
Abstract
Aims: Our aim was to assess vascular response after polymer-free sirolimus-eluting stent (SES) implantation by using an optical coherence tomography (OCT)-derived vascular healing score (HS), quantifying the deficiency of healing.
Methods and results: In a prospective, multicentre, single-arm, open-label study, OCT examinations were performed at three months in 45 patients (47 lesions). Per protocol, 24 lesions which had not reached adequate vascular healing according to study criteria were scheduled for OCT examination at six months. The HS was calculated at two time points. Serial OCT imaging demonstrated that the proportion of covered stent struts increased from a median of 87.1% at three months to 98.6% at six months (p<0.001). The neointimal thickness increased from a median of 82.8 µm to 112.2 µm (p<0.001), whereas the median percentages of malapposed struts were 0.2% and 0.0% at the two respective time points. Neointimal volume obstruction increased from 6.3% to 12.8%, and the HS decreased from a median of 28.1 at three months to 2.4 at six months.
Conclusions: In patients who had inadequate vascular healing three months after polymer-free SES implantation, serial OCT showed almost complete vascular healing at six months. (ClinicalTrials.gov Identifier: NCT01925027)
Abbreviations
BMS: bare metal stent
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
HS: healing score
ISA: incomplete stent apposition
LLL: late lumen loss
OCT: optical coherence tomography
%NVO: percentage of in-stent neointimal volume obstruction
RUTTS: ratio of uncovered to total stent struts per section
SES: sirolimus-eluting stent
Introduction
Optical coherence tomography (OCT) provides excellent imaging resolution of implanted devices and coronary arteries. At follow-up, OCT sheds light on the vascular healing process for the various coronary stent platforms1-3. Post-mortem data, demonstrating the relationship between uncovered stent strut and stent thrombosis, have been reported4. The impact of permanent polymer drug-eluting stents (DES) on long-term complications (late and very late stent thrombosis) from delayed vascular healing has been well described in the literature5,6. Consequently, intensive clinical research has focused on the potential relationship between OCT findings (uncovered strut and stent malapposition) and clinical outcomes.
Complications created by the polymer have motivated researchers to design new coronary stent platforms without a polymer coating. To evaluate the status of vascular healing systematically, the healing score (HS) has been introduced in clinical research7,8. The advantage of the HS is that it permits a relative assessment of the speed and degree of vascular “healing” in patients treated with different types of stent and at different points in time. The Nano+™ polymer-free stent (Lepu Medical Technology Co., Ltd., Beijing, China) is a polymer-free sirolimus-eluting stent (SES) which elutes the drug sirolimus from a reservoir made of abluminal nanomeric cavities, aimed at minimising long-term complications that have been related to the polymer coating. Prior OCT examinations three months after Nano+ implantation in 47 lesions showed that the strut coverage rate was 93.0%9.
The aim of our present study was to provide mechanistic insights into the vascular healing process after implantation of polymer-free SES, as assessed in the vascular healing process by serial assessment of lesions with OCT that were treated with polymer-free SES at three and six months.
Methods
PATIENT POPULATION
The Nano+ OCT study is a prospective, multicentre, single-arm, open-label study in coronary artery disease patients. The inclusion and exclusion criteria have been described previously9. Briefly, the inclusion criteria are: 1) stable angina or silent ischaemia demonstrated by positive functional study with a de novo target lesion of >50% diameter stenosis (%DS); 2) planned intervention in up to two de novo lesions in different epicardial vessels; 3) lesion length of less than 18 mm; 4) native coronary artery of 2.5-4.0 mm in diameter. All patients had OCT assessment scheduled at three-month follow-up. Whenever the pre-specified criteria were not fulfilled on three-month OCT imaging, the patients were scheduled for additional OCT assessment at six months. For the purposes of this study which aimed at assessing vascular healing serially at three months and six months, the data were exclusively reported in lesions which had OCT assessment at both time points. The study protocol was approved by all institutional ethics committees and informed consent was obtained from every patient before any intervention was performed.
DEVICE DESCRIPTION
The Nano+ stent is a drug-eluting stainless steel stent with a strut thickness of 91 μm. A large number of pores with a diameter of 400 nm are present on the abluminal stent surface. The device releases sirolimus from the abluminal pores directly (i.e., without using a polymer coating) into the vessel wall. A total of 85% of the drug is released within 30 days.
OCT ACQUISITION AND ANALYSIS
The details of OCT acquisition have been previously described9. All OCT images were analysed at an independent core laboratory (Cardialysis BV, Rotterdam, The Netherlands) with QIvus 2.2 software (Medis, Leiden, The Netherlands). The stent and lumen areas were semi-automatically traced at 1 mm intervals.
At three-month follow-up, all lesions were evaluated by pre-specified OCT criteria, derived from the literature4,7,10,11. These criteria were: (i) percentage of covered struts >90%; (ii) no frame with a ratio of uncovered to total stent struts (RUTTS) of >30%; (iii) incomplete stent apposition area (ISA) ≤2 mm2; (iv) intraluminal defect area (ILD) <300 µm2. If these were not fulfilled, the patients were scheduled for additional OCT assessment at six months. A covered strut was defined as having a neointimal thickness >0 μm8,12. A RUTTS was calculated in every cross-section from the number of uncovered struts divided by the total number of struts4. Incomplete strut apposition was defined as a separation between strut and vessel wall with a distance greater than the thickness of the strut (i.e., >91 μm). An intraluminal defect was defined as an irregularly shaped structure, either free from the vessel wall or attached to the vessel wall or the stent.
To quantify the degree of vascular healing, the healing score (HS) was calculated at every time point. Details of the HS components have been described8. The score consisted of five parameters, as follows: (a) percentage of intraluminal defect (%ILD); (b) percentage of malapposed and uncovered struts (%MU); (c) percentage of uncovered struts alone (%U); (d) percentage of malapposition alone (%M); (e) presence of neointimal volume obstruction more than 30% (%NVO). The parameters were weighted according to the following formula: HS=[% ILD×4]+[% MU×3]+[% U×2]+[% M]+[% NVO –30%]. Whenever %NVO was less than 30, the last component was set as 0.
TREATMENT ALLOCATION AND FOLLOW-UP PROCEDURE
All patients in this trial underwent stent implantation according to standard procedures. All patients received aspirin 75-100 mg per day indefinitely and a daily dose of clopidogrel 75 mg or prasugrel 10 mg or ticagrelor 90 mg bid for at least four months after the index procedure.
Following the OCT examination at three months, all OCT pullbacks were assessed off-line by an independent core lab. When all pre-specified OCT criteria were met, patients were allowed to stop DAPT at four months but were advised to continue aspirin indefinitely according to standard guidelines13. In contrast, if any of the OCT criteria were not met, the patients were scheduled for repeat angiography and OCT examination at six months according to the protocol. If the six-month OCT findings met all criteria, the patients were allowed to stop DAPT at seven months; otherwise, the patients were requested to continue DAPT for at least 12 months after the index procedure.
Patients who stopped DAPT at either four or seven months according to the OCT findings were followed up at hospital or by a telephone call one month after their discontinuation of DAPT (five or eight months) in order to assess their vital status and to confirm the absence of serious adverse events after the discontinuation of DAPT.
QUANTITATIVE CORONARY ANALYSIS
Two-dimensional quantitative coronary analysis (QCA) was performed at an independent core lab (Cardialysis BV) with the CAAS system (CAAS 5.9; Pie Medical BV, Maastricht, The Netherlands), using validated quantitative methods14.
STUDY ENDPOINTS
The primary endpoint was the change of HS from three to six months. The secondary angiographic endpoints were binary restenosis, late lumen loss (LLL), minimal lumen diameter (MLD) and %DS at six months. The secondary OCT endpoints were neointimal area and volume; mean stent lumen diameter, area and volume; minimal stent lumen diameter, area, and volume; mean neointimal thickness of the strut coverage; percentage of covered struts; and percentage of incomplete strut apposition area at six months.
Statistical analysis
The statistical analysis was performed by SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). Categorical variables were summarised with frequencies and percentages. The continuous variables were reported as mean±standard deviation (SD) or median and interquartile ranges (IQR 1st-3rd) as appropriate. The Wilcoxon signed-rank test was used to compare continuous variables between serial OCT and QCA data. The categorical variables were compared by Fisher’s exact test. A two-sided p-value <0.05 was considered statistically significant.
Results
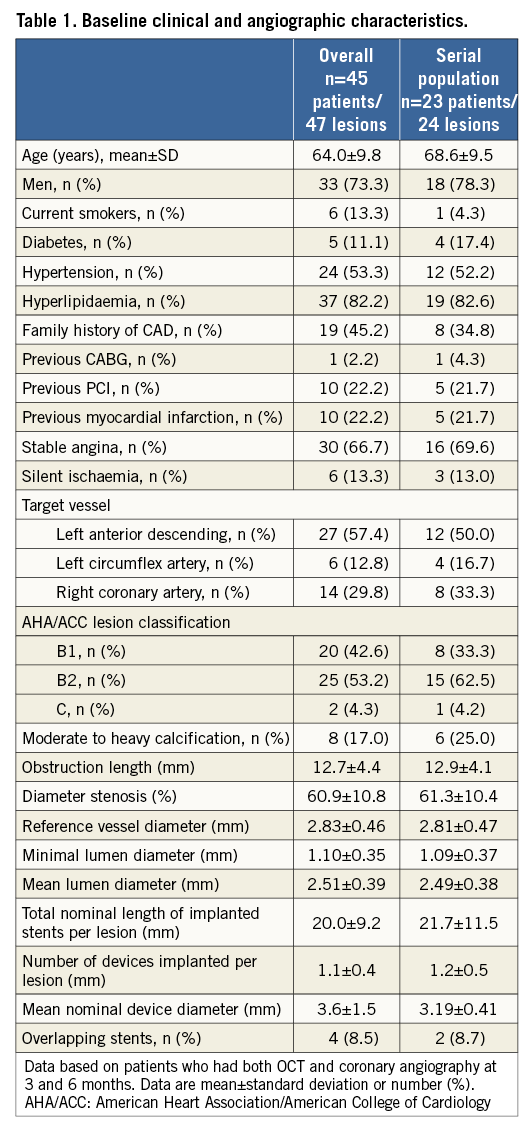
BASELINE CLINICAL AND ANGIOGRAPHIC CHARACTERISTICS
The study population is described in Figure 1. A total of 45 patients with 47 lesions received Nano+ stents and all patients underwent repeat coronary angiography and OCT examination at three months. Twenty-seven patients (28 lesions) did not meet all OCT criteria at three months and were scheduled for OCT examination at six months. Out of 27 patients, three patients refused to undergo six-month invasive angiography while one patient underwent clinically indicated target lesion revascularisation without OCT assessment at 185 days. Finally, 23 patients with 24 lesions had serial coronary angiography and OCT assessment at three and six months. Baseline clinical and angiographic characteristics are presented in Table 1.
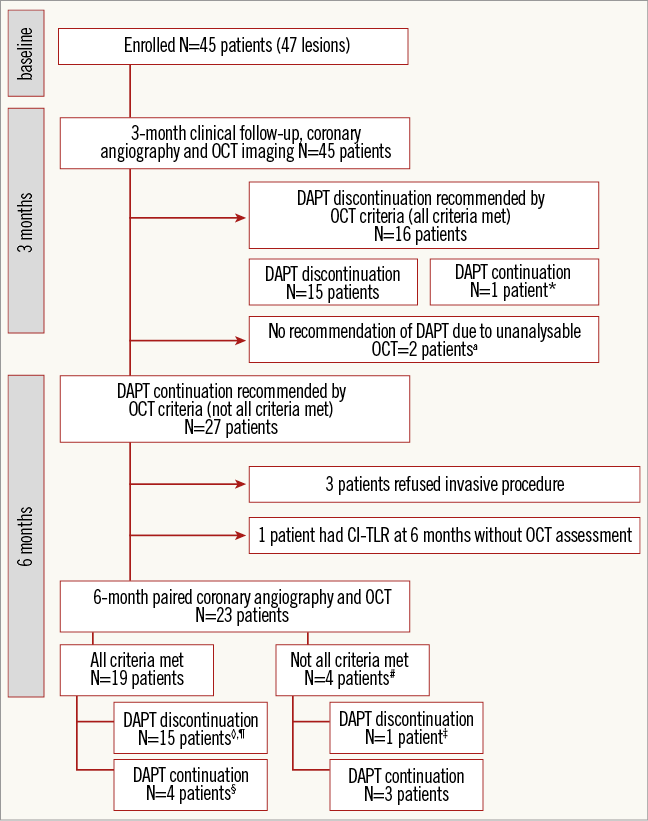
Figure 1. Study flow chart and follow-up. *One patient was advised to continue DAPT according to his physician’s opinion. aTwo patients stopped clopidogrel after six-month OCT assessment due to fulfilment of OCT criteria. #No definite, probable, possible stent thrombosis reported at eight-month clinical follow-up. ‡Clopidogrel was stopped due to dental procedure. ◊Two patients had indication to use anticoagulant after stent implantation. One patient discontinued aspirin at one month and the other discontinued aspirin at three months after the index procedure. Both patients took the combination of anticoagulant and clopidogrel until six-month follow-up. After clopidogrel was discontinued at six months, the patients took only anticoagulant therapy. ¶One patient underwent CABG due to progression of distal left main disease. §One patient had non-clinically indicated TLR at 184 days. CI-TLR: clinically indicated target lesion revascularisation; DAPT: dual antiplatelet therapy; OCT: optical coherence tomography
SERIAL ANGIOGRAPHIC AND OCT EXAMINATIONS AT THREE MONTHS AND SIX MONTHS
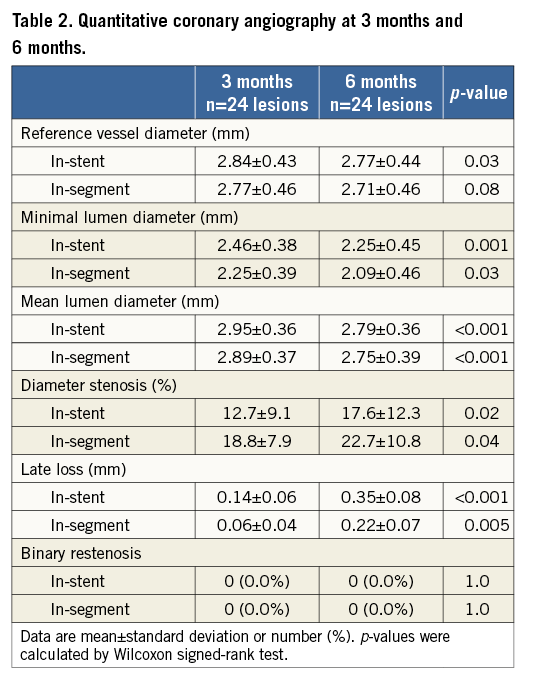
The QCA assessment at three and six months is shown in Table 2. The in-stent MLD was 2.46±0.38 mm at three months and 2.25±0.45 mm at six months. The in-stent LLL was 0.14±0.06 mm at three months and 0.35±0.08 mm at six months.
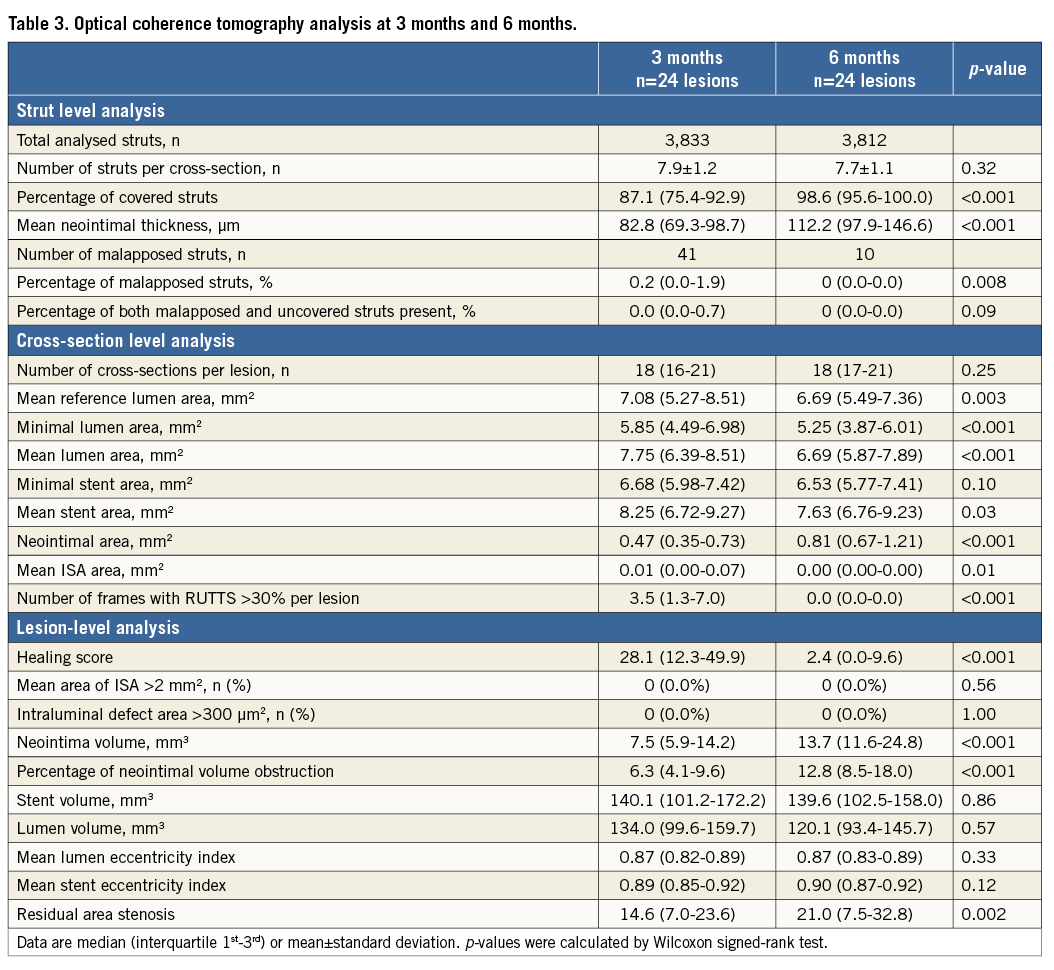
The OCT analyses are shown in Table 3. At three months, 95.8% (23 lesions) had at least one frame with RUTTS >30% and decreased to 12.5% (three lesions) at six months. Of note, 14 lesions (58.3%) had a percentage of covered strut <90% at three months while at six months only two lesions (8.3%) had such an incomplete coverage. The median number of frames with RUTTS >30% decreased from 3.5 frames (range 1.3-7.0) at three months to 0.0 frames at six months. There was no case with an intraluminal defect >300 µm2 at either time point.
The proportion of covered stent struts increased from a median of 87.1% (75.4%-92.9%) at three months to 98.6% (95.6%-100.0%) at six months (Figure 2). The median of neointimal thickness increased from 82.8 µm (69.3-98.7) to 112.2 µm (97.9-146.6) between three and six months. Only one case still had persistent malapposition at six months and there was no newly detected stent malapposition. The median percentage of malapposed struts at three and six months was 0.2% (IQR 0.0%-1.9%) and 0.0% (IQR 0.0%-0.0%), respectively. The %NVO increased between three and six months from 6.3% (IQR 4.1%-9.6%) to 12.8% (IQR 8.5%-18.0%). The percentage of residual area stenosis increased from 14.6% (IQR 7.0%-23.6%) to 21.0% (IQR 7.5%-32.8%), p=0.002. The HS significantly decreased from a median of 28.1 (IQR 12.3-49.9) at three months to 2.4 (0.0-9.6) at six months (Figure 3, Figure 4).
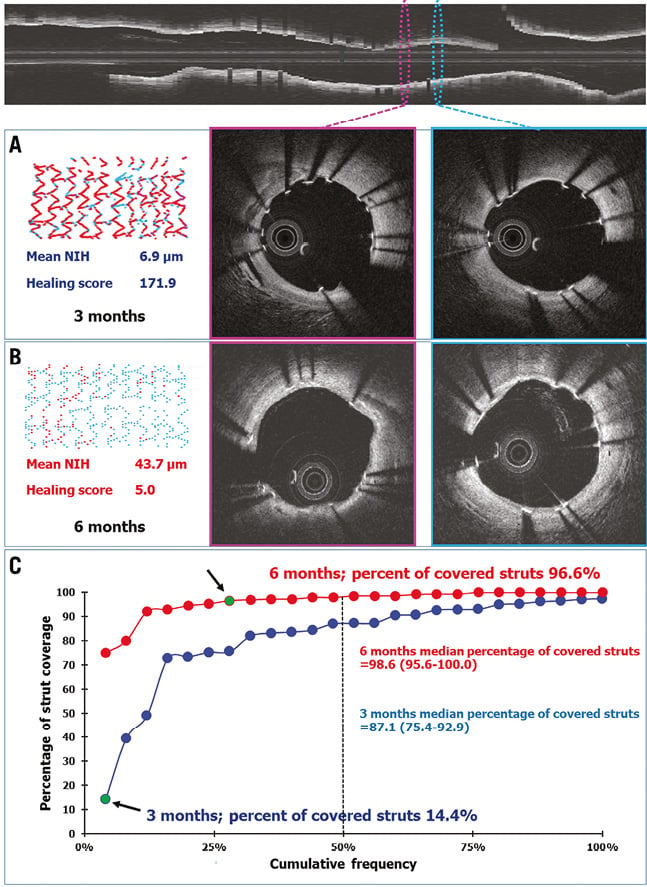
Figure 2. Example of optical coherence tomography and cumulative frequency curve of percentage of covered struts at three months and six months in 24 paired lesions. Top panel shows the longitudinal view with corresponding cross-section samplings from two different in-stent segments (pink and blue ellipse). The follow-up OCT cross-sections at three months (middle and right-hand panel A) and six months (middle and right-hand panel B) as shown in coloured frames corresponding to the coloured cross-sections in the longitudinal view. The coverage of struts improved from three to six months. Panel A (left) shows a colour-coded spread-out sheet depicting a high proportion of uncovered struts (red dots) to covered struts (blue dots). At six-month follow-up (panel B, left), the spread-out sheet shows an improvement of strut coverage (fewer red dots). The HS dramatically decreased from 171.9 at three months to 5.0 at six months. The cumulative frequency curves of the percentage of covered struts at three months (blue) and six months (red) are shown in panel C. The green dots indicated by black arrows illustrate a shift from low to high percentage of strut coverage from three months to six months.
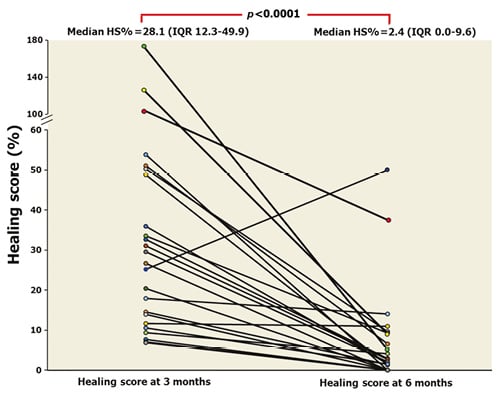
Figure 3. Temporal change of healing index at three-month and six-month follow-up in 24 paired lesions.
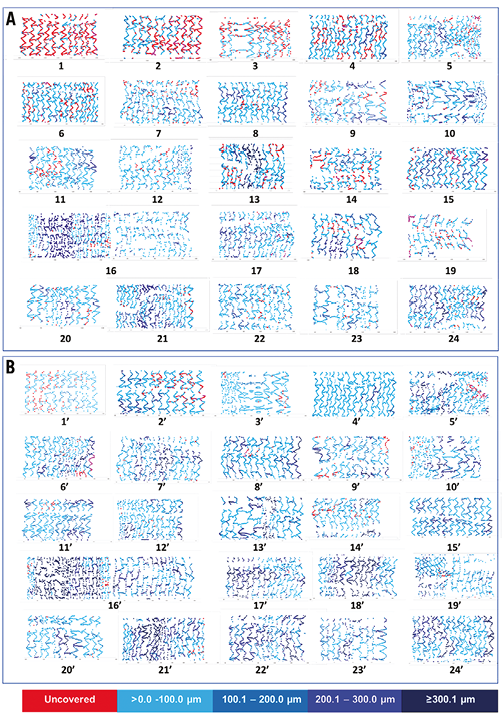
Figure 4. Spread-out sheets demonstrating the coverage status of the individual stents/patients at two time points (three and six months). Upper panel (A) demonstrates the coverage status of the individual stents at three months, while the lower panel (B) demonstrates the coverage status of the same stents at six months. Struts are colour-coded according to their coverage status. Uncovered struts are depicted as red while covered struts are depicted as blue, with deepening colour of blue indicative of a thicker neointima (light blue colour indicates a neointimal thickness more than 0 μm to 100.0 μm, sustained blue indicates a neointimal thickness 100.1-200.0 μm, navy blue indicates a neointimal thickness 200.1-300.0 μm, dark blue indicates a neointimal thickness more than 300.1 μm).
ANTIPLATELET THERAPY
Amongst 23 patients who received six-month repeat OCT imaging, 21 patients had taken the following DAPT at six months: aspirin and clopidogrel 18 (85.7%) patients, aspirin and ticagrelor 2 (9.5%) patients, and aspirin and prasugrel in one (4.8%) patient.
CLINICAL OUTCOMES
Throughout the eight-month period, no definite, probable or possible stent thrombosis was reported among the 27 patients. There were three patients who underwent revascularisation after six-month angiographic follow-up. Two patients received target lesion revascularisation and one patient underwent coronary bypass due to stable angina and progression of distal left main disease.
Discussion
The present study was to provide a mechanistic interpretation of the vascular healing process in polymer-free SES in which the relative lack of neointimal growth was documented at three months. The main findings are: 1) an improvement in vascular healing, as assessed by the HS, was documented by a decrease of HS from 28.1 at three months to 2.4 at six months; 2) the angiographic late lumen loss and %NVO on OCT at six months were 0.35 mm and 12.8%, respectively; and 3) the absence of any definite or probable stent thrombosis up to eight months.
Initially, there was heterogeneity in the healing process at three months; three lesions had a percentage of covered struts <50%. The extremely low coverage might be related to: i) the presence of ISA, as malapposed struts generate high shear flow disturbance that is known to be a factor of retardation of neointimal growth15; ii) unfavourable characteristics of circulating endothelial progenitor cell in individual patients16; and iii) a heterogeneity in drug effect, potentially due to defective manufacturing of a particular stent batch.
CAN PATHOLOGICAL CRITERIA BE APPLIED AS AN INDICATOR OF OPTIMAL VASCULAR HEALING?
The OCT quantitative criteria used in the present study were derived from a post-mortem histopathological study that showed a strong correlation of late stent thrombosis with RUTTS and the number of uncovered struts4. Our OCT analysis at three months showed that 95.8% of lesions had RUTTS >30% while the percentage of strut coverage <90% was observed in 58.3%. These findings reflect the weak relationship between OCT surrogate markers and their clinical prognostic value. The anatomopathological criteria seem to overestimate the deficiencies of vascular healing status and they appear to be too stringent. The issues of applying the pathological criteria to clinical research are the following. 1) The criteria were derived from a post-mortem study of patients with late stent thrombosis and may thus reflect a certain selection bias since the denominator (patients alive having uncovered and malapposed struts) is unknown while the numerator (autopsied patients with stent thrombosis) is known. To prove the prognostic value of uncovered and malapposed struts in clinical practice, a huge sample size to show statistical significance would be needed. Since the incidence of stent thrombosis with a combination of uncovered or malapposed struts is very low, the correlation may never be established. 2) The criteria were derived in the era of first-generation DES which had thicker struts that promoted recirculation zones with low endothelial shear stress behind the struts – a biological trigger for stent thrombosis17. 3) The conventional DAPT used in these anatomopathological cases (ticlopidine or clopidogrel) was not as potent as contemporary antiplatelet agents such as ticagrelor or prasugrel.
CAN OCT CRITERIA BE APPLIED AS GUIDANCE FOR DUAL ANTIPLATELET DURATION?
The current analysis showed that only one third of lesions had adequate vascular healing at three months whereas vascular healing was almost complete at six months, without stent thrombosis. The present study did not pretend to detect lesions that were going to develop stent thrombosis: the study protocol recommended continuation of DAPT in order potentially to prevent patients from developing stent thrombosis. Previously, there has been sufficient evidence showing that abbreviated DAPT duration of three months18 or six months19-22 after polymer-coated DES implantation for low to moderate-risk patients is safe. It has recently been demonstrated that DAPT duration can even be abbreviated to only one month after polymer-free DES implantation, as reported in the LEADERS FREE trial23. In the LEADERS FREE trial, a polymer-free umirolimus-coated stent was compared with a bare metal stent (BMS) in high risk of bleeding patients who underwent PCI. The results showed that a polymer-free umirolimus-coated stent was superior to a BMS with respect to the composite of cardiac death, myocardial infarction, or stent thrombosis when used with a one-month course of DAPT. The rate of stent thrombosis did not differ significantly between the two groups, and more than half of the stent thromboses in both groups occurred during the first 30 days, when patients were prescribed DAPT. Consequently, invasive imaging is not a pragmatic requirement to determine whether DAPT has to be continued longer than three or six months in polymer-free DES, as reported in the present study. However, the consideration of abbreviated DAPT duration should be applied in specific stent platforms and in relation to similar lesion characteristics to those assessed in clinical trials.
ANGIOGRAPHIC LATE LUMEN LOSS AND OCT FINDINGS IN POLYMER-FREE DES
The LLL of the Nano+ stent at three and six months was 0.17 mm and 0.35 mm, respectively. These results are comparable to previous data of a polymer-free rapamycin-eluting stent that had an LLL of 0.16 mm24 and 0.47 mm25 at three months and six to eight months, respectively. Although Nano+ had a higher angiographic in-stent LLL as compared to the first and second generation of DES (both permanent and biodegradable polymer-coated)26-28, the risk of target lesion revascularisation may still be low (<5% to 10%), according to the findings of a previous study with early-generation DES29. A higher LLL may not necessarily translate into a higher target lesion revascularisation rate among different DES platforms30.
A recent study that compared FFR and MLA by OCT has clearly demonstrated that, with the exception of left main disease, an MLA >1.96 mm2 is not generally associated with an FFR <0.8031. In our present study, the MLA was, in general, much higher than this meta-analysis-derived threshold that was recently proposed by D’Ascenzo et al31.
Limitations
The limitations of this prospective study are: (1) a relatively small sample size that was not powered to demonstrate the relationship between OCT findings and clinical outcomes, as stated in the protocol; (2) the lesions that were treated in the present study were simple lesions, therefore favourable vascular healing might be expected in these relatively short and less complex lesions; (3) a serial OCT follow-up was solely performed in inadequate vascular healing lesions at three months, which is a potential cause of selection bias; (4) the lack of a direct comparison between the study devices and contemporary DES that are used in routine clinical practice; and (5) the criteria of covered stent was defined as having a neointimal thickness more than 0 µm, which may not be practical in clinical use.
Conclusion
In patients treated with polymer-free SES, serial OCT showed almost complete vascular healing at six months, even when coverage was insufficient at three months. This suggests an adequate safety and efficacy profile of the device at that point in time. The implication of these findings should be evaluated in a large clinical trial.
| Impact on daily practice The Nano+ is a polymer-free DES that elutes sirolimus from a reservoir made of abluminal nanomeric cavities without the presence of a polymer. The healing score (HS), derived from OCT assessment, showed that the polymer-free SES develops an almost complete vascular healing within six months. |
Guest Editor
This paper has been guest edited by Carlo Di Mario, MD, PhD, FESC, FACC, FRCP, FSCAI; NIHR Biomedical Research Unit, Royal Brompton & Harefield NHS Foundation Trust, London, United Kingdom.
Acknowledgements
The authors wish to thank Eric van Remortel, Monique Schuijer and Frank van Otterloo for their invaluable technical support.
Funding
The study was sponsored by Lepu Medical, Beijing, China.
Conflict of interest statement
C. von Birgelen is a consultant to Boston Scientific and Medtronic and has received lecture fees from MSD and AstraZeneca; the research department has received institutional research grants from Biotronik, Boston Scientific, and Medtronic. P.W. Serruys and Y. Onuma are members of the advisory board of Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

