
Abstract
Aims: We aimed to investigate the effects of an initial learning period with mandatory optical coherence tomography (OCT) guidance for the implantation of everolimus-eluting bioresorbable vascular scaffolds (BVS).
Methods and results: We analysed procedural and clinical outcomes of all BVS implantations at a single centre where OCT guidance was mandatory in the initial rollout (OCT-mandatory) phase. We compared these data with the later phase where use of OCT was at operator discretion (OCT-selective or angiography). We implanted 406 BVS in 306 vessels (201 OCT, 105 angiography) in 272 patients. Follow-up duration was 38±10 months. Annualised rates of device-oriented cardiac events (DOCE) and scaffold thrombosis (ScT) were 1.4% and 0.4%, respectively. The risks of DOCE (HR 1.06, 95% CI: 0.33-3.34; p=0.71) and ScT (HR 0.48, 95% CI: 0.07-3.85; p=0.49) were not significantly different when comparing the OCT and angiography groups.
Conclusions: Routine use of OCT to guide and optimise BVS implants results in very acceptable outcomes. Further, the benefits of such an early OCT-mandatory “learning” period persist after cessation of routine OCT usage when imaging is not routinely used. A period of mandatory OCT usage for BVS implants may therefore be beneficial in improving patient outcomes with these devices.
Abbreviations
BVS: bioresorbable vascular scaffold
CABG: coronary artery bypass graft
DAP: dose-area product
DAPT: dual antiplatelet therapy
DES: drug-eluting stents
DOCE: device-oriented cardiac events
ISA: incomplete scaffold apposition
MI: myocardial infarction
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
QCA: quantitative coronary angiography
RVD: reference vessel diameter
SD: standard deviation
ST: scaffold thrombosis
TLR: target lesion revascularisation
TVR: target vessel revascularisation
Introduction
The everolimus-eluting bioresorbable vascular scaffold (Absorb™ BVS; Abbott Vascular, Santa Clara, CA, USA) represents a novel treatment option for coronary artery disease with promising early clinical results1. However, an observed increase in the risk of scaffold thrombosis (ScT) and myocardial infarction in BVS compared with metallic drug-eluting stents (DES) in trial populations and registries has raised concerns, culminating in and contributing to commercial withdrawal of this first-generation device2,3.
Predisposing factors for excess ScT in BVS observed in clinical series include incomplete lesion coverage, underexpansion and both strut malapposition and fracture4. Deficiencies in implantation technique appear to be important for BVS failure: a recent methodical review of all ScT cases in a real-world registry has demonstrated that ScT rates can be mitigated through rigorous attention to optimal implant technique – the “PSP” algorithm (1:1 predilatation, appropriate sizing and post-dilatation with a non-compliant balloon to high pressure)5. Invasive imaging guidance may play an important role in achieving these three goals: pre-implant optical coherence tomography (OCT) can guide balloon and scaffold sizing and allows unrivalled post-implant visualisation of scaffold strut apposition and expansion, as well as detecting edge dissections, all of which can be addressed to improve the long-term BVS result6.
At our institution, we elected to use OCT systematically in the initial phase of our BVS programme. After a run-in period with mandated OCT guidance, operators defaulted to angiographic guidance with OCT at operator discretion to resolve ambiguous cases. We were interested to discover whether switching implantation strategy after an OCT learning curve maintained BVS performance in the long term. In this study, we report a single-centre prospective registry of all BVS implantations, comparing the routine mandated OCT-guided implantation with a subsequent phase of implantation guided by either OCT or angiography alone, at operator discretion.
Methods
STUDY POPULATION
All patients receiving the Absorb BVS at the Royal Papworth Hospital, Cambridge, UK, between July 2012 and January 2016 were included. Clinical and lesion characteristics and procedural details were prospectively recorded in a database during each procedure and were verified by inspection of individual case records before analysis. The decision to implant a BVS was at operator discretion, guided by the patients’ presumed ability to tolerate at least 12 months of dual antiplatelet therapy (DAPT), according to patient/lesion characteristics and appropriate consent. Formal training on the recommended implantation technique for BVS was mandatory, including five proctored cases before implanting independently. It was arbitrarily decided to change from an OCT-mandatory to OCT-selective approach at 24 months after initiation of the BVS programme, after approximately 100 procedures had been performed. All patients provided informed consent for the procedure and subsequent data collection and analysis.
OPTICAL COHERENCE TOMOGRAPHY
Intracoronary imaging was performed with optical coherence tomography (OCT) (C7 Dragonfly™; St. Jude Medical, St. Paul, MN, USA). Imaging acquisition was performed during manual contrast injection after a 100 mcg intracoronary bolus dose of glyceryl trinitrate. The reference vessel diameter and area were measured <5 mm proximal and distal to the shoulders of the diseased target segment to guide BVS size selection7.
OCT was used primarily for sizing of the scaffolds before implantation - diameter being the most important factor, with length being of secondary importance. Plaque and lesion characteristics were noted by the operator but, with the exception of the requirement for additional adjunctive lesion preparation techniques (e.g., rotational atherectomy), there was no mandated protocol to follow, apart from best practice accepted guidance of avoiding landing devices in areas of lipid-rich plaque or concentric calcification. After scaffold deployment, OCT was used to identify underexpansion and incomplete scaffold apposition (ISA). The target for OCT-guided deployment of the BVS was a minimal scaffold area greater than 90% of the reference vessel area and minimal or no ISA. Additional scaffolds were implanted for evidence of significant edge dissection (scaffold edge dissections >60 degree arc of vessel circumference or causing MLA <4 mm2)8.
In angiography-guided cases, scaffold size was determined by visual estimation or by gauging vessel size by assessing angiographic expansion of the predilatation balloon. The decision to post-dilate was at operator discretion and was guided, in part, by angiographic appearance in two orthogonal projections. Quantitative coronary angiography (QCA) was performed only for the purposes of the registry and was not used to guide scaffold sizing at the time of implantation.
QUANTITATIVE CORONARY ANGIOGRAPHIC ANALYSIS
Offline QCA analysis was performed using CAAS 5.10.2 software (Pie Medical Imaging BV, Maastricht, the Netherlands). In each case, the treated and peri-treated regions (defined as 5 mm proximal and distal to the scaffold edge) were analysed, with minimal luminal diameter and reference vessel diameter measured.
LESION TYPES AND PROCEDURAL CHARACTERISTICS
Lesions were defined by the AHA/ACC classification9. 1:1 predilatation was defined as the use of a predilatation balloon of at least the same diameter as the reference vessel diameter (RVD) or the nominal scaffold diameter. Ostial lesions were defined as BVS implants less than 5 mm from the ostium of the LAD, LCX or RCA. Procedural times, X-ray screening times, X-ray dose (dose-area product [DAP]) and contrast volume used were recorded for all cases but only compared for routine single-vessel cases: multiple-vessel cases and research protocol cases were excluded to allow fair and appropriate comparisons to be made.
CLINICAL OUTCOMES
Clinical follow-up was performed on all subjects by clinic visit or telephone interview. Follow-up was closed in December 2017. Outcome endpoints were device-oriented cardiac events (DOCE: cardiac death, target vessel-related myocardial infarction, target lesion revascularisation) and definite or probable in-scaffold thrombosis (ScT), as defined by the Academic Research Consortium definition9. Death was considered cardiac in origin unless obvious non-cardiac causes were identified. Target vessel revascularisation (TVR) was defined as unplanned repeat PCI or coronary artery bypass graft (CABG) in the target vessel, and target lesion revascularisation (TLR) as repeat PCI (at a remote time point from the index implantation) or CABG for the lesion in the previously treated segment or in the adjacent 5 mm. There were no patients within the study who had different lesions treated with BVS guided in a different fashion (i.e., patients had either all BVS implants OCT-guided, or none).
STATISTICAL ANALYSIS
Values are presented as mean±standard deviation (SD) for continuous variables or as counts and percentages for categorical variables. Continuous variables were compared by independent sample t-tests or ANOVA; categorical variables were compared by the chi-square statistic. Time-to-event curves were generated using the Kaplan-Meier method and compared with the log-rank Mantel-Cox test. Univariate Cox regression was performed to assess the relationship between clinical/procedural variables and DOCE. A p-value of <0.05 was considered to be statistically significant and all reported p-values are two-sided. Analyses were carried out using SPSS for Windows, Version 19.0 (IBM Corp., Armonk, NY, USA). Graphs were plotted with GraphPad Prism version 5.02 (GraphPad Software Inc, San Diego, CA, USA).
Results
Overall, 406 BVS were implanted in 306 vessels in 272 patients during the study period. OCT was used to guide BVS implantation in 99 vessels (100%) in the mandatory phase and 102/207 (49%) in the selective phase, with the remainder of implants guided by angiography alone. OCT guidance was principally used early in the clinical experience, with OCT use being 100% in 2012 and 2013, 82% in 2014 and 28% in 2015/6.
CLINICAL AND LESION CHARACTERISTICS
Clinical and lesion characteristics are summarised in Supplementary Table 1. There were no significant differences between OCT-mandatory, OCT-selective and angiography groups for any of the characteristics recorded. In the overall study group, no difference in lesion type was noted over time: the proportion of B2/C lesions treated was 60% in 2012-2013 and 56% in 2014-2016 (p=0.57).
PROCEDURAL DETAILS
Procedural characteristics are summarised in Supplementary Table 2. Balloon predilatation was performed in 97% of all cases. A non-compliant balloon was used for predilatation in a quarter of cases in both groups, more commonly in the latter stages of the study. Relative to both deployed scaffold size and post hoc QCA measurement of reference vessel diameter, 1:1 sizing of the predilatation balloon was performed more frequently in OCT cases than in the angiography cases (57% vs. 30%; p<0.001, and 84% vs. 63%; p<0.001). In the OCT cases, larger predilatation balloon diameters were used (2.86±0.39 vs. 2.75±0.36 mm; p=0.01). More non-compliant predilatation balloons were used in the OCT-mandatory than in the OCT-selective phase (18% vs. 28%, p=0.03).
Fewer BVS were used per vessel (1.23±0.45 vs. 1.43±0.65, p=0.02) and total scaffold length (26.4±10.0 mm vs. 31.1±14 mm, p=0.02) was shorter in the early OCT-mandatory phase compared to the OCT-selective phase. Both number and length of BVS deployed did not differ subsequently between OCT and angiographic guidance in the OCT-selective phase. Post-dilatation was performed more frequently in the OCT-selective phase of the study, particularly when OCT guidance was used (99% vs. 91%; p=0.01).
All patients received at least one year of DAPT after implantation. There was a significantly higher use of ticagrelor in the cases performed later in the study period (19% in 2012-2013 versus 51% in 2014-2015; p<0.001), reflecting changes in local practice regarding DAPT prescription for PCI after presentation with acute coronary syndrome. Ticagrelor was used more frequently in acute coronary syndromes (67/141 [48%]) than in stable angina cases (42/165 [25%], p<0.001).
IMPACT OF OCT IMAGING ON PROCEDURES
In the 201 cases where OCT was used to guide implantation, a median of 2 (1-4) OCT acquisitions were performed per vessel. Overall, OCT was performed before predilatation in 29%, after predilatation in 76% and after BVS implantation and post-dilatation in 98%. In 17 cases, OCT was used only after deployment and post-dilatation of the BVS. In four cases, OCT after BVS was no longer possible, twice because of technical failure of the OCT system or catheter, twice because of the inability to pass the OCT catheter through the scaffolded segment. In the OCT-mandatory phase, OCT was used both before and after BVS implant in all cases. In the OCT-selective phase, use was more heterogeneous, reflecting “real-world” use of intravascular imaging. In 34 cases (17%), the intended implant strategy was changed after the last OCT run: supplementary post-dilatation for eccentric scaffold expansion and strut malapposition was performed in 20 cases (10%), implantation of additional BVS for inflow or outflow dissections in 12 cases (6%) and administration of intravenous tirofiban for intracoronary thrombus visualised in the vessel distal to the deployed BVS in two cases (1%), both of which had presented with acute coronary syndrome.
To assess the impact of OCT guidance, we analysed procedural and X-ray screening times, X-ray dose (DAP) and contrast volume used for elective single-vessel procedures only to eliminate bias (Figure 1). There were no differences in procedural or X-ray screening time between OCT-mandatory and OCT-selective groups (82±18 vs. 76±20 min; p=0.06, and 13±4 vs. 10±2 min; p=0.10). However, when comparing angiography alone (n=80) versus all procedures using OCT (n=123), procedure and X-ray screening times were significantly shorter when using angiographic guidance alone (80±18 vs. 60±18 min; p<0.001, and 12.9±5.0 vs. 9.2±3.7 min; p<0.001, respectively). X-ray exposure and contrast volume used were lower in the OCT-selective compared with the OCT-mandatory strategy (9,153±4,680 vs. 10,441±4,422 cGy·cm²; p=0.04, and 179±79 vs. 234±68 ml; p<0.001) (Figure 1), again driven by significantly lower X-ray and contrast use in the angiography-guided cases (DAP 7,470±2,802 cGy·cm²; p<0.001, and 136±53 ml; p<0.001, respectively) compared to OCT-guided cases.
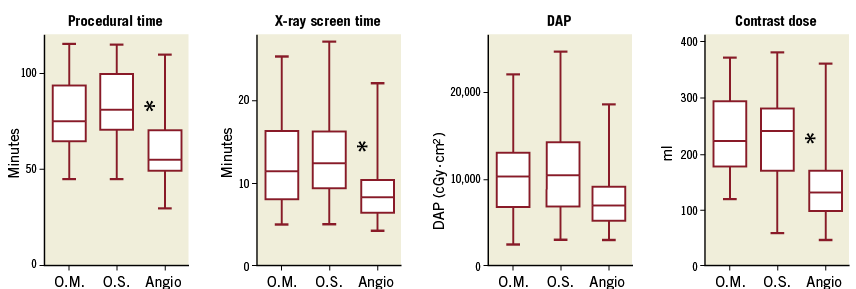
Figure 1. Comparison of procedure time, X-ray screening time and dose, and contrast volume used in OCT-mandatory, OCT-selective and angiography cases. Box-and-whisker plots comparing maximum, minimum, median and upper and lower quartiles for procedural times, X-ray screening times, X-ray dose (dose area product [DAP]) and contrast volume used for all elective single-vessel procedures between OCT-mandatory (n=63), OCT-selective (n=65) and angiographic (n=75) strategies. * indicates p<0.05. O.M.: OCT-mandatory; O.S.: OCT-selective
CLINICAL OUTCOMES
Clinical follow-up was complete in all 272 patients; outcomes are summarised in Table 1. Mean follow-up duration was 38±10 months and was significantly longer in the OCT-mandatory group. For the overall population, median follow-up duration for all patients was 38 months. For all patients alive on the follow-up closing date, the follow-up duration range was 22-65 months. There were no significant differences in outcome rates for DOCE between the OCT-mandatory, OCT-selective and angiography groups and, in particular, no difference in scaffold thrombosis (ScT) rates (annual DOCE rate 1.5, 1.3 and 1.5%, respectively; annual ScT rate: 0.5%, 0% and 0.3%, respectively) (Figure 2).
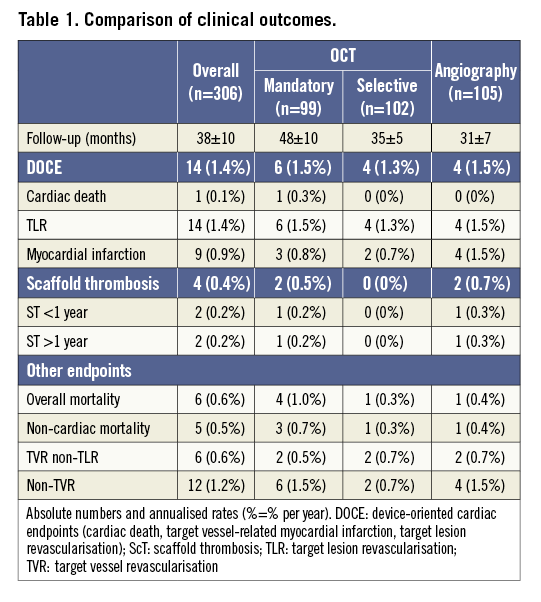
Figure 2. Kaplan-Meier survival curves for freedom from DOCE. Number at risk: number at risk at each year of follow-up. p-value shown for log-rank test comparison.
Univariate Cox regression was performed to identify associations between clinical, procedural and lesion characteristics and DOCE (Figure 3). The following parameters were associated with an increased risk of DOCE: ostial lesions (HR 8.26, 95% CI: 2.54-27; p<0.001), QCA RVD less than 2.5 mm (HR 5.88, 95% CI: 1.92-18; p=0.002), diabetes mellitus (HR 3.69, 95% CI: 1.2-11; p 0.02) and lack of predilatation (HR 5.26, 95% CI: 1.1-25; p=0.03). The limited number of endpoints did not allow multivariate analysis. There was a relationship between RVD and DOCE rates; DOCE were recorded for eight out of 71 (11.2%) vessels with an RVD smaller than 2.5 mm versus five out of 235 (2.1%) larger vessels (p<0.001).
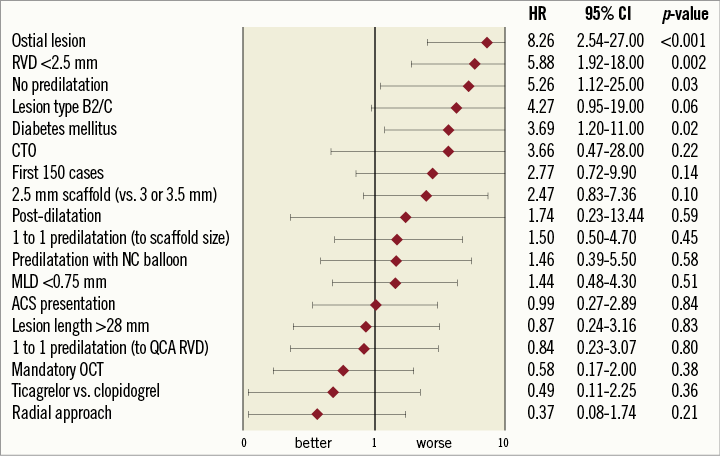
Figure 3. Forest plot indicating univariate regression hazard ratio (HR) and 95% confidence intervals (CI) of DOCE for clinical, lesion and procedural characteristics. ACS: acute coronary syndrome; CTO: chronic total occlusion; NC: non-compliant; QCA: quantitative coronary angiography; RVD: reference vessel diameter
SCAFFOLD THROMBOSIS
In total, four cases of scaffold thrombosis were identified during follow-up (overall annualised ScT rate 0.4%) (Table 1); all cases were “definite” ScT as per the ARC definition. In two patients, ScT occurred subacutely (after 24 hours but within one month after implantation), when both patients were on DAPT. ScT occurred very late (after more than one year) in two patients, and one patient was still on DAPT. Three of the ScT cases were associated with ScT-elevation myocardial infarction. One patient died one day after presentation with ScT and all were re-stented with metallic stents at re-presentation. Angiographic and OCT images and details of three ScT cases are presented in Figure 4.
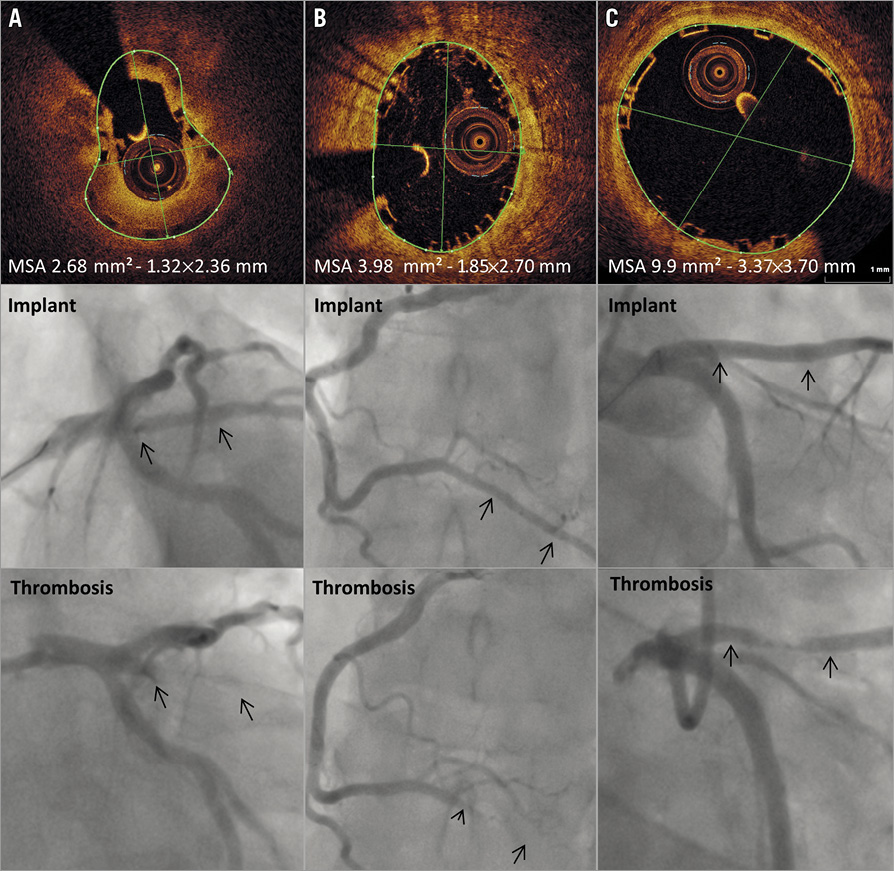
Figure 4. Angiographic and OCT imaging screenshots at the level of minimal scaffold dimensions in scaffold thrombosis cases. A) Subacute ScT in intermediate vessel (day 4 post implantation): OCT run after thrombectomy shows eccentric “pear-shaped” scaffold underexpansion. B) Subacute ScT in posterior descending artery (day 7): OCT run at implantation after final post-dilatation shows good strut apposition but moderate eccentricity and scaffold underexpansion. C) Very late ScT in left anterior descending artery (day 674): OCT run at implantation showing good scaffold expansion and strut apposition; patient off DAPT at time of event. Arrows indicate position of the BVS markers.
Discussion
We report on our initial experience with the Absorb BVS in 406 consecutive implants. Our centre’s policy had been to implant BVS with OCT guidance systematically in the early stages, and to evolve towards a selective policy of OCT guidance with a routine angiography-alone strategy after this initial training period. In the overall population, the rate of DOCE and ScT was low and broadly comparable with metallic everolimus drug-eluting stents used in a “real-world” setting10. We confirmed that the risk of DOCE was higher in ostial lesions, vessels with diameter less than 2.5 mm, presence of diabetes mellitus and in cases where no predilatation was performed, similar to other reports11,12. In the angiography group, there was a significantly lower X-ray exposure, X-ray time and contrast use when compared to the mandatory OCT group. Mandatory use of OCT was associated with larger diameter predilatation, more 1:1 predilatation implantation at higher pressures and, critically, use of fewer BVS, in terms of both number and overall length deployed. Post-dilatation appeared to be less frequent in the OCT-mandatory phase, and potent DAPT was more frequently used in the OCT-selective phase, which may be confounding factors, and probably reflects the evolution of BVS practice during this study period. Nevertheless, these differences did not appear to influence clinical or device event rates. Our data suggest that an initial learning period with OCT guidance followed by a selective approach to use of intracoronary imaging guidance may provide very acceptable longer-term BVS results.
Recent randomised controlled trial data comparing BVS to everolimus-eluting metallic drug-eluting stents have shown unacceptable target vessel failure and myocardial infarction rates in the BVS group driven by increased scaffold thrombosis, despite no signal in terms of mortality2,3. At present, the commercialisation of the Absorb BVS has been stopped. Lack of operator adherence to evolving recommendations on patient and lesion selection, implantation technique and the importance of imaging to guide implantation may have been factors explaining these disappointing data.
Our data paint a different picture of the “real-world” performance of BVS: our population was comparable to the GHOST-EU cohort with similar rates of diabetes patients (23% versus 26%), acute coronary syndrome presentation (47% versus 46%) and lesion types (B2/C 58% versus 52%)13. Despite this, our outcome data demonstrate that BVS performed rather better, with annualised TLR rates of 1.4% vs. 7.0% and an annualised ST rate of 0.4% vs. 3.4% in GHOST-EU. A key difference that we believe explains the superior performance is the frequency and strategy of intravascular imaging use to guide BVS implantation in our study, bringing with it improved BVS implantation and optimisation. In GHOST-EU, only 28% of implants were guided by intravascular imaging (OCT or IVUS) versus 66% in our study (all OCT). Balloon post-dilatation was also performed in only 49% of cases in GHOST-EU compared to 90% in our cohort. The importance of post-dilatation was demonstrated by Puricel et al in a multicentre registry investigating ScT in 1,305 BVS patients, where systematic introduction of an implantation protocol involving mandatory effective 1:1 predilatation, adequate scaffold sizing and systematic post-dilatation was associated with an ScT rate decrease from around 3.0% to 1.1%5.
OCT carries an additional cost that may need to be justified. Our data indicate that a BVS implantation strategy with a training period informed by compulsory OCT guidance, followed by selective OCT use in only half of cases, allows interventionists to obtain acceptable BVS results. Published data from the ILUMIEN III trial show that OCT-guided PCI improves both mean and minimum stent expansion compared with angiography-guided stenting alone, and we believe that this can also be the case for scaffold implantation6. Further, it is encouraging that the risk of ScT in our overall cohort (0.4% per year) was as low as that observed with second-generation DES and that the ScT rate did not increase when the frequency of OCT usage dropped. An important consideration is the fact that half of the ScT cases were very late (>1 year post implant) and are less likely to be a result of a mechanical issue with the BVS that could have been recognised and addressed at implantation.
Despite the preceding extensive training period with OCT, it is of interest to note procedural behaviour changes in the OCT-selective group: there was a decrease in the use of 1:1 predilatation. This may be important since 1:1 predilatation has been shown to improve BVS expansion, and a rigorous approach to implant technique has been demonstrated to reduce ScT5. Similarly, we demonstrated an increased risk of DOCE in those who did not receive post-dilatation. Nevertheless, OCT use in the OCT-selective group was associated with more 1:1 predilatation and post-dilatation and this, along with a longer duration of DAPT, may have mitigated the associated risk from failure to predilate 1:1 in our cohort.
Limitations
This was a single-centre, non-randomised study and patient selection bias is therefore inevitable to some degree. As experience increased, the complexity of the cases selected for BVS implantation may have changed. Similarly, whilst case complexity may have been higher when OCT was used in the OCT-selective cases, the prior OCT-guided experience and DAPT choice are counter-confounders, along with the imbalance in follow-up periods between the two groups implicit in the temporal nature of the two implant strategies used. However, the secondary analysis comparing the two components of the OCT-selective strategy mitigates this criticism to some degree. The size and design of our study did not allow a non-inferiority analysis to be performed between OCT- and angiography-guided implantation. Our findings should therefore be viewed as hypothesis-generating until confirmed by a randomised study.
Conclusions
When implanting BVS in real-world populations, an initial learning period with mandated OCT guidance results in very acceptable clinical outcomes and facilitates future use of a more selective strategy of intracoronary imaging without diminution of BVS performance. Despite such an approach, the risk of adverse events remained higher in ostial lesions, smaller vessels, in diabetics and when predilatation was not performed, highlighting the importance of patient/lesion selection and of optimum implant technique for this novel technology. Additionally, OCT use to guide BVS implantation decreased the number and total length of scaffolds implanted, encouraged increased use of higher diameter post-dilatation and altered overall procedural strategy in 17% of cases, at a cost of increased procedural time, contrast volume administered, and patient radiation dose.
| Impact on daily practice BVS implantation requires appropriate scaffold sizing, lesion preparation and post-implant optimisation. OCT guidance may facilitate these steps. When implanting BVS, an initial learning period with mandated OCT guidance results in very acceptable clinical outcomes and facilitates future use of a more selective strategy of intracoronary imaging without diminution of BVS performance. |
Conflict of interest statement
N. West has acted as a consultant for Abbott Vascular (significant) and St. Jude Medical (modest). S. Hoole has acted as a consultant for Abbott Vascular (modest) and St. Jude Medical (modest). The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Clinical and lesion characteristics.
Supplementary Table 2. Procedural details.
To read the full content of this article, please download the PDF.

