more symmetric distribution but, on the other hand, the lumen area was reduced at this time point4. Similarly, in our analysis we found a more symmetric distribution of the neointima at two years but no differences in the lumen area between six months and two years, a fact that was attributed to scaffold expansion. This unique combination –scaffold expansion, a more symmetric neointima distribution and the preservation of luminal dimensions– is described for the first time in an endoluminal device, allowing us to see neointimal proliferation from a different perspective and identify beneficial effects resulting from this process. Recently it has been suggested that the developed fibromuscular tissue can shield high-risk plaque4,18. The PROSPECT Trial has shown that the presence of a thin-cap fibroatheroma (TCFA) is an independent predictor of future adverse events in patients who had an acute coronary syndrome19. Hence, it could be advocated that the intentional shielding of the detected TCFAs would lead to a better prognosis. Based on this concept, the SECRITT study was recently conducted and demonstrated the feasibility of covering high-risk plaques with a dedicated self-expanding stent (vProtect™ Luminal Shield; Prescient Medical, Inc., Doylestown, PA, USA)20. This device reduces the vessel wall trauma as it does not require balloon inflation. On the other hand it maintains all the other drawbacks that a typical metallic stent has, including the impaired physiological function of the vessel wall (e.g., cyclic strain), the alteration of the coronary geometry and the risk of late stent thrombosis1. Absorb BVS potentially overcomes these limitations, allowing complete circumferential covering of the underlying plaques, and appears to be a more attractive option for the invasive treatment of vulnerable plaques (Figure 3)4,18. Our analysis indicates that two years after an Absorb BVS implantation the majority of the studied frames (91%) are fully covered by neointimal tissue. In addition, at this time point the minimum thickness was found to be >65 μm in 76% of the analysed frames, suggesting complete sealing and potential passivation of the underlying plaques. These results are comparable to those reported in the SECRITT study, where at six months the cap thickness was increased by 150 μm; 8.1% of the vProtect Luminal Shield struts were uncovered and 7.6% were malapposed.
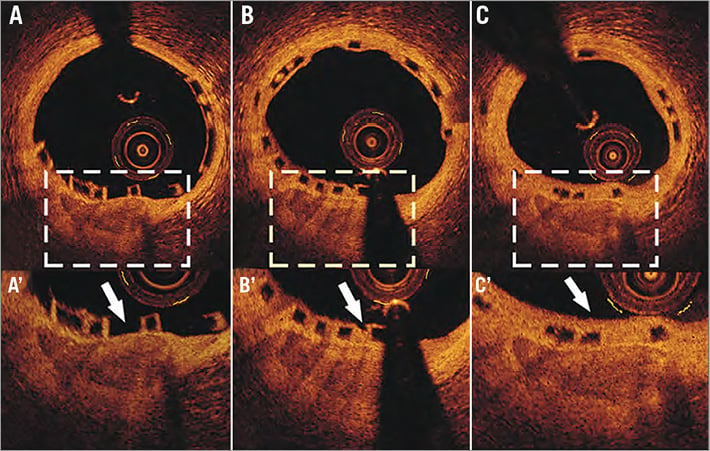
Figure 3. Optical coherence tomographic images obtained at baseline, post device implantation (A), at six-month (B) and at two-year (C) follow-up. There is a superficial calcified plaque seen at 6 o’clock. The thickness of the fibrous cap that covers the plaque is only 30 μm at baseline (A’ – arrow). At six months there is a thin layer of neointima tissue overlaying the plaque that has a thickness of 160 μm (B’ – arrow). At two years the neointima covers the whole circumference of the vessel wall while the thickness of the fibrous cap is increased and measured 220 μm (C’ – arrows).
Another question that needs to be answered is whether the neointima is able to provide permanent passivation of the underlying plaque or if it can evolve to a high-risk plaque. Cumulative evidence has demonstrated that the previously considered “benign” neointima in metallic stents may progress and transform into neoatherosclerosis21. Based on these findings, it may be hypothesised that neoatherosclerosis can also develop after BVS implantation. There are, however, some significant differences between the BVS and metallic stents. Firstly, the full resorption of the scaffold noted in BVS eliminates the risk of late inflammation triggered by a hypersensitivity reaction to a foreign body, such as a durable polymer, e.g., the polymer poly (butyl methacrylate) which can promote atherosclerosis22,23. In addition, BVS permits the restoration of vessel wall physiology; it does not cause a permanent alteration of vessel geometry and allows the smooth muscle cells to maintain their contractile phenotype24,25. Finally, histological findings in porcine models and in humans have shown that the neointima, developed after BVS implantation, has features associated with plaque stability as it is rich in connective tissue and smooth muscle cells2,24,25. Thus, while neoatherosclerosis cannot be excluded, it may be less likely to occur in BVS as these devices overcome the limitations of the traditional metallic stents and generate an atheroprotective environment that may prevent the degeneration of a stable to a high-risk plaque.
A significant limitation of the current study is the small number of patients included. In addition, we used the distance from the distal end of the Absorb BVS to identify correspondence between frames obtained at different time points. It was decided to implement this approach in order to include all the analysed frames, although this methodology is less accurate than the identification of correspondence based on the presence of anatomical landmarks, such as side branches or calcium deposits. In addition, we did not evaluate the neointima distribution in segments with malapposed struts. However, device malapposition was seen in only 3% of the frames acquired at follow-up, and thus it is unlikely that this methodological limitation would have affected the reported results.
Conclusions
This study demonstrates that neointimal proliferation continues six months after an Absorb BVS implantation. This leads to the formation of a fibromuscular layer that covers the whole circumference of the vessel wall in 91% of cases, even though it has non-uniform distribution. Further research is needed to assess the compositional characteristics of the developed neointima and to examine the potential value of the Absorb BVS in sealing vulnerable plaques.
Guest Editor
This paper was Guest Edited by Antonio Colombo, MD, Scientific Institute S. Raffaele, Milan, Italy.
| Impact on daily practice Neointima formation in metallic stents results in a significant reduction of lumen area and has been associated with future cardiovascular events. In the Absorb BVS, neointimal proliferation does not compromise the luminal dimensions as the scaffold expands and thus is able to accommodate the developed neo-tissue which seals the underlying plaques, changing the phenotype of the treated lesions to more stable forms. These unique characteristics of the Absorb BVS render this device the ideal scaffold for the invasive passivation of high-risk prone-to-rupture plaques. |
Funding
C. Bourantas wishes to acknowledge the funding support of the Hellenic Heart Foundation (ELIKAR), Athens, Greece.
Conflict of interest statement
C. Dorange and R. Rapoza are employees of Abbott Vascular. None of the other authors has any conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

