
Over the better part of two decades, lessons learned from stent failure have led to technical advances that have resulted in current-generation metallic drug-eluting stents (DES) with very low rates of target lesion failure (TLF) and stent thrombosis1. Continued efforts have been directed at achieving iterative improvements in the design of DES to enhance adaptive vascular healing following vascular injury. By promoting early strut coverage with minimal neointimal hyperplasia but a functioning endothelial layer, stent thrombosis may be minimised and, by reducing the inflammatory response to the foreign polymer and metallic struts, TLF may be reduced.
To this end, although there has been limited success in developing novel antiproliferative drugs that differentially inhibit proliferation and migration of vascular smooth muscles yet promote them in endothelial cells2, progress has been made in altering antiproliferative elution kinetics, using biocompatible/biodegradable polymers for drug elution, and/or designing thinner struts while maintaining the radial strength required for scaffolding support. Bioresorbable vascular scaffolds (BVS) represent a paradigm shift, designed to provide temporary mechanical support during vascular remodelling, followed by complete resorption over the next several years, thus removing the nidus for very late adverse events while restoring cyclic pulsatility and physiologic vasomotion. Unfortunately, enthusiasm for BVS has been tempered by the inferior performance of the first-in-class Absorb™ scaffold (Abbott Vascular, Santa Clara, CA, USA) compared to contemporary metallic DES3. The shortcomings of the Absorb BVS have been attributed to large strut thickness and width, translating into a large device footprint in the vessel lumen, which in turn may affect the local haemodynamic microenvironment and accelerate neointima formation and promote device thrombosis4. Hence, minimising strut thickness while preserving radial strength and altering scaffold resorption rates may be key prerequisites to improving the performance of iterative BVS technology.
In the current issue of EuroIntervention, three reports (one case report5 and two small size randomised clinical trials6,7) examine the impacts of the aforementioned advances in the design of the metallic DES or BVS on vascular healing following stent/scaffold implantation, while a fourth observational study reports neointima patterns in the metallic DES >10 years after implantation8. All four studies use optical coherence tomography (OCT) to assess neointima morphology and utilise the presence of scaffold/strut coverage as surrogates for vascular healing post implantation.
While OCT allows high-resolution imaging, accurate dimensional measurements and automatic analysis of many steps during percutaneous coronary intervention9, it is limited in its ability to evaluate the cellular responses that contribute to vascular healing due to imaging resolution. As such, caution should be exercised when using OCT to assess vascular healing for a number of reasons. First, strut coverage and re-endothelialisation are not synonymous (Figure 1)10. Second, a number of preclinical and clinical studies have cast doubt on the validity of strut coverage and neointima patterns on OCT as surrogates for assessment of post-implantation vascular healing. As shown in experimental models2, the endothelial monolayer covering the struts or the luminal side of the neointima is 1-2 µm in thickness and is therefore considerably beyond the detection limit of the current commercial OCT systems with a resolution of ~15 µm. At this point, the endothelium may only be identified using experimental micro-OCT systems11. Third, fibrin-targeted near-infrared fluoroscopy combined with OCT in a preclinical model has shown that a sizeable percentage of struts deemed covered by OCT are actually covered by fibrin, particularly in DES12, thus representing a thrombogenic nidus. Indeed, exposure of fibrin or collagen to the vessel lumen is the predominant feature identified in acute coronary syndromes caused by intact fibrous cap, the so-called “erosions”, also diagnosed by OCT. Fourth, clinically, a relationship between strut coverage and endothelium-dependent vasorelaxation has not been established13. Finally, the neointimal patterns on OCT do not always agree with specific tissue types on histopathology. Smooth muscle cells within collagenous/proteoglycan matrix, healed neointimal rupture or erosion, and superficial macrophage accumulation may each correlate with various neointimal patterns on OCT14.
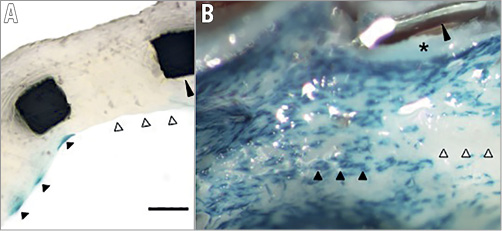
Figure 1. Neointimal strut coverage and re-endothelialisation. A) Partial cross-section of stented vessel from atherosclerotic apolipoprotein-E knockout mouse with endothelial Tie2-LacZ reporter showing complete stent strut coverage (long black arrowhead) but only partial re-endothelialisation. Blue cells depicted by short black arrowheads are endothelial cells. Short white arrowheads show strut covered with neointimal hyperplasia, but absent endothelium. B) En face imaging of the same stented vessel confirms partial absence of endothelium. Blue cells on the surface of the stented vessel (short black arrowheads) are endothelial cells. Large gaps in endothelialisation (white arrowheads) are present despite complete neointimal coverage. The long arrowhead shows a cut stent strut previously embedded in neointima (asterisk). (With permission from Elsevier).
Bearing in mind these limitations of OCT to evaluate and quantify vascular healing, a case report by Mitomo et al5 demonstrated that, nine months post implantation, neointima inside the MAGNITUDE® BVS (Amaranth Medical, Mountain View, CA, USA) was thinner, smoother and more homogenous compared to the one visualised in an Absorb BVS implanted in another artery in the same patient, thus excluding the effects of patient-related factors in vascular healing.
The MAGNITUDE BVS has the thinnest strut (<100 µm) among 22 types of existing BVS. The resorption kinetics of ultra-high molecular weight poly-l-lactide used in the MAGNITUDE BVS are quicker than those of the Absorb BVS. Although limited by the sample size, the OCT findings of Mitomo et al suggest that reducing strut thickness and polymer degradation time may improve healing compared to the first-generation Absorb BVS.
The study by de la Torre Hernandez et al sought to compare the neointima morphology and strut coverage between DES with BP (BP-DES) from three different manufacturers and the Absorb BVS in 120 patients7.
The subjects had at least two comparable lesions with one stent type implanted in each lesion in order to compare the healing response in the same patient. Although the thinner struts of the DES and the use of BP were expected to improve vascular healing indices, the authors found markedly lower rates of uncovered scaffolds in the Absorb BVS than in the BP-DES group on OCT, both at six and at 12 months. While preclinical studies suggest a higher degree of fibrin deposition in the thick-strut BVS compared to metallic DES15, which may account for the higher strut coverage seen in this report, it is well established that late lumen loss is greater in BVS compared to metallic DES16. These findings highlight the aforementioned fundamental flaws in OCT-based assessment of vascular healing, in that they are divergent from the clinical outcomes of BVS versus DES17. If OCT-defined healing is better in the Absorb BVS, why are clinical outcomes worse? The inability of OCT to distinguish tissue types (i.e., fibrin from neointima) remains a critical limitation of this technology in the assessment of healing (Figure 2).
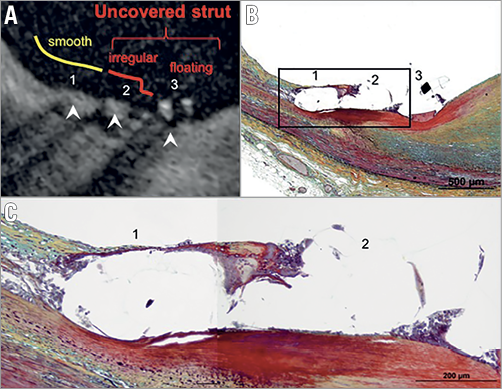
Figure 2. Correlation of strut coverage on OCT with histopathology. A) Optical coherence tomography image showing covered (1, 2) and uncovered (3) struts confirmed by histology (B). C) Magnified histological image corresponding to the image in panel A and histological image in panel B shows strut 1 covered by a thin layer of neointima with overlying endothelium, whereas strut 2 is mainly surrounded by a few inflammatory cells and accumulated fibrin in the absence of endothelium (Movat, ×400). (With permission from Elsevier).
In another study utilising OCT, Asano et al6 investigated percent strut coverage one or two months after implantation of the BuMA™ Supreme stent (SINOMED, Tianjin, China) that contains a BP (resorbed within six weeks) with fast sirolimus elution (released within 28 days) as compared to XIENCE DES (Abbott Vascular) that elute everolimus up to 120 days.
At one month, there was higher strut coverage and higher neointima area in BuMA stents compared to XIENCE DES, while at two months the strut coverage was similar between the two groups. The faster drug release may have contributed to faster strut coverage within the first month after implantation of the BuMA BP-DES compared to XIENCE DES, which in turn may theoretically reduce the risk of early stent thrombosis. Nonetheless, the faster drug elution may also lead to an increase in excessive neointima hyperplasia at a later stage, resulting in the observed higher in-stent late lumen loss with BuMA BP-DES compared to the Resolute™ zotarolimus-eluting stent (Medtronic, Minneapolis, MN, USA) with slower drug elution18, although the lumen loss did not appear to affect coronary flow as assessed by computational fluid dynamics19.
In a retrospective observational study, Usui et al8 assessed the OCT findings >5 years after implantation of a sirolimus-eluting DES (114 stents between five to 10 years and 66 stents >10 years) and reported a higher frequency of neoatherosclerosis (NA) >10 years after implantation compared to the earlier time point of five to 10 years.
Multivariate regression analysis identified stent age >10 years as the only independent predictor of NA. These findings support the notion that NA is a continuous process occurring in parallel with de novo atherosclerosis in the non-stented segments of coronary arteries20, and indicate that mechanisms for stent thrombosis may shift from suboptimal stent implantation and delayed healing at early post-implantation stages to NA in the longer term21. The study by Usui et al thus emphasises the need for intensifying therapies to prevent atherosclerosis progression, not only in non-stented segments of the coronary artery, but also to prevent very late stent failure due to NA.
In conclusion, the OCT-based studies in this issue of EuroIntervention suggest that the newer generation of BVS with thinner scaffolds may mitigate some of the issues encountered with the first-generation BVS and result in better biocompatibility and healing process. The potential impact of BP-DES on improving healing remains unknown. Although a BP-DES with fast drug elution (BuMA) showed faster strut coverage compared to XIENCE DES, this difference disappeared rapidly over time; it may be countered by an increase in late neointima hyperplasia and lumen loss. Metallic DES remain a nidus for very late events, most likely by an increase in NA. Lastly, we reiterate that caution is needed in interpreting vascular healing by commercial OCT systems alone. Density-22 or light property-based analysis23, micro-OCT11 and hybrid imaging24 hold the promise of providing more accurate assessment of vascular remodelling in response to stent implantation.
Conflict of interest statement
Z. Ali reports research grants from St Jude Medical, Boston Scientific and Cardiovascular Systems Inc., and honoraria from Abbott Vascular, Boston Scientific, Medtronic and Cardinal Health. A. Finn reports research grants from Abbott Vascular, Boston Scientific, Celonova, Edwards, Medtronic, Sinomed, Gore, Cook and Lutonix/Bard, and honoraria from Abbott Vascular, Boston Scientific, Celonova, Sinomed, Cook and Lutonix/Bard. K. Karimi Galougahi has no conflicts of interest to declare.

