
Abstract
Aims: We sought to compare vascular healing with bioresorbable everolimus-eluting vascular scaffolds (BVS) and drug-eluting stents with bioabsorbable polymers (BP-DES) at six and 12 months both implanted in the same patients.
Methods and results: This was a multicentre and prospective study including patients with at least two comparable lesions to treat. In every patient both BVS and BP-DES (SYNERGY, Orsiro or BioMatrix Flex) were implanted by lesion randomisation. Patients included were evaluated with optical coherence tomography at six or 12 months (2:1). Finally, 68 patients had an examination at six months and 27 patients at 12 months. The rates of uncovered struts at six months were 1.7±3.2% for BVS and 5.3±5.6% for BP-DES (p=0.0001), and at 12 months 0.48±0.72% and 4.8±5%, respectively (p=0.001). Rates of strut malapposition were significantly lower with BVS. There was no significant intra-patient correlation with BP-DES/BVS for endpoints. Evaginations were more frequent and larger with BVS. Discontinuities in BVS were observed in 19.4% at six months and 14.3% at 12 months.
Conclusions: Vascular healing with BVS and BP-DES could be more device-specific than patient-specific. At follow-up, BVS presented fewer uncovered or non-apposed struts than BP-DES but more frequent and larger evaginations. Discontinuities in BVS were relatively frequent at both time points.
Abbreviations
BP-DES: bioabsorbable polymer drug-eluting stents
BVS: bioresorbable vascular scaffolds
EES: everolimus-eluting stents
MLA: minimum lumen area
MSA: minimum stent area
OCT: optical coherence tomography
Introduction
Drug-eluting stents with bioabsorbable polymers (BP-DES) were designed to decrease polymer-triggered unfavourable vascular responses and, ultimately, the risk of very late stent thrombosis. The development of bioresorbable vascular scaffolds (BVS) was aimed at preventing long-term stent-related events. Nonetheless, recent data show that their use is associated with a higher rate of thrombosis1,2.
The arterial healing process depends on device features, but it could be influenced by biological factors that are highly variable among individuals. Accordingly, we designed a study in which both BVS and BP-DES were implanted randomly in selected lesions of the same patient, enhancing the comparability with respect to a per-patient randomised design.
We sought to evaluate and compare the vascular healing process using optical coherence tomography (OCT) at six and 12 months with BVS and different models of BP-DES. The study was supported by the research agency of the Spanish Society of Cardiology.
Methods
The ESTROFA (grupo de EStudio de la TROmbosis de stents FArmacoactivos) OCT BVS vs. BP-DES study was a multicentre prospective study conducted in 15 centres, designed to compare the healing process at six and 12 months between BP-DES and BVS.
STUDY POPULATION
Patients were eligible for the study if they met all of the following clinical and angiographic criteria.
Clinical inclusion criteria: a) indication for percutaneous revascularisation out of the setting of primary angioplasty; b) adequate candidates for a dual antiplatelet therapy period of at least 12 months.
Angiographic inclusion criteria: a) patients should have at least two lesions to be treated. If in the same vessel, it should be feasible to treat both lesions without overlapping of stents and leaving a gap >20 mm; b) lesions should be suitable to be treated with stents >8 mm in length and ≥2.5 mm in diameter.
Angiographic exclusion criteria: a) restenosis; b) left main disease; c) chronic total occlusion; d) bifurcation; e) ostial location; f) presence of clear angiographic signs of complication (rupture, dissection, ulceration or thrombus).
The study protocol was approved by the local research ethics committee of all participating centres. A specific informed consent was obtained in all patients included in this study. The study was promoted by the Spanish Society of Cardiology.
STUDY DEVICES
The BVS used was the Absorb GT1™ (Abbott Vascular, Santa Clara, CA, USA) and the BP-DES group comprised the BioMatrix Flex™ stent (Biosensors Interventional Technologies, Singapore), the SYNERGY™ stent (Boston Scientific, Marlborough, MA, USA) and the Orsiro™ stent (Biotronik, Berlin, Germany). Technical details are provided in Supplementary Appendix 1.
PROCEDURE
In every patient the first target lesion to treat was by protocol randomly allocated to BVS or BP-DES treatment through an on-site system, treating the second target lesion with the other study device so that every patient had both types of study device implanted. In case of the presence of three or more lesions to treat, all of these additional lesions were treated with BP-DES. Among lesions assigned to treatment with BP-DES, subtype selection was carried out following an on-site alternate sequence, 2:1:1 for SYNERGY, Orsiro and BioMatrix, respectively.
Adequate lesion preparation, device sizing and post-dilatation were highly recommended, especially for BVS. Dual antiplatelet therapy was indicated for a minimum period of 12 months. Angiographic and OCT examination at follow-up was scheduled at six or 12 months (2:1) using an alternate sequence.
ANGIOGRAPHIC ANALYSIS
Serial angiographic studies were obtained after intracoronary administration of nitroglycerine in two well selected orthogonal matching views at baseline, post-procedure, and follow-up. Quantitative analysis was performed with validated 2D software for QCA analysis (QAngio XA version 7.3; Medis, Leiden, the Netherlands).
OCT ACQUISITION AT FOLLOW-UP
Per protocol OCT acquisition was planned at six-month or 12-month angiographic follow-up with a variation of ±15 days. All OCT recordings were collected for analysis in a centralised core lab (Hospital Clinico San Carlos, Madrid, Spain). A more detailed description of the OCT acquisition procedure is provided in Supplementary Appendix 1.
OCT ANALYSIS AND STUDY ENDPOINTS
Co-primary endpoints were: a) rate of uncovered struts at six months for BVS and BP-DES; b) rate of uncovered struts at 12 months for BVS and BP-DES.
Off-line analysis of the stented segment was performed at 1 mm intervals with a dedicated analysis system (QIvus®; Medis, Leiden, the Netherlands) in a core lab.
ASSESSMENT OF COVERAGE
The struts of the BP-DES were classified as uncovered if any part of the strut was visibly exposed to the lumen and the struts of the BVS were classified as uncovered if the thickness of the coverage from the endoluminal border of the black box to the lumen contour was <30 µm3,4.
Assessment methods for other findings are fully described in Supplementary Appendix 1. Investigators in the core lab were obviously not blinded to the type of stent (BVS or BP-DES) but they were blinded for the time of examination.
STATISTICAL ANALYSIS
The sample size calculation was based at the initiation of the study on the limited available data at that time5-8. A detailed description of the sample calculation and the statistics applied is provided in Supplementary Appendix 1.
Results
A total of 120 patients were enrolled in the study. Clinical and procedural characteristics are shown in Supplementary Table 1. The study flow diagram is shown in Figure 1. Clinical outcomes at 12 months are presented in Supplementary Table 2. The quantitative angiographic analysis at baseline, six- and 12-month follow-up did not show significant differences (Supplementary Table 3). Findings in planimetric OCT analysis are presented in Supplementary Table 4. In the BVS group, a smaller minimum lumen area was noted at six months, as well as a lower BVS area than expected from the nominal stent area ratio at both time points.
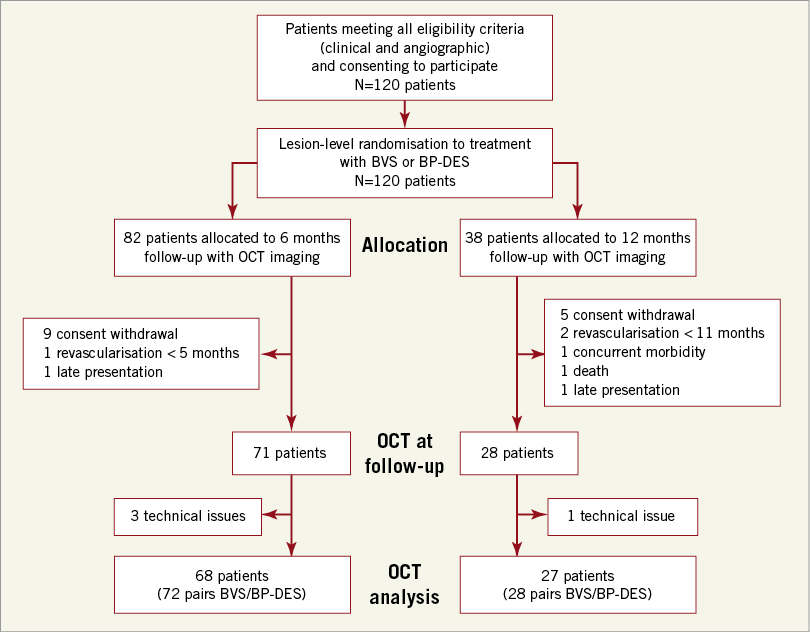
Figure 1. Flow diagram of the study.
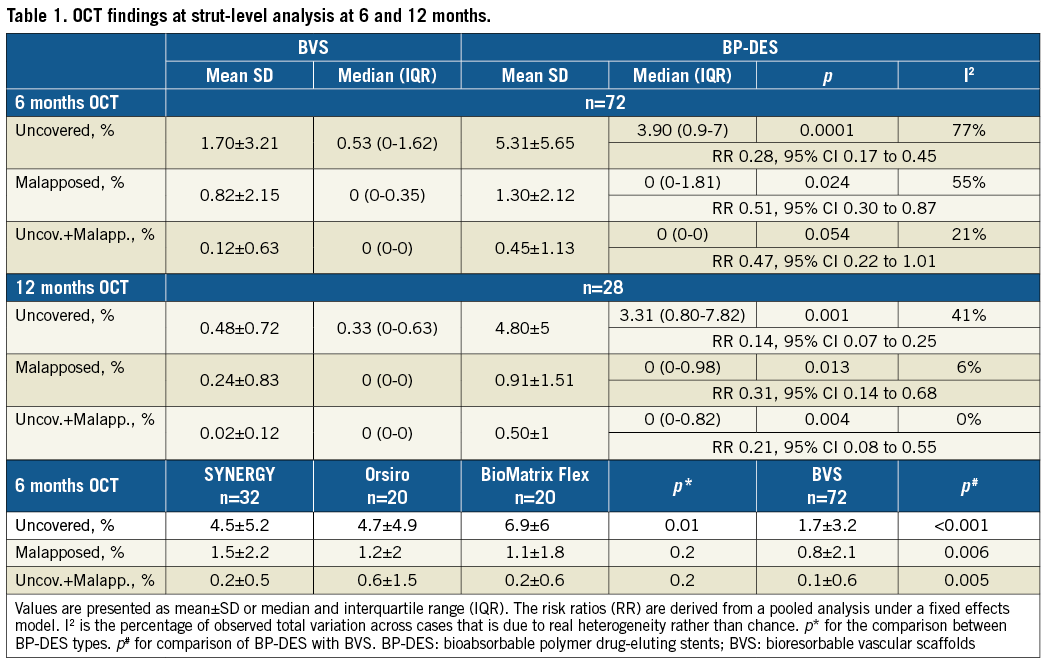
The OCT analysis at strut level is shown in Table 1. The kappa statistic for the interobserver agreement was 0.86 for strut uncoverage and 1 for strut malapposition. A significantly lower rate of uncovered and/or malapposed struts was observed with BVS at six and 12 months. However, significant heterogeneity was found for uncoverage. Notably, only 4-10% of uncovered struts with BVS or BP-DES either at six or 12 months had concomitant malapposition. Among BP-DES, uncoverage was significantly lower with SYNERGY and Orsiro. The clusters for uncoverage and malapposition are shown in Supplementary Table 5. Overall, the independent predictors for an uncoverage rate over 1% were the BVS (OR 0.13, 95% CI: 0.05 to 0.29; p<0.0001) and stent length >18 mm (OR 2.34, 95% CI: 1.04 to 5.25; p=0.039).
The relationship between uncoverage or non-apposition with BVS and BP-DES within the same patient is illustrated in Figure 2 and Figure 3. No significant correlation was found for any strut-level endpoint. The correlative graphics for uncovered strut rates at six months with BVS vs. each model of BP-DES are shown in Supplementary Figure 1. The strut-level endpoints at six and 12 months for BVS and BP-DES groups are presented in Figure 4.
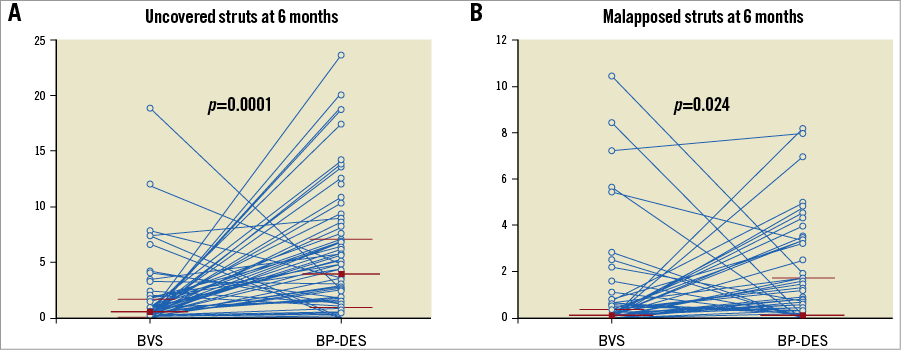
Figure 2. Strut-level endpoints in paired BVS and BP-DES at six months. A) Rates of uncovered struts at six months, correlation coefficient –0.21 (95% CI: –0.43 to 0.016). B) Rates of malapposed struts at six months, correlation coefficient 0.098 (95% CI: –0.14 to 0.32). Blue lines connect values from the same patient. Median and interquartile range is shown for BVS and BP-DES cohorts.
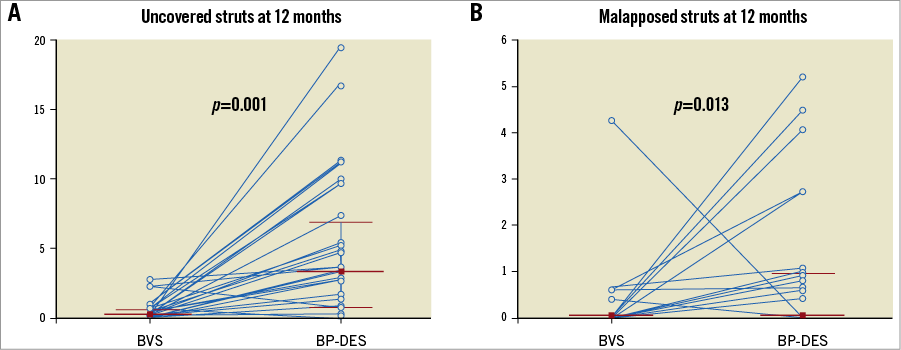
Figure 3. Strut-level endpoints in paired BVS and BP-DES at 12 months. A) Rates of uncovered struts at 12 months, correlation coefficient 0.14 (95% CI: –0.25 to 0.49). B) Rates of malapposed struts at 12 months, correlation coefficient 0.11 (95% CI: –0.27 to 0.47). Blue lines connect values from the same patient. Median and interquartile range is shown for BVS and BP-DES cohorts.
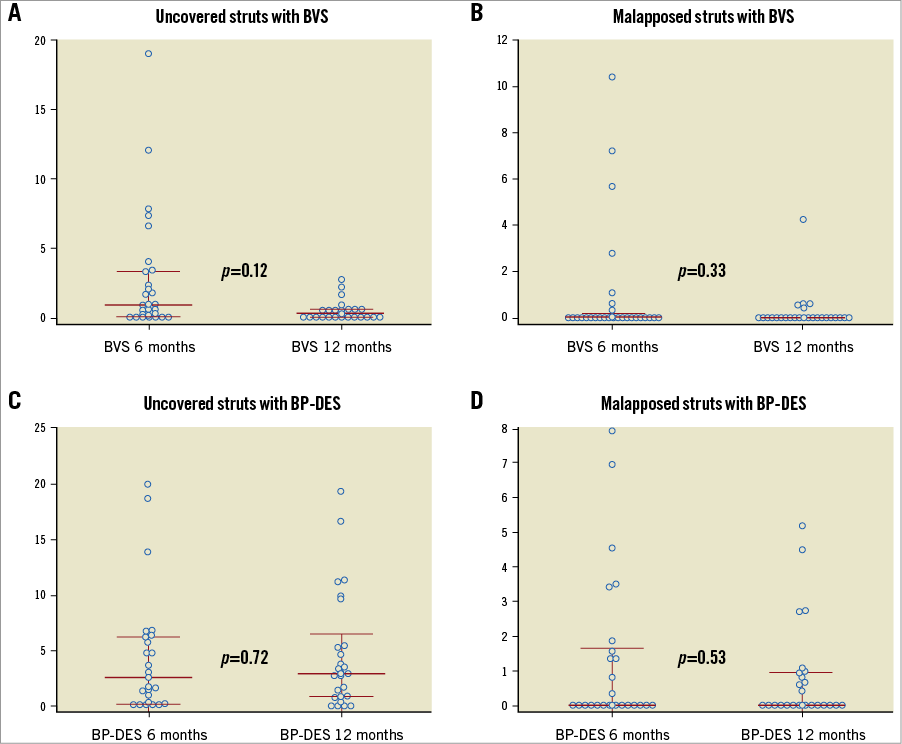
Figure 4. Strut-level endpoints from six to 12 months. A) Rates of uncovered struts with BVS. B) Rates of malapposed struts with BVS. C) Rates of uncovered struts with BP-DES. D) Rates of malapposed struts with BP-DES. Median and interquartile range is shown for the six- and 12-month cohorts.
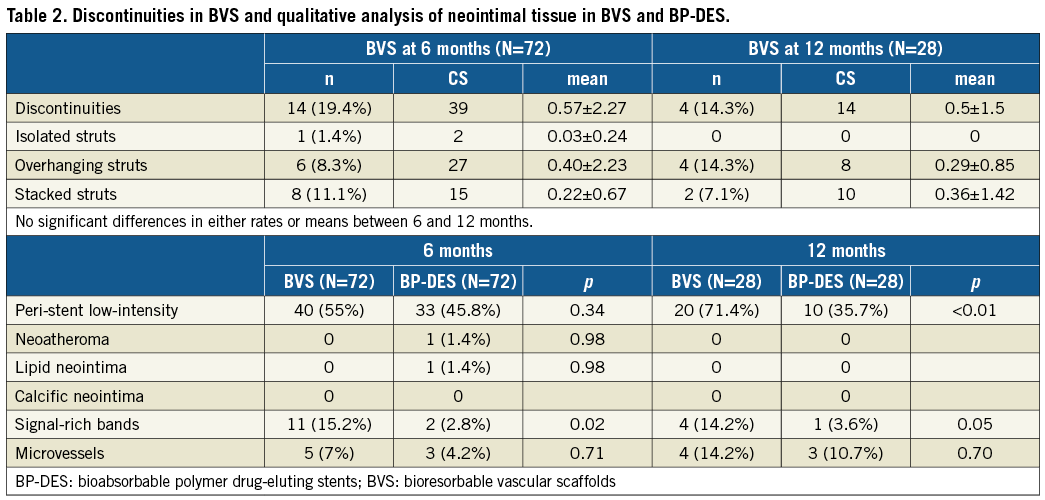
Analysis of discontinuities in BVS and qualitative analysis of the neointimal tissue are presented in Table 2. Peri-strut low-intensity areas were found similarly at six months but were significantly more prevalent with BVS at 12 months. BVS discontinuities were relatively frequent, even at six months, and mostly evident as overhanging and stacked struts.
Evaginations were observed more frequently and resulted in being larger with BVS, especially at six months (Supplementary Table 6). The overall rate of evaginations in BVS was comparable between those patients showing or not showing evaginations in BP-DES (83% vs. 73%, p=0.1). The rate of evaginations was comparable between BP-DES types but the magnitude was smaller with SYNERGY. In both groups, the evaginations did not appear to be related to underexpansion of the devices but to a lower degree of intimal proliferation and more malapposition in BP-DES (Supplementary Table 7).
Discussion
We found that impaired vascular healing after BP-DES and BVS implantation appears to be predominantly device-specific. We documented that strut uncoverage is less frequent in BVS and that it is influenced by BP-DES design. Of note, the rates of strut uncoverage and malapposition were not significantly different at six- and 12-month follow-up. Discontinuities were relatively frequent with BVS, even at six months, and peri-stent vascular evaginations were more frequently observed with BVS.
VASCULAR HEALING AFTER IMPLANTATION OF BVS AND METALLIC DES
At six months, the proportion of uncovered struts with BVS in series of 12-25 patients has been 2-5.3%5-7, at 12 months 3.3%4, at 24 months 1% and at 36 months 1.7%7,9. In a post hoc analysis of 44 unmatched patients, comparable rates of uncovered struts at 12 months were found for BVS and second-generation DES10. In the EVERBIO II trial, BVS showed a lower uncoverage rate at nine months compared with BP biolimus-eluting stents11. In the recently published TROFI II trial12, a better healing score was observed with BVS at six months compared with a durable polymer everolimus-eluting stent, implanted in primary angioplasty.
The proportion of uncovered struts with a BP biolimus-eluting stent ranges from 17% at six to eight months to 9% at eight to 12 months13,14. Uncoverage rates in small series treated with the Orsiro stent were 1.3% at three months and 1.8% at six months15, and with the SYNERGY stent 5.5% and 3.4% at three and six months, respectively16.
A more complete extension of coverage could have been expected in our study with the thin-strut BP-DES than with BVS. However, the degree of BVS coverage was in the range of that previously reported. Nonetheless, strictly speaking, vascular healing cannot be accurately assessed by means of OCT since no distinction can be made between endothelial and fibrin strut-covering layers. A recent investigation, using OCT-derived light property analysis, showed that tissue maturation was comparable but lipidic change of neointima was less prominent after BVS implantation compared to metallic everolimus-eluting stents, suggesting a more stable superficial neointima on the BVS17. On the other hand, the thicker BVS struts could promote a more extensive peri-strut deposition of fibrin, explaining the higher early strut coverage18,19. Moreover, the higher prevalence of peri-strut low-intensity area observed with BVS at 12 months could be related to more fibrin deposition and inflammatory activity.
EVAGINATIONS AND DISCONTINUITIES
In a recent publication, the incidence of evaginations in 102 BVS at 12 months was high (54%) but major evaginations were infrequent (0.9%)20. The presence of evaginations was strongly associated with malapposition but not with uncoverage and these were related with more fractures and more peri-stent low-intensity area. In our study, in agreement with the data mentioned above, evaginations were more frequently seen with BVS and were present in scaffolds showing more fractures and peri-stent low-intensity area. Regarding the mechanisms involved, evaginations in BP-DES were related to less intimal proliferation and higher rates of strut uncoverage and malapposition. In BVS, these were related to the right coronary artery location and a smaller lumen area stenosis. Nonetheless, the absence of baseline OCT prevents drawing any conclusions about their mechanisms.
Late strut discontinuity of the polymeric struts has been observed in up to 40% of patients at three years21. In our study, discontinuities were less common but not infrequent even at six months. The different rates between studies could be related mainly to the different times of assessment. The prognostic relevance of discontinuities was inferred in the previously mentioned study from a small sample size (51 patients) of the ABSORB cohort B. Nonetheless, the recently published INVEST registry, including 36 patients with very late BVS thrombosis at a median time of 20 months, demonstrated that the leading mechanism underlying very late thrombosis with BVS was scaffold discontinuity, which could suggest an unfavourable resorption-related process22.
BVS STRUT COVERAGE AND RISK OF LATE/VERY LATE THROMBOSIS
The endpoint of strut coverage by OCT has thus far been considered an adequate surrogate for the risk of late thrombosis with metallic DES. The high rates of coverage for BVS struts reported herein and in previous studies and, on the other hand, the increased risk of late thrombosis reported with BVS could be seen as contradictory. However, these are not necessarily in contradiction with clinical evidence regarding BVS thrombosis. As clearly shown in patient-level meta-analysis of the ABSORB trials, the risk of BVS thrombosis is concentrated in two periods, the first 30 days and between 18 and 36 months23. As previously mentioned, factors other than coverage could account for the increased late/very late thrombosis risk with BVS22.
The reported findings regarding discontinuities and evaginations could most probably count as risk factors for very late thrombosis events. Therefore, the endpoint of strut coverage by OCT might not be as valid as a surrogate for late/very late thrombosis risk with bioresorbable scaffolds as it is for metallic DES.
Limitations
The lack of baseline OCT examination precluded any definitive conclusion regarding the cause of the incomplete stent apposition and evaginations found at follow-up. The absence of mandatory post-procedural OCT was mainly due to the intention to assess vascular healing in conditions closer to real practice where no systematic use of imaging is carried out.
We acknowledge the limitations of the methodology employed to evaluate tissue coverage in BVS, and it is plausible that there may have been a certain rate of false positive findings; nonetheless, we used the most accepted technique (OCT) and we followed the most established standards. Assessment of coverage with OCT portends certain limitations that could be overcome to some extent with the use of coronary angioscopy; however, this technique is affected by relevant limitations which notably restrain its use in trials. The study design isolates quite well the device-specific effects on vascular healing but it does not permit establishing the relative contribution of the patient-specific vs. device-specific effects.
Conclusions
We found that vascular healing after BP-DES and BVS implantation could be predominantly device-specific. BVS showed a lower rate of uncovered and/or non-apposed struts at six months and 12 months. No intra-patient correlation for endpoints was found between BVS and BP-DES. Evaginations were more frequent and larger with BVS, particularly at six months. Discontinuities in BVS were relatively frequent at both time points. These results suggest that we should focus on specific imaging risk features in specific devices rather than evaluating the same features in all of them.
| Impact on daily practice The study provides new insights into the vascular healing process after implantation of BVS and BP-DES, proposing a study model which allows a more accurate device comparison. The study casts doubt on the validity of the commonly used endpoint of strut coverage as determined by OCT to inform about the risk of late/very late thrombosis with bioresorbable devices, pointing out the value of other findings such as evaginations or discontinuities. It is crucial to achieve a deep knowledge about the bioresorbable coronary devices if we want this promising technology to succeed. |
Acknowledgements
(Supplementary Appendix 2).
Funding
The study was funded by unrestricted grants from Boston Scientific (Study Reference #: ISROTH10090), Abbott Vascular, St. Jude Medical, Biotronik and Biosensors.
Conflict of interest statement
J.M. de la Torre Hernandez has received unrestricted grants for research from Boston Scientific, Abbott Vascular, St. Jude Medical, Biotronik and Biosensors, and consulting fees from Medtronic and Philips Volcano Inc. N. Gonzalo has received personal fees from Abbott Vascular for educational events. J. Escaned has received personal fees from Boston Scientific and Abbott Vascular for educational events. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Methods.
Supplementary Appendix 2. Acknowledgements.
Supplementary Figure 1. Uncoverage in BVS and different types of BP-DES at 6 months.
Supplementary Table 1. Clinical and procedural characteristics.
Supplementary Table 2. Major adverse cardiac events at 12 months.
Supplementary Table 3. Quantitative angiographic data.
Supplementary Table 4. OCT planimetric findings at 6- and 12-month follow-up.
Supplementary Table 5. Clusters for uncoverage and malapposition in OCT.
Supplementary Table 6. Evaginations in OCT.
Supplementary Table 7. OCT findings in devices with and without evaginations.
To read the full content of this article, please download the PDF.

