
Abstract
Aims: We sought to investigate two-year clinical and serial optical coherence tomography (OCT) outcomes after implantation of a fully bioresorbable vascular scaffold (BVS) or a cobalt-chromium everolimus-eluting stent (CoCr-EES).
Methods and results: In the ABSORB Japan trial, 400 patients were randomised in a 2:1 ratio to BVS (N=266) or CoCr-EES (N=134). A pre-specified OCT subgroup (N=125, OCT-1 group) underwent angiography and OCT post procedure and at two years. Overall, the two-year TLF rates were 7.3% and 3.8% in the BVS and CoCr-EES arms (p=0.18), respectively. Very late scaffold thrombosis (VLST) beyond one year was observed in 1.6% (four cases: all in non-OCT-1 subgroups) of the BVS arm, while there was no VLST in the CoCr-EES arm. In three cases, OCT at the time of or shortly after VLST demonstrated strut discontinuities, malapposition and/or uncovered struts. However, the vessel healing by two-year OCT was nearly complete in both BVS and CoCr-EES arms with almost fully covered struts, and minimal malapposition. The flow area by two-year OCT was smaller in the BVS arm than in the CoCr-EES arm, mainly due to tissue growth inside the device. However, there were no differences between the BVS and CoCr-EES with regard to the quality of homogenous tissues growing inside the devices.
Conclusions: The rate of TLF was numerically higher in the BVS arm than in the CoCr-EES arm, although this difference was not statistically significant. VLST was observed only in the BVS arm at a rate of 1.6% between one and two years. Further studies are mandatory to investigate the risk of BVS relative to metallic stents for VLST, and the underlying mechanisms of BVS VLST.
Introduction
The fully bioresorbable coronary scaffold is a new device treatment for coronary artery stenosis which provides temporary mechanical support with drug elution, potentially without the limitations of permanent metallic implants1. The potential of this technology to induce late lumen enlargement and plaque reduction has been demonstrated in a few seminal observations up to five-year follow-up using multiple imaging modalities2. The longitudinal imaging observations were, however, made in the absence of a comparator.
A recent meta-analysis of the four randomised trials (ABSORB II, III, Japan and China)2-5 comparing an everolimus-eluting polylactide bioresorbable vascular scaffold (BVS) and a cobalt-chromium everolimus-eluting metallic stent (CoCr-EES) demonstrated non-inferiority of BVS to CoCr-EES in terms of target lesion failure at one year6. To date, however, there is little evidence for very late events beyond one year in the context of randomised trials7. Actually, despite the theoretical long-term advantage of the technology, anecdotal case reports demonstrated a rare occurrence of very late stent/scaffold thrombosis (VLST) with late scaffold discontinuities on optical coherence tomography (OCT)8,9. These observations warrant a report of long-term outcomes from a randomised trial to compare the rate of VLST between BVS and metallic drug-eluting stents (DES). Furthermore, a serial imaging investigation at baseline and at follow-up (with and without VLST) would provide important information to explore the mechanisms of VLST after BVS implantation.
Therefore, we conducted a randomised clinical trial comparing BVS with CoCr-EES with serial imaging substudies by OCT and intravascular ultrasound (IVUS). In the current manuscript, we report the two-year clinical outcomes in the entire study population and the serial OCT findings at post-procedure and two-year follow-up in an imaging subgroup from the ABSORB Japan trial.
Methods
STUDY DESIGN AND POPULATION
ABSORB Japan was a prospective, multicentre, randomised, single-blind, active-controlled clinical trial randomising 400 patients in a 2:1 ratio to treatment with the Absorb BVS (Abbott Vascular, Santa Clara, CA, USA: N=266) or CoCr-EES (XIENCE PRIME/Xpedition; Abbott Vascular: N=134). The details of the trials have been published elsewhere5. All patients were maintained on a thienopyridine for at least 12 months, and aspirin indefinitely. As an imaging substudy, 125 patients were also allocated randomly to the OCT-1 group to undergo serial OCT follow-up at post-procedure and at two- and three-year follow-up.
The ethics committees approved the protocol at the participating institutions. All patients provided written informed consent and were blinded to their treatment assignment up to five-year follow-up.
ENDPOINTS AND DEFINITIONS
A complete list of endpoints is provided elsewhere5. Definitions of clinical endpoints, including stent/scaffold thrombosis (ST), were based on the Academic Research Consortium criteria. Target lesion failure (TLF), the primary endpoint, was defined as a composite of cardiac death, target vessel myocardial infarction (MI), or ischaemia-driven target lesion revascularisation (ID-TLR). Independent study monitors performed on-site verification of 100% of the case report form data. Death, MI, TLR/target vessel revascularisation (TVR), and ST were adjudicated by an independent blinded clinical events committee (Harvard Clinical Research Institute, Boston, MA, USA). A data safety monitoring board monitored patient safety.
The major imaging endpoints for the present analysis included a nitrate-induced vasoreactivity test at two years on angiography and changes in average lumen (flow) area from post procedure to two years on OCT.
STENTING PROCEDURE AND TWO-YEAR IMAGING FOLLOW-UP
The details of implantation technique were previously published5. In the patients sub-randomised to the OCT-1 group, post-procedural OCT was performed using a frequency domain imaging system (C7/C8 system; LightLab Imaging, Westford, MA, USA or Lunawave system; Terumo Corporation, Tokyo, Japan). When significant incomplete strut apposition was observed on OCT, the performance of additional post-dilatation was allowed. Whenever additional post-dilatation was performed, angiography and OCT were repeated. At two years, coronary angiography was repeated in the same projections as at post procedure. OCT was performed in the target lesion, including 5 mm distal and 5 mm proximal to the stent/scaffold.
The imaging data were analysed in the independent core laboratories (quantitative coronary angiography [QCA]: Beth Israel Deaconess Medical Center, Boston, MA, USA, and OCT: Cardialysis, Rotterdam, The Netherlands).
OCT ANALYSIS
The final OCT recordings were analysed off-line using QIVUS software, version 3.0 (Medis, Leiden, The Netherlands). We were unable to blind the analysts to the device type because of the specific appearance of BVS and CoCr-EES struts by OCT. Taking into account the difference in optical properties of CoCr and polylactide, OCT analysis was performed using comparative methods10,11. The details of OCT methods are described in Online Appendix 1. Definitions of OCT imaging endpoints were reported previously5. For the OCT endpoint analysis, stent area and derived measures are based on the abluminal stent contour6. Quantitative assessment of OCT was performed at 1 mm intervals, while the qualitative assessment of acute disruption and/or late discontinuities was performed frame by frame.
Statistical analysis
Two-year events were counted up to 758 days, the end of the follow-up window. The clinical endpoint was evaluated in the intent-to-treat population whereas the imaging endpoint was evaluated in the final analysis set population, excluding patients who did not receive the assigned treatment.
For binary variables, counts, percentages, and 95% confidence intervals (CI) were calculated. Pearson’s chi-squared test or Fisher’s exact test was performed when appropriate. Continuous variables were presented as mean and standard deviation or medians and interquartile ranges whenever appropriate. For time-to-event variables, survival curves were constructed using Kaplan-Meier estimates, and were compared by the log-rank test. All statistical analyses were performed using SAS, versions 9.2 and 9.3 (SAS Institute Inc., Cary, NC, USA).
ROLE OF THE FUNDING SOURCE
The sponsor was involved in study design, data collection, data analysis, data interpretation, and the writing of this report. The corresponding author had full access to the analysed data in the study and accepts full responsibility for the integrity of the study and the decision to submit the manuscript for publication.
Results
CLINICAL OUTCOMES IN THE ENTIRE STUDY POPULATION
The baseline characteristics and the study flow chart of two-year follow-up are presented in Online Table 1 and Figure 1. At two years, 391 patients (98%) had clinical follow-up. Almost half of the patients were on a dual antiplatelet regimen (BVS: 52.3%, CoCr-EES: 50.7%, p=0.78). The two-year TLF rate was not significantly different between the BVS and CoCr-EES arms. The numerically higher rate of TLF in the BVS arm was mainly driven by ID-TLR as well as target vessel MI. However, the rate of all TLR was similar in both arms. The definite/probable ST rates were 3.1% and 1.5% (p=0.51), respectively (Table 1, Figure 2).
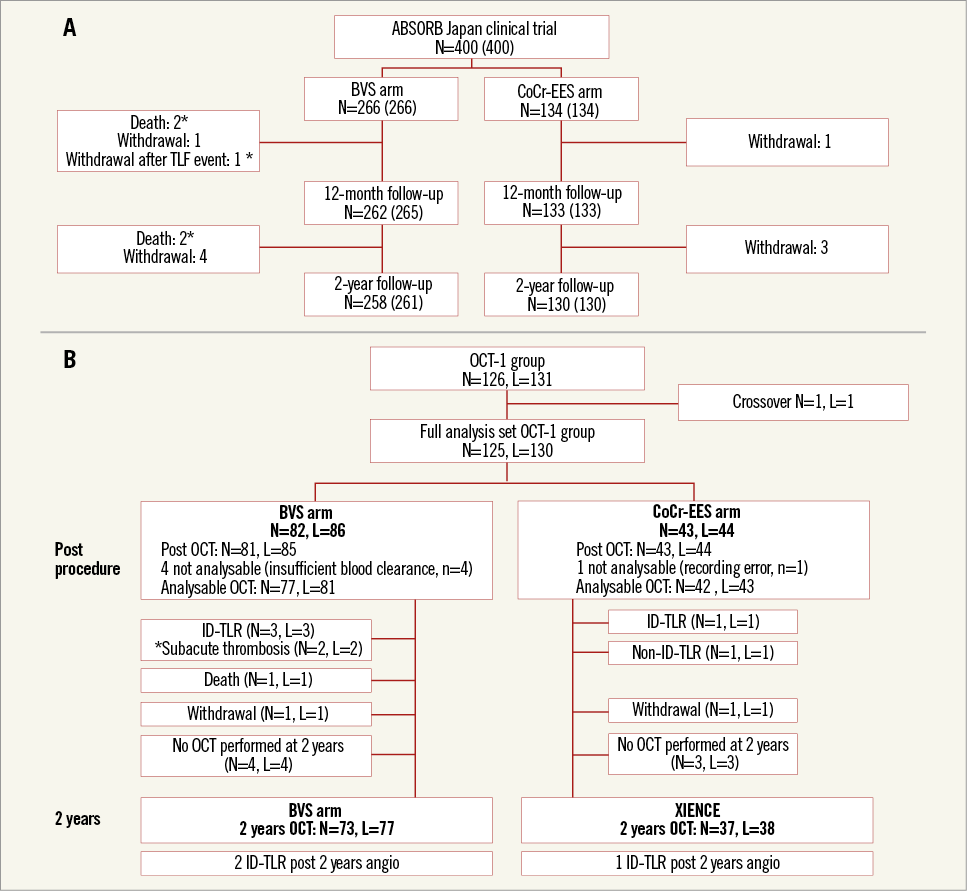
Figure 1. Study flow charts at two years. Clinical follow-up in the entire study population (A) and imaging follow-up in the OCT-1 subgroup (B). The actual number of patients analysed is indicated in brackets including subjects (*) who died or withdrew from the study after known myocardial infarction or revascularisation. BVS: bioresorbable vascular scaffold; CoCr-EES: cobalt-chromium everolimus-eluting stent; ID: ischaemia-driven; OCT: optical coherence tomography; TLR: target lesion revascularisation
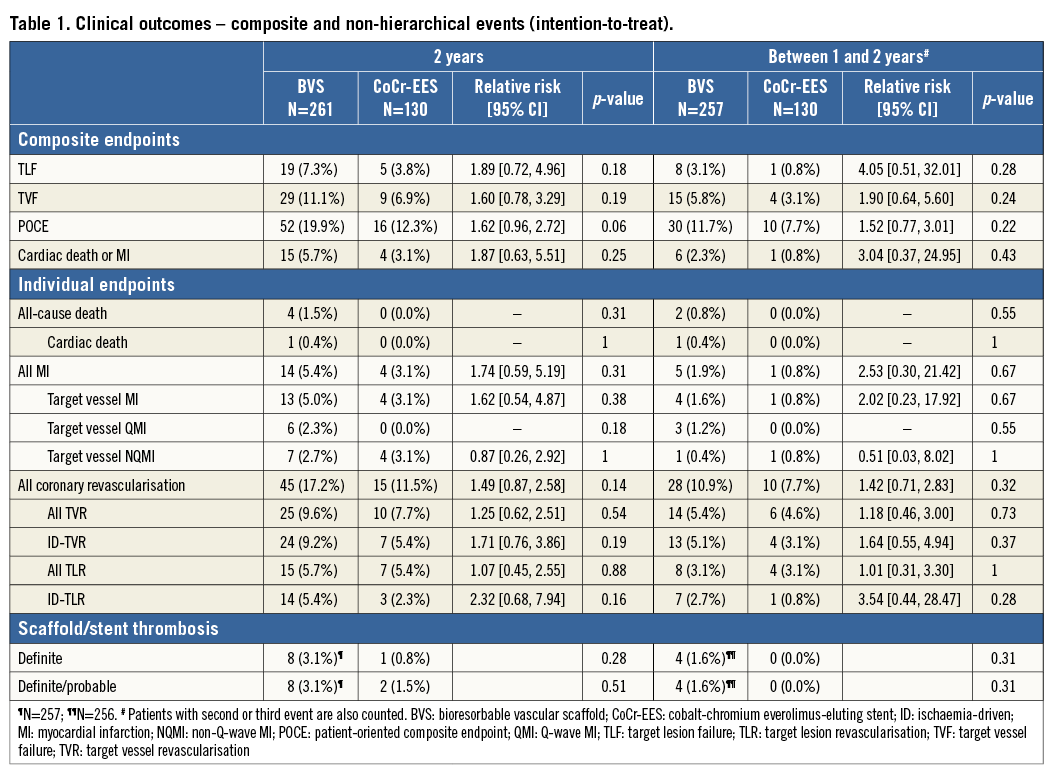
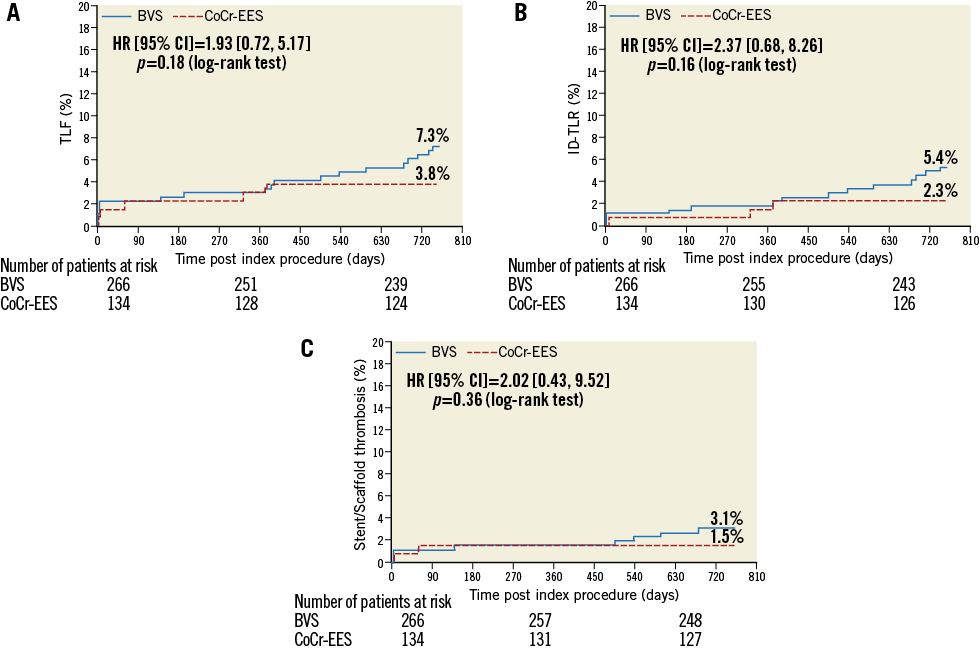
Figure 2. Kaplan-Meier estimates for TLF (A), ID-TLR (B) and definite or probable ST (C). BVS: bioresorbable vascular scaffold, CoCr-EES: cobalt-chromium everolimus-eluting stent; HR: hazard ratio; ID: ischaemia-driven; TLF: target lesion failure; TLR: target lesion revascularisation; ST: scaffold/stent thrombosis
From one to two years, there were four MI cases (1.6%, two non-Q-wave MI and two Q-wave MI) in the BVS arm related to VLST occurring from 494 to 679 days after the index procedure (Table 1).
CHARACTERISTICS OF PATIENTS WITH VLST AND OCT FINDINGS AT THE TIME OF VLST
The clinical, angiographic, and procedural characteristics, OCT findings at the time of VLST, and narratives of these four VLST cases with OCT images are presented in Figure 3 and Online Appendix 2. Of note, all four VLST cases did not belong to the OCT-1 subgroup, and therefore did not undergo OCT imaging at post procedure. These four VLST cases had a relatively large angiographic reference vessel diameter, and the scaffolds were widely patent at one-year follow-up angiography in all cases. One case was not on any antiplatelet therapy at the time of VLST. On post-VLST OCT performed in three cases, malapposition and late strut discontinuities were observed in all cases (Figure 3, Online Table 2).
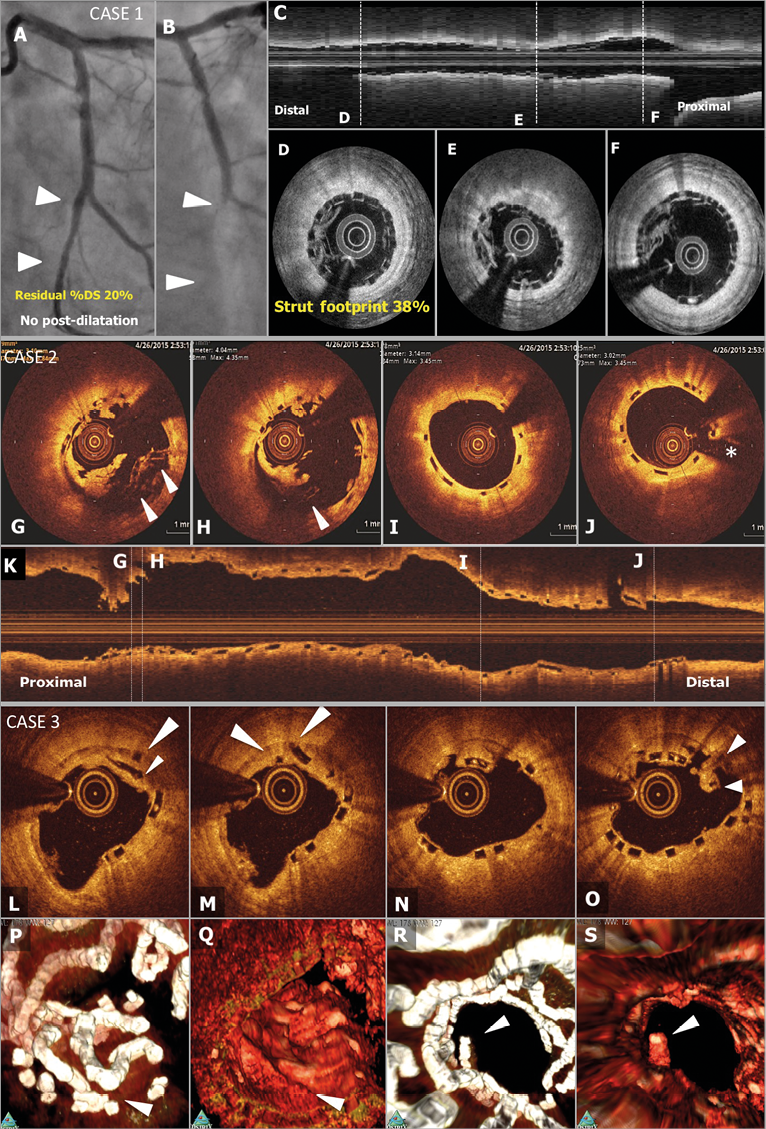
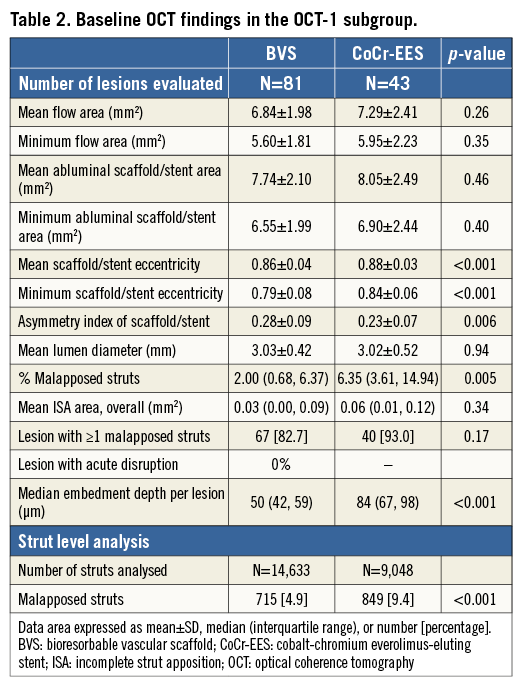
Figure 3. Representative angiographic and OCT images. Case 1. A case of subacute ST (A-F): a patient received a 2.5 mm BVS in a left circumflex coronary artery without post-dilatation (A). Post-procedural optical frequency domain imaging (C-F) showed severe underexpansion of the proximal part of the scaffold with a minimum lumen area of 2.48 mm2 and mean scaffold area of 3.47 mm2 with a high density of polymeric struts (D & E). On day 4, the patient developed subacute ST (B). The full description of the two subacute ST cases is provided in Online Appendix 3. Case 2. OCT images at the time of VLST on day 494 (G-K): OCT after thrombus aspiration demonstrated overhanging struts and uncovered malapposed struts (G & H). The middle and distal parts of the scaffold showed no remarkable findings (I & J). The full description of the four VLST cases is provided in Online Appendix 2. Case 3. A case of late strut discontinuities without any clinical sequel (L-S): OCT at two years demonstrated covered and overhanging struts (arrowheads in L & M) and malapposed and overhanging struts with back tissue bridge (arrowheads in O), indicating late discontinuities. In the corresponding 3-dimensional OCT reconstructions with (Q) or without tissue enhancement (P), two rings were overlapping with tissue coverage. One ring was protruding into the lumen from the vessel wall (R), but this was integrated in the vessel wall by tissue extending behind struts (S).
SERIAL OCT AND ANGIOGRAPHIC FINDINGS IN THE OCT-1 SUBGROUP
The baseline characteristics and the patient flow in the OCT-1 subgroup are presented in Online Table 1 and Figure 1. Two patients in the OCT-1 subgroup experienced subacute ST. In one case, post-procedure OCT revealed severe underexpansion of the scaffold and a dense cluster of strut footprints, while in the other case OCT showed acceptable scaffold expansion with a small malapposition (Figure 3, Online Appendix 3).
Quantitative measurements of device and flow area at post procedure were comparable between the BVS and CoCr-EES arms (Table 2). The BVS scaffold area was more eccentric than the CoCr-EES. Malapposed struts were less frequently seen with BVS than with CoCr-EES, although the incomplete stent apposition (ISA) area was small and not different in both arms. The BVS struts were less embedded than the CoCr-EES struts. In qualitative analysis, there was no acute disruption of polymeric struts.
OCT at two years demonstrated a uniform tissue coverage in most of the lesions in both the BVS and CoCr-EES arms (Figure 4). The dimensions of stent and scaffold were stable over two years after implantation (Figure 5), while there was a trend towards greater neointimal growth with BVS than with CoCr-EES (Table 3). As a result, the mean and minimum flow area was smaller in the BVS arm than in the CoCr-EES arm, which was consistent with the smaller in-device minimum lumen diameter by QCA (Online Table 3). Paired OCT data demonstrated a larger reduction of mean flow area in the BVS arm than in the CoCr-EES arm (BVS: –1.49±1.13 mm2 vs. CoCr-EES: –0.80±0.74 mm2, p=0.001).
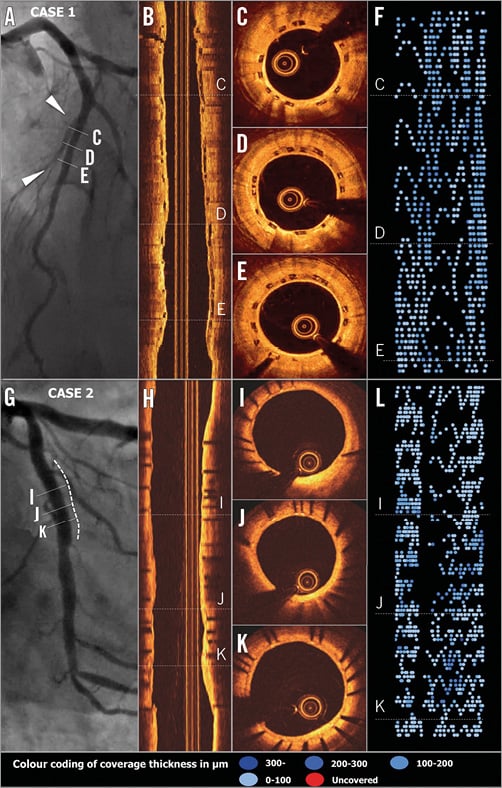
Figure 4. Typical two-year OCT images of BVS (A-F) and CoCr-EES (G-L). Case 1. A patient received a BVS in the mid left anterior descending coronary artery. On two-year OCT imaging (longitudinal view: B and cross-sections: C-E), the polymeric struts were completely covered and apposed, resulting in a healing score (HS) of 0. The foldout representation of OCT (F) illustrates graphically the distribution of neointimal thickness per strut throughout the scaffold. The extent of the thickness in microns is categorised and colour-coded at the bottom of the Figure. Case 2. A patient received a CoCr-EES in the left circumflex coronary artery. OCT at two years showed minimal neointimal hyperplasia (angiography: G, longitudinal and cross-sectional OCT: H & I-K, foldout view: L) with excellent neointimal coverage (HS=0).
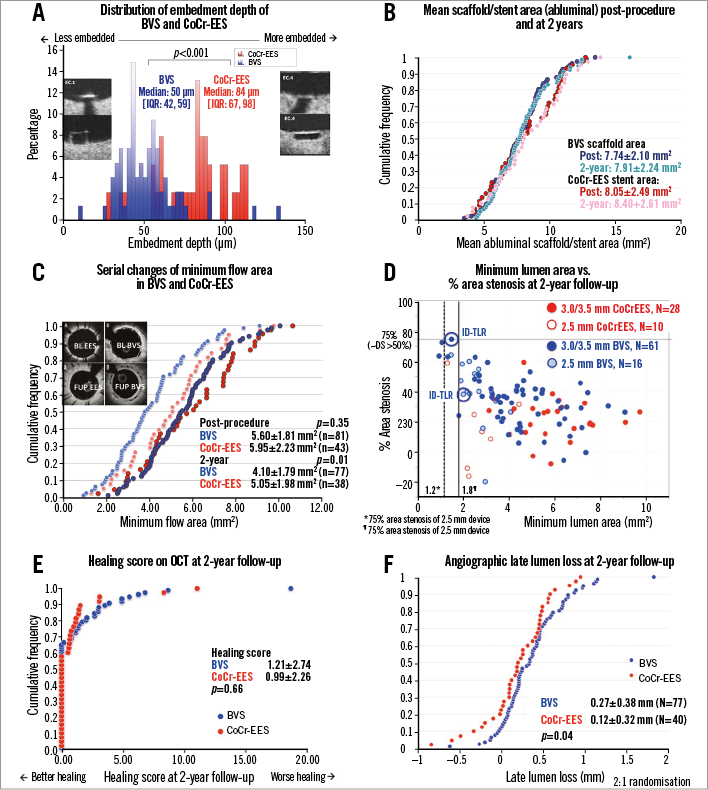
Figure 5. Quantitative OCT and angiographic analysis. A) Distribution of embedment depth of BVS and CoCr-EES on post-procedural OCT. B) Cumulative distribution function curves (CDFC) of scaffold area and stent area as assessed on OCT post procedure and at two years. C) CDFC of minimal flow area at post procedure and two years comparing BVS with CoCr-EES. D) Scatter plot of minimum lumen area and % area stenosis as assessed by OCT at two years. E) OCT healing scores at two years comparing BVS with CoCr-EES. F) CDFC of angiographic in-device late lumen loss at two years comparing BVS with CoCr-EES. BVS: bioresorbable vascular scaffold; CoCr-EES: cobalt-chromium everolimus-eluting stent
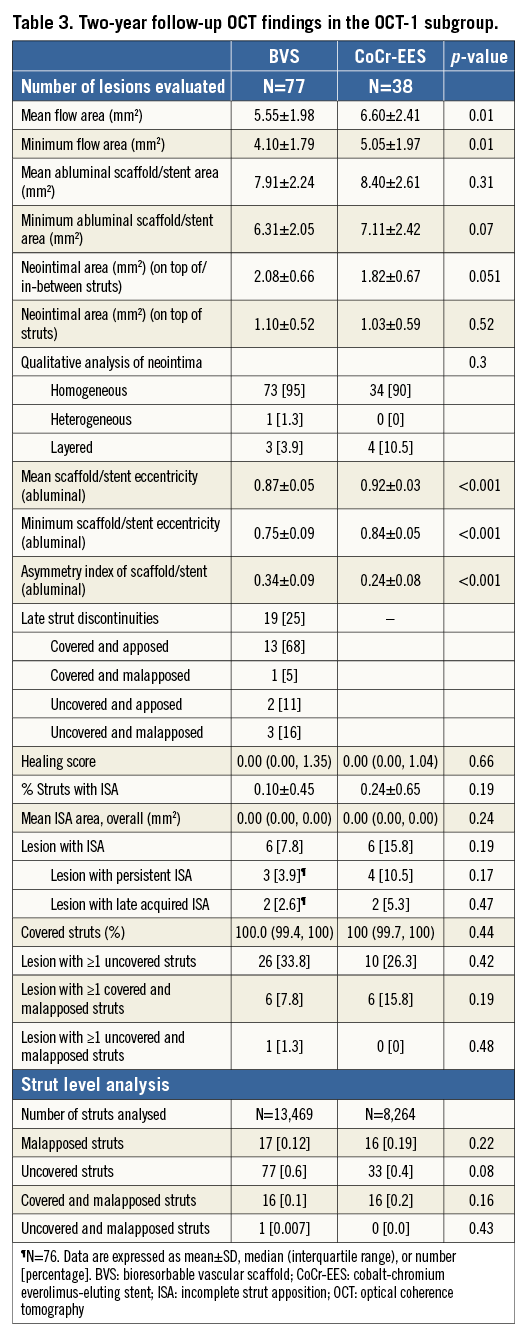
The vessel healing on OCT was complete and similar in both the BVS and CoCr-EES arms. The tissue characteristics at the site of minimal scaffold/stent area were similar in both arms, with most of the characterised tissues being homogeneous (Table 3). Coverage of the struts was nearly complete in both arms, while ISA areas were minimal, resulting in minimal healing scores in both arms.
The serial OCT of the scaffold segment allowed the assessment of strut discontinuities, whether they were persistent or late acquired. Overhanging or stacked struts were found at two years in approximately one quarter of the BVS arm, although there was no acute strut disruption at post-procedure OCT. However, the majority of such struts were well covered and apposed (Table 3). In only three cases, such struts were uncovered and malapposed (Figure 3). There were no adverse events associated with these findings.
An angiographic vasomotion test before and after nitrate administration demonstrated no significant differences between the two arms in vasodilatation (0.06±0.14 mm vs. 0.07±0.17 mm, p=0.89).
Discussion
The present two-year follow-up of the ABSORB Japan trial demonstrated the following: (1) the rate of TLF was numerically higher in the BVS arm than in the CoCr-EES arm, although this difference was not statistically significant; (2) VLST after one year was observed in 1.6% of the BVS arm, while there was no VLST in the CoCr-EES arm; (3) in three cases, OCT at the time of or shortly after VLST demonstrated strut discontinuities, malapposition and/or uncovered struts; (4) in the imaging subgroup with serial OCT, the vessel healing on two-year OCT was complete in both the BVS and CoCr-EES arms with almost full strut coverage, minimal ISA, and homogenous in-device tissue growth.
The current study showed statistically non-different two-year TLF rates between the BVS and CoCr-EES arms. The TLF rate in the BVS arm was in parallel with the rates reported in the ABSORB cohort B and ABSORB II trials, although the event rates were numerically higher in the BVS than in the CoCr-EES arm8. The numerical differences in TLF rates between BVS and CoCr-EES might be derived from the low event rate in the CoCr-EES arm (3.8%), which is currently regarded as the best-in-class DES12,13.
Four patients (1.6%) in the BVS arm had VLST between one and two years. There is a scarcity of data on VLST stemming from randomised trials, except for the ABSORB II trial that showed two-year definite VLST rates of 0.6% in the BVS arm and 0% in the CoCr-EES arm. Among the BVS registries reporting outcomes beyond one year, the reported VLST rates ranged from 0.6 to 0.8%8,14, which might be slightly higher than those for CoCr-EES (approximately 0.2%)15,16. The results from future pooled analyses of randomised trials would provide further insights into the risk of BVS relative to metallic DES for VLST.
In the four VLST cases, the scaffolds were widely patent at one-year angiographic follow-up, suggesting that the occurrence of VLST could relate to structural abnormalities undetectable on angiography. Due to the bioresorption, radial mechanical support of BVS decreases at ≈6 months and is minimal at 12 months. After one year, the mechanical support of BVS is almost absent. Increasing plaque proliferation, which could be induced by uncontrolled cardiovascular risks, and the progressive reduction in vessel support could facilitate such structural abnormalities. In three VLST cases, OCT at the time of or shortly after VLST demonstrated strut discontinuities, malapposition and/or uncovered struts. These findings are in line with the previous reports by Karanasos et al and Räber et al, demonstrating that incomplete lesion coverage, malapposition, strut discontinuities and underexpansion of the scaffold were frequently observed by OCT in patients presenting with definite BVS VLST. The causal relationship of such OCT abnormalities and VLST, however, still remains undetermined. Firstly, after the mechanical integrity of the scaffold disappears at six months after implantation, the scaffold structure becomes malleable so that wiring, thrombus aspiration, ballooning, or imaging procedure at late follow-up could induce strut discontinuities or malapposition17. Secondly, single OCT imaging only at the time of the event could not differentiate the persistent acute disruption/malapposition and late acquired discontinuities. In general, late discontinuities occur frequently as part of the programmed process of bioresorption17. The question remains whether the late discontinuities are bystander findings or the cause of the late event and, if so, what the trigger is to induce VLST.
At baseline, malapposed struts were less frequently observed in the BVS arm than in the CoCr-EES arm (strut-level, BVS: 4.9% vs. CoCr-EES: 9.4%), while at two years there was a low frequency of malapposition in both arms (0.12% vs. 0.19%). On OCT, malapposition is judged taking into account the thickness of the struts. As long as the same endoluminal expansion is achieved, thicker struts would have better apposition. The observed difference in malapposition rate post procedure may therefore be due to the thicker struts of BVS than those of XIENCE. Recently, Mangiameli et al reported a case of OCT-defined neoatherosclerosis observed in the BVS scaffold18. However, the vessel healing in the OCT-1 subgroup was almost complete in both the BVS and CoCr-EES arms with almost complete strut coverage, minimal ISA, and homogenous in-device tissue growth, suggesting that it would be unlikely for VLST to be causally related to ubiquitously seen adverse vascular responses to BVS. Of note, VLST did not occur in the OCT-1 subgroup where post-implantation OCT was performed. The role of post-procedure OCT in preventing VLST should be evaluated in future investigations. Approximately one half of patients still received DAPT at two years. The protocol mandated 12 months of dual antiplatelet therapy (DAPT), while DAPT after 12 months was at the discretion of investigators. The observed high rate of DAPT at two years is in line with other Japanese all-comer stent trials and may reflect Japanese clinical practice. For example, in the RESET trial (Randomized Evaluation of Sirolimus-Eluting Versus Everolimus-Eluting Stent Trial), 73% of patients were still on DAPT at two years.
The larger reduction of mean flow area and similar vasoreactivity of BVS compared to CoCr-EES were demonstrated in the present study as major imaging endpoints. The larger reduction of mean flow area could be explained by the greater neointimal growth in-between the struts related to the higher strut thickness of BVS (157 µm) than CoCr-EES (89 µm) (p=0.051). The neointimal area on top of the struts was comparable between both devices; the next-generation BVS with thinner strut thickness may overcome the issue of flow area reduction. Regarding the vasomotion, BVS demonstrated a larger vasoreactivity of 0.06±0.14 mm than that observed in the ABSORB first-in-man trial at two years (0.034±0.09 mm)19. Based on the literature20, the metallic stent is not supposed to exhibit a change in dimensions after intracoronary injection of nitrate. However, CoCr-EES also showed some vasomotion, which resulted in the comparable levels of vasoreactivity between BVS and CoCr-EES. The methodological approach computing the change in lumen diameter prior to and following the intracoronary administration of nitrate with different X-ray gantry positions could have introduced confounding factors in the calibration process and blurred the difference in vasomotoricity between BVS and CoCr-EES.
Limitations
Several limitations have to be acknowledged. First, the study is severely underpowered to detect differences in clinical outcomes between BVS and CoCr-EES at two years, especially in low-frequency events. Second, the included patients mainly had stable CAD and non-complex lesions, precluding the generalisability of the study findings to patients with complex lesions. A recent meta-analysis suggested that an ethnicity difference might confound the results of strut coverage21. Therefore, careful interpretation is needed when extrapolating the current results to a Western population22. The strut coverage of the current trial is, however, consistent with the ABSORB cohort B trial analysed in the same core lab. Third, the absence of VLST in the OCT-1 subgroup did not allow us to identify any OCT imaging correlates at post procedure associated with subsequent VLST.
Conclusions
In conclusion, at two years after implantation, the rate of TLF was numerically higher in the BVS arm than in the CoCr-EES arm, although this difference was not statistically significant. VLST was observed at a rate of 1.6% between one and two years only in the BVS arm. Further studies are mandatory to investigate the risk of BVS relative to metallic stents for VLST, and the underlying mechanisms of BVS VLST.
| Impact on daily practice Scarce data are available on midterm (>1 year) outcomes after implantation of a fully bioresorbable everolimus-eluting vascular scaffold (BVS) in comparison with a cobalt-chromium everolimus-eluting stent (CoCr-EES). In the ABSORB Japan randomised trial comparing BVS and CoCr-EES, vessel healing as assessed by optical coherence tomography at two years was almost complete in both arms, with complete strut coverage and minimal strut malapposition. The two-year target lesion failure rate was not statistically different but numerically higher in the BVS arm than in the CoCr-EES arm. VLST after one year was observed in 1.6% of the BVS arm, which warrants further investigation. |
Guest Editor
This paper was guest edited by Tommaso Gori, MD, PhD; Zentrum für Kardiologie, Universitätsmedizin Mainz, University Medical Center, Mainz and DZHK Rhein-Main, Germany.
Conflict of interest statement
Y. Onuma and P.W. Serruys are members of the Advisory Board of Abbott Vascular. G.W. Stone is Chairman of the Advisory Board of Abbott Vascular and a consultant to Reva Corp. J.J. Popma reports grants from Abbott Vascular and personal fees from Abbott Vascular. A. Namiki, T. Ueno, K. Ando, K. Igarashi, K. Kozuma, K. Tanabe, and T. Kimura report personal fees for advisory agreements with Abbott Vascular Japan. H. Kusano, R. Raposa and C. Simonton are employees of Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor, Tommaso Gori, has received speaker’s honoraria from multiple companies including Abbott Vascular.
SUPPLEMENTARY DATA
Online Appendix 1. OCT analysis methods
Quantitative assessment of the stented/scaffolded coronary segment of the final OCT pullback was performed in an independent core laboratory (Cardialysis BV, Rotterdam, The Netherlands) using QIvus software, version 3.0 (Medis, Leiden, The Netherlands). Taking into account the difference in optical properties of cobalt-chromium and polylactide, OCT analysis was performed using comparative methods11. OCT imaging endpoints included the mean and minimal scaffold/stent and diameter, area and volume, the frequency of incomplete strut apposition including area and volume, the percentage of uncovered struts, the percentage of malapposed struts, the mean neointima thickness and neointimal hyperplasia area on top of the strut and inter-strut, and volume, the mean flow area and volume, the malapposition area and volume, and intraluminal defect area and volume5.
The malapposition distance was assessed as the distance between the interpolated lumen contour and the midpoint of the backside of the malapposed polymeric or metallic struts (abluminal reflective frame in polymeric struts or virtual backside of the metallic struts based on the strut thickness [XIENCE: 89 µm])11. A malapposition distance greater than zero was the criterion of malapposition. Struts were classified as uncovered if any part of the strut was visibly exposed to the lumen or as covered if a layer of tissue was visible over all the reflecting surfaces23. Regarding polymeric struts, the threshold for coverage at follow-up was defined as 30 microns, which corresponds to the average interobserver measurement (difference in 300 struts analysed two times, 35±6 μm) of the endoluminal light backscattering frame of the strut24. Eccentricity index (EI) was calculated per cross-section as the ratio of the projected minimal and maximal lumen or scaffold/stent diameter25,26. Asymmetry index (AI) was calculated per lesion as (1 –projected minimum lumen or scaffold/stent diameter/projected maximal lumen or scaffold/stent diameter throughout an entire pullback)26.
Additionally, the following analyses were performed. Frequency of acute disruption and late discontinuities were assessed according to the previously published methods8. Qualitative assessment of the pattern of coverage was assessed at the site of the maximal in-stent/in-scaffold area obstruction in each lesion27. To quantify the degree of vascular healing status, the healing score (%HS) was calculated using published methods10,28. The degree of strut embedment was quantified using QCU-CMS (LKEB, Leiden, The Netherlands)29.
Online Appendix 2. Detailed description of VLST cases (non-OCT subgroup)
VLST CASE 1 (DAY 494)
The subject is a 79-year-old male, former smoker with a history of dyslipidaemia and hypertension, both requiring medication, type 2 diabetes mellitus treated with oral hypoglycaemics, Canadian Cardiovascular Society (CCS) class I stable angina. The core lab assessed a moderately calcified 17.86 mm lesion in the proximal left anterior descending artery (LAD) involving a side branch, with reference vessel diameter (RVD) of 3.65 mm proximal and 2.76 mm distal (Online Figure 1A). On 18 December 2013, the subject was enrolled in the trial and received one 3.5×28 mm bioresorbable vascular scaffold (BVS) at 10 atm in the proximal LAD followed by post-dilation using a 3.5×15 mm non-compliant balloon at 18 atm. Final in-scaffold minimal lumen diameter (MLD) was 2.57 mm with 18% diameter stenosis (%DS) without angiographic complications (Online Figure 1B). Protocol-required 13-month coronary angiography showed no restenosis at the target lesion (13.0% in-scaffold %DS, and 22.6% in-segment %DS).
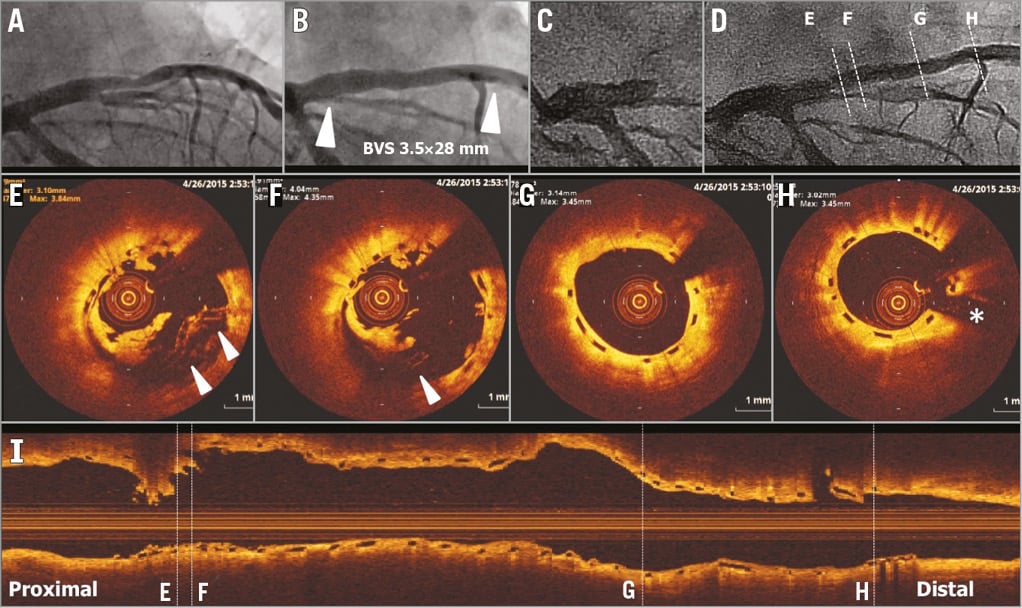
Online Figure 1. VLST case 1 (day 494).
On day 494, the subject returned to the hospital due to acute Q-wave myocardial infarction. Coronary angiography showed occlusion of the LAD at the proximal edge of the scaffold (Online Figure 1C). After successful manual thrombus aspiration (Online Figure 1D), OCT showed overhanging struts and uncovered malapposed struts with a disruption of circularity at the proximal part of the scaffold (Online Figure 1E, Online Figure 1F). The middle and distal parts of the scaffold showed no remarkable findings (Online Figure 1G, Online Figure 1H, Online Figure 1I). A metallic stent was implanted without complications. Clopidogrel and cilostazol had already been discontinued after one-year follow-up, whereas aspirin was discontinued one week prior to the event but no reason was indicated.
VLST CASE 2 (DAY 536)
The subject is a 44-year-old male, with a history of hypertension and dyslipidaemia, both requiring medication, type 2 diabetes mellitus treated with oral hypoglycaemics, CCS class I stable angina. Core lab assessed a moderately calcified 6.93 mm lesion in the mid right coronary artery (RCA) involving a small ventricular branch, with RVD of 3.15 mm proximal and 3.58 mm distal (Online Figure 2A). On 27 June 2013, the subject received a 3.5×18 mm BVS at 15 atm in the mid RCA without post-dilatation. Final in-segment MLD was 2.79 mm with a %DS of 16.2% without angiographic complications (Online Figure 2B). The patient was sub-randomised to the intravascular ultrasound (IVUS) subgroup and therefore post-procedural IVUS was performed. On IVUS, no apparent malapposition was observed and minimal lumen area was 7.95 mm2 (Online Figure 2C-Online Figure 2E). Protocol-required 13-month coronary angiography showed no restenosis (22% in-scaffold %DS).
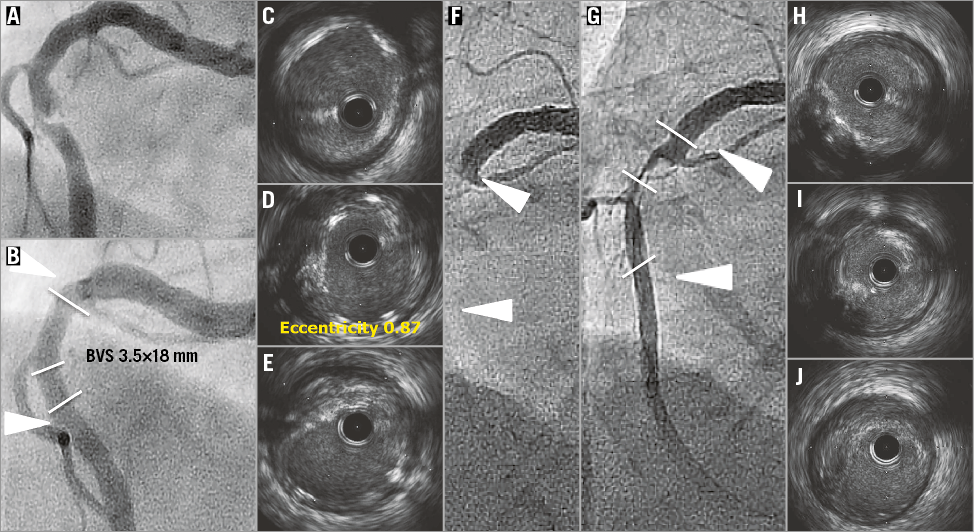
Online Figure 2. VLST case 2 (day 536).
On day 536, the subject presented with an ST-segment elevation myocardial infarction (STEMI) without abnormal Q-waves. Coronary angiography revealed occlusion of the RCA at the proximal edge of the scaffold (Online Figure 2F). After successful manual thrombus aspiration (Online Figure 2G), IVUS revealed protruding tissues with attenuation in the scaffolded segment without apparent strut malapposition (Online Figure 2H-Online Figure 2J). A bare metal stent was implanted. Clopidogrel had been discontinued at one year, whereas aspirin was ongoing after interruption in days 433-461 due to planned surgery.
VLST CASE 3 (DAY 595)
The subject is a 72-year-old male, former smoker with a history of type 2 diabetes mellitus treated with oral hypoglycaemics, CCS class III stable angina. Core lab assessed a mildly calcified 6.91 mm lesion in the distal RCA with an RVD of 3.07 mm proximal and 2.81 mm distal (Online Figure 3A). The patient received a 3.0×18 mm BVS at 8 atm in the distal RCA followed by post-dilatation with a 3.25×10 mm non-compliant balloon at 16 atm. Final in-segment MLD was 2.19 mm with a %DS of 20.5% (Online Figure 3B). Protocol-required 13-month coronary angiography demonstrated in-scaffold %DS of 8.4%.
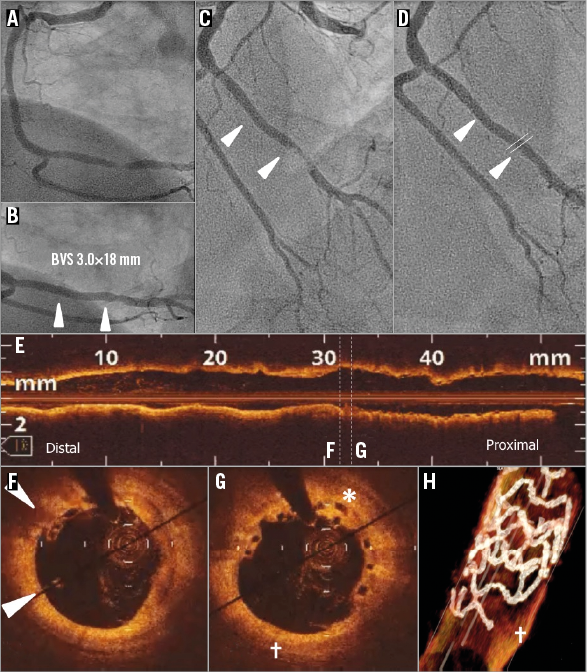
Online Figure 3. VLST case 3 (day 595).
On day 595, the patient presented with an acute myocardial infarction (Online Figure 3C). Coronary angiography revealed an occlusive thrombus at the distal edge of the scaffold. Manual thrombus aspiration was performed, which improved the Thrombolysis In Myocardial Infarction (TIMI) flow up to 2 with a distal embolisation of the atrioventricular branch. Five days after VLST, the patient came back to the catheterisation laboratory (Online Figure 3D), which revealed restoration of flow (TIMI 3). OCT demonstrated malapposed struts in the distal part of the scaffold (arrowhead, Online Figure 3F) and covered overhanging struts (asterisk, Online Figure 3G). One half of the distal ring was not discernible (+ in Online Figure 3H) by three-dimensional OCT reconstruction, and it is speculated that the ring was dislocated by wire and thrombus aspiration. Distal to the scaffold, OCT revealed a calcified and light-attenuating plaque without significant stenosis.
VLST CASE 4 (DAY 679)
The subject is a 59-year-old male, smoker with a history of dyslipidaemia requiring medication, type 2 diabetes mellitus treated with oral hypoglycaemics, CCS class II stable angina. Core lab assessed a moderately calcified 10.84 mm lesion in the distal RCA (Online Figure 4A), with RVD of 3.08 mm proximal and 3.20 mm distal. The subject received a 3.5×18 mm BVS at 14 atm in the distal RCA without post-dilatation (Online Figure 4B). Final in-segment MLD was 3.12 mm with in-scaffold %DS of 7.6% and no dissection was observed. Protocol-required 13-month coronary angiography demonstrated no significant stenosis at the target lesion (2.2% in-scaffold %DS and 11% in-segment %DS).
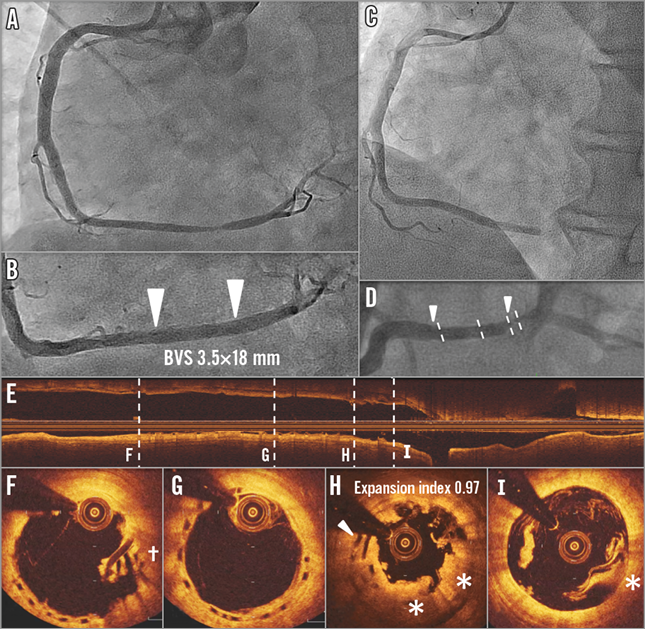
Online Figure 4. VLST case 4 (day 679).
On day 679, the subject returned to the hospital due to STEMI. Coronary angiography showed occlusion of the RCA at the distal edge of the scaffold (Online Figure 4C, Online Figure 4D). OCT after manual thrombus aspiration (Online Figure 4E-Online Figure 4I) revealed overhanging struts (Online Figure 4I and arrowhead in Online Figure 4H) with coverage by the light-attenuating tissue (* in Online Figure 4H and Online Figure 4I) or overhanging struts without coverage (+ in Online Figure 4F), demonstrating the presence of strut discontinuities. The procedure was completed after ballooning without implanting a metallic scaffold. Clopidogrel was discontinued at day 669 and aspirin was continued. The patient came back to the catheterisation laboratory 24 days later due to recurrent angina. OCT revealed overhanging struts without coverage remaining in the distal part of the scaffold. A metallic drug-eluting stent was implanted.
Online Appendix 3. Detailed description of subacute ST cases in the OCT-1 subgroup
SUBACUTE ST CASE 1
A 64-year-old male smoker with a history of dyslipidaemia and hypertension, type 2 diabetes mellitus treated with oral hypoglycaemics, and CCS class II stable angina was treated with a 2.5×18 mm BVS implantation in the mid RCA with post-dilatation by a 2.75 mm balloon. Post-procedural OCT (Online Figure 5A-Online Figure 5C) showed a good expansion of the scaffold (minimal LA: 5.55 mm2) with a small malapposition in the middle part of the scaffold. On day four, the patient presented with non-Q-wave myocardial infarction, with angiographic occlusion in the proximal edge of the scaffold. Post-thrombectomy OCT (Online Figure 5A’-Online Figure 5C’) showed an attachment of tissue in the proximal part with resolution of malapposition in the middle part. Aspirin and clopidogrel were ongoing.
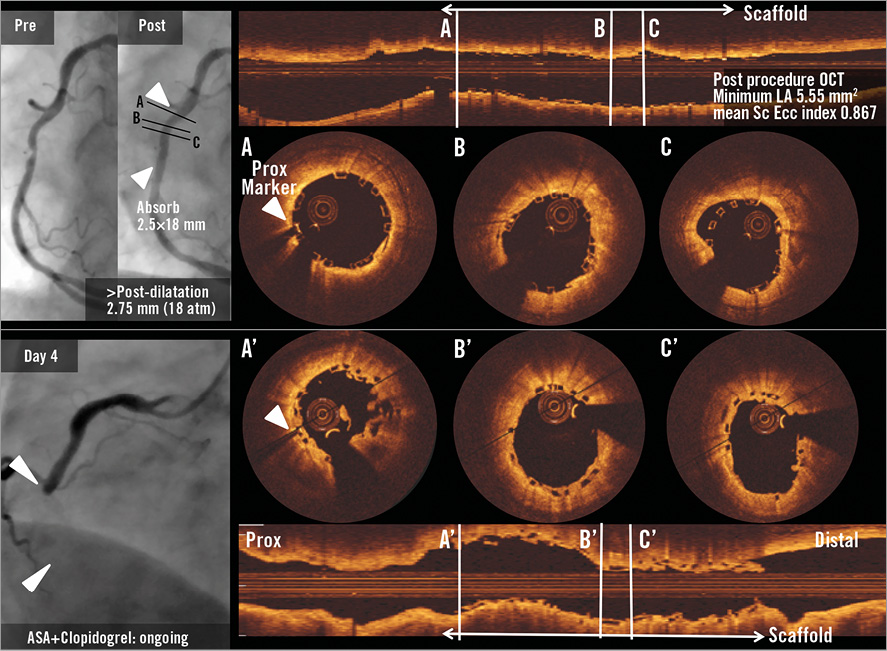
Online Figure 5. Subacute ST case 1.
SUBACUTE ST CASE 2
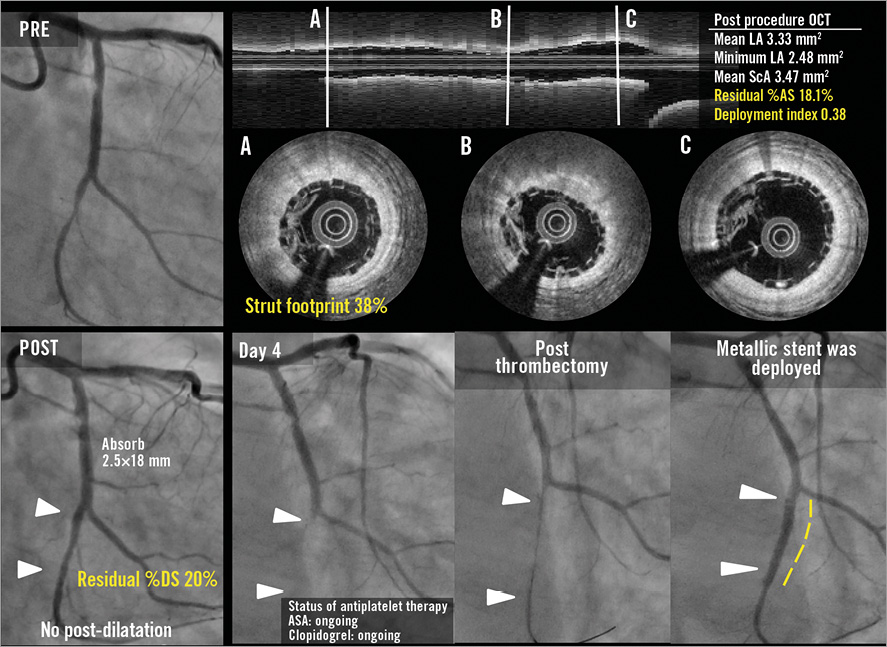
A 64-year-old male with a history of dyslipidaemia requiring medication, hypertension, previous drug-eluting stent implantation in a non-target vessel, former tobacco use presented with a CCS class II stable angina. The patient received a 2.5×18 mm BVS scaffold in a relatively small left circumflex coronary artery without post-dilatation. Post-procedural OCT showed an underexpansion of the 2.5 mm device with a minimum lumen area of 2.48 mm2 and mean scaffold area of 3.47 mm2 with a high density of polymeric struts (Online Figure 6A-Online Figure 6C). Post-procedural angiographic residual stenosis was 20%. The patient developed STEMI on day four, and coronary angiography showed in-scaffold occlusion at the proximal edge of the scaffold. The subacute scaffold thrombosis was treated by implantation of a metallic stent. Aspirin and clopidogrel were ongoing.
Online Figure 6. Subacute ST case 2.

