
Abstract
Aims: The DESSOLVE III OCT substudy aimed to compare serially neointimal hyperplasia volume obstruction (%VO) between the thin-strut MiStent with early polymer elimination and nine-month sustained drug release from microcrystalline sirolimus and the durable polymer-coated everolimus-eluting XIENCE stent at six and 24 months after implantation.
Methods and results: The efficacy endpoint was %VO, calculated as abluminal neointimal volume/stent volume. Thirty-six patients (MiStent 16 patients, 16 lesions; XIENCE 20 patients, 22 lesions) underwent serial OCT evaluation at both six and 24 months. At six months, mean abluminal %VO was significantly lower in the MiStent group than in the XIENCE group (14.54±3.70% vs 19.11±6.70%; p=0.011), whereas the difference in %VO between the two groups decreased at 24 months (20.88±5.72% vs 23.50±7.33%; p=0.24). There was no significant difference in percentage malapposed struts and percentage uncovered struts between the two groups at both time points.
Conclusions: In the serial comparative OCT analysis of the MiStent versus the XIENCE, the MiStent showed a more favourable efficacy for preventing neointimal formation with comparable strut tissue coverage, as compared with the XIENCE at six months, but this difference in %VO decreased at 24 months so that the difference in neointima at 24 months was no longer significant.
Introduction
Conventional drug-eluting stents (DES) combine short-term drug release (<90 days) with the permanent presence of a polymer on the stent surface, which could contribute to late progression of neointimal thickness, hypersensitivity reactions and neoatherosclerosis formation with a subsequent increased risk for stent thrombosis, acute myocardial infarction, sudden death and revascularisation at long-term follow-up1,2. In contrast, a slow release of drug with prolonged retention in the tissue and early elimination of the polymer coating has the potential to reduce neointimal proliferation and a risk of both early and late stent failure3,4,5,6.
The aim of the DESSOLVE III OCT substudy was to analyse abluminal in-stent neointimal hyperplasia volume obstruction (%VO) – a histomorphometric surrogate of neointima – at six and 24 months after implantation of a three-month absorbable polymer-coated, thin-strut coronary stent system using a crystalline form of sirolimus (MiStent®; Micell Technologies, Durham, NC, USA). Here, we present the serial comparison of optical coherence tomography (OCT) imaging results between the MiStent and the commercially available durable polymer-coated everolimus-eluting XIENCE stent (Abbott Vascular, Santa Clara, CA, USA).
Methods
STUDY DESIGN
The DESSOLVE III OCT study is a substudy of the DESSOLVE III randomised controlled trial (RCT), which was designed to compare the MiStent with the XIENCE stent in an all-comers population (n=1,398)7,8,9. The current OCT substudy was performed at three interventional cardiology centres in Poland. Patients for the OCT substudy consented to undergo six- and 24-month follow-up with repeat catheterisation and OCT imaging. Participants had to meet the inclusion criteria of the DESSOLVE III main study (NCT02385279) and specific criteria related to the OCT substudy. The main inclusion criterion was one single de novo lesion in a native coronary artery with stenosis of ≥50% successfully treated with the study stents. The main exclusion criteria for the OCT substudy included bifurcation lesions, total occlusions, left main lesions, lesions with overlapping stents, severely tortuous lesions, calcified or angulated anatomy of the study vessel that, in the opinion of the investigator, could result in suboptimal imaging or excessive risk of complication from placement of an OCT catheter.
OCT imaging was performed with the use of an OCT system (ILUMIEN™ imaging system) and Dragonfly™ catheters (both Abbott Vascular) using a non-occlusive technique with intracoronary injection of contrast agent and following standard techniques recommended by the manufacturer.
The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practices after approval of the local ethics committee.
DEVICE DESCRIPTION
The detailed design of the MiStent has been described elsewhere10. Briefly, the MiStent is a thin-strut (64 µm) cobalt-chromium stent coated with biodegradable polylactide-co-glycolic acid (PLGA) that contains a crystalline form of sirolimus. The PLGA begins to flow off the stent in 45-60 days and is fully absorbed within 90 days, embedding microcrystals of sirolimus in the vessel wall with a therapeutic drug presence of up to nine months4. The XIENCE V® cobalt-chromium stent (Abbott Vascular) is covered with a durable fluoropolymer that releases 80% of everolimus within the first 30 days after deployment11 (Figure 1).
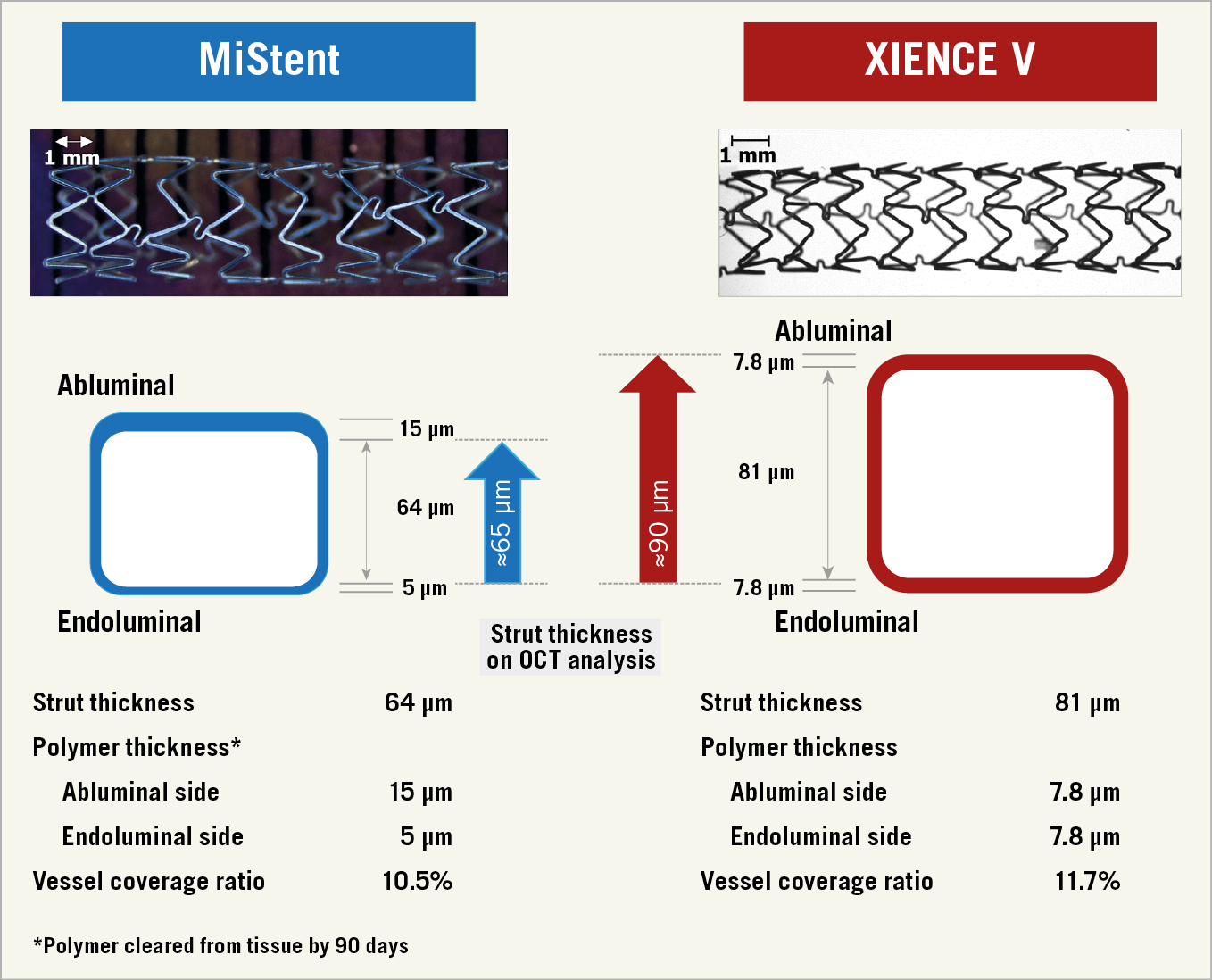
Figure 1. Strut thickness calculation on the follow-up OCT analysis. At six months, the asymmetric bioresorbable polymer has disappeared so that the true thickness of the MiStent is approximately 65 µm, whereas with the XIENCE the symmetric durable coating will persist so that the thickness of a combination of metallic struts and coating is approximately 90 µm (81+7.8 ≈ 90). These two values of strut thickness were used in the abluminal OCT assessment. The endoluminal polymer thickness should be negligible for assessment due to the blooming of the metallic strut on OCT.
OCT AND QUANTITATIVE CORONARY ANGIOGRAPHY (QCA) ANALYSIS
There are two types of measurement of neointimal hyperplasia post stenting. One considers the endoluminal optical leading edge of the strut and connects by interpolation of these leading edges to form a circular boundary (endoluminal stent contour). This circular boundary and the luminal contour comprise the circumferential neointima covering the struts. The second assessment (abluminal stent contour) takes into account the virtual abluminal backside of the struts – that vary in thickness as a function of the stent design (64≈65 µm for MiStent; 81+7.8≈90 µm for XIENCE) – to delineate automatically the abluminal backside of the struts (Supplementary Figure 1),12. On OCT, the abluminal stent contour can indicate the original lumen border and allow quantification of neointimal hyperplasia12. Quantitative OCT assessment was performed at every 1.0 mm interval using QIvus software, version 3.0 (Medis, Leiden, the Netherlands).
In QCA analyses, the stented segment and the peri-stent segments (defined by a length of 5 mm proximal and distal to the stent edge), as well as their combination (in-segment analysis), were analysed13. Late loss was defined as the difference in minimum lumen diameter (MLD) between post procedure and follow-up. QCA assessment was performed using the Coronary Angiography Analysis System, version 5.11 (Pie Medical Imaging, Maastricht, the Netherlands).
Both analyses were performed by an independent core laboratory (Cardialysis B.V., Rotterdam, the Netherlands) and the analysts were blinded to the device type.
OCT ENDPOINTS AND DEFINITIONS
The primary endpoint was the pre-specified six-month abluminal in-stent %VO, calculated as the mean abluminal neointimal volume/mean abluminal stent volume ×100. Quantitative OCT assessment on the following endpoints was also performed: (1) mean and minimal lumen area, (2) mean and minimal stent area, (3) stent symmetry, (4) stent expansion, (5) mean neointimal hyperplasia area, (6) percentage of malapposed struts, (7) percentage of covered and uncovered struts, and (8) neointimal healing score. The calculation method of the healing score is presented in Supplementary Appendix 1,14.
STATISTICAL ANALYSIS
The details of the statistical analysis are presented in Supplementary Appendix 2.
Results
STUDY SUBJECTS
Twenty-five (25) patients (25 lesions) in the MiStent group and 28 patients (30 lesions) in the XIENCE group underwent repeat catheterisation with QCA and OCT assessment at six-month follow-up. Subsequently, 16 patients (17 lesions) in the MiStent group and 21 patients (23 lesions) in the XIENCE group underwent QCA and OCT at 24 months. Serial evaluation was available in 16 patients (16 lesions) in the MiStent group and 20 patients (22 lesions) in the XIENCE group due to the refusal of nine patients, three adverse events between six and 24 months (one death in the MiStent group; one death and one target lesion revascularisation [TLR] in the XIENCE group), and two non-analysable cases (Figure 2).
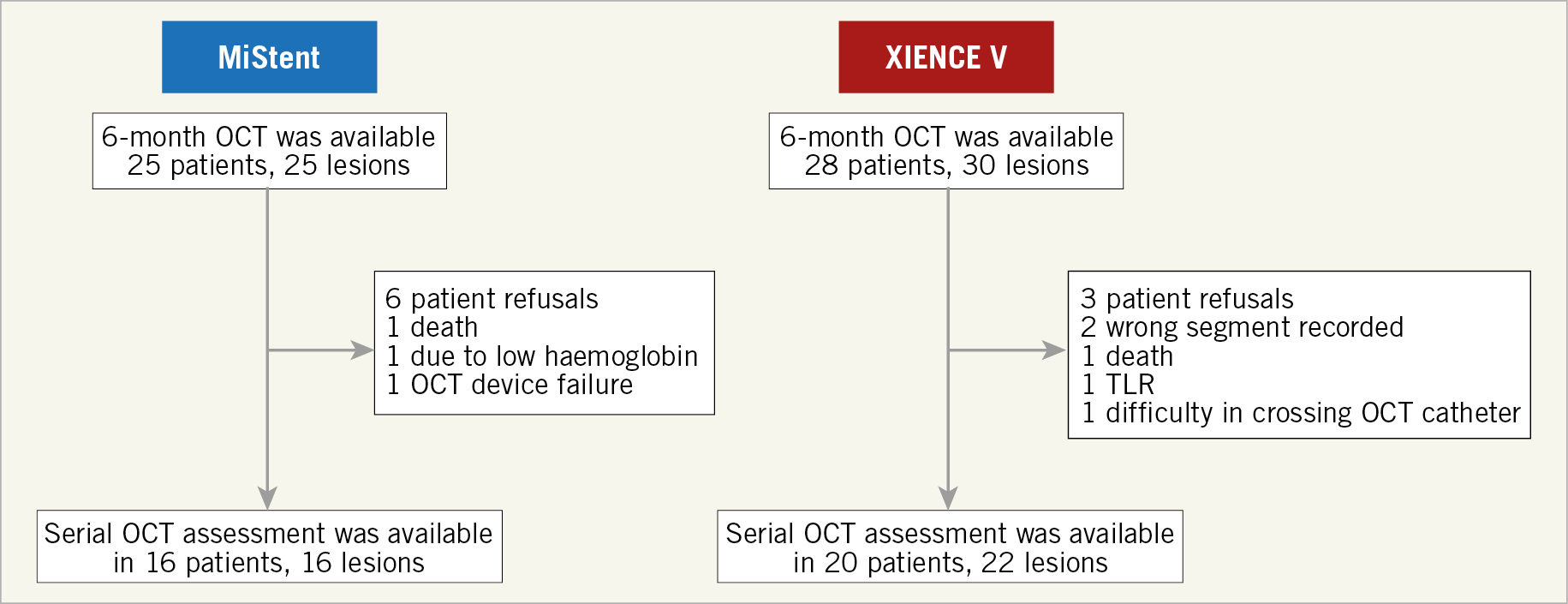
Figure 2. Flow diagram of participants in the DESSOLVE III OCT substudy.
BASELINE CHARACTERISTICS AND PROCEDURAL DATA
There was no significant difference in baseline clinical characteristics and procedural data except for the mean maximum pressure used for predilatation between the MiStent and the XIENCE group (Table 1). There were no significant differences in coronary lesion distribution between the two stents.
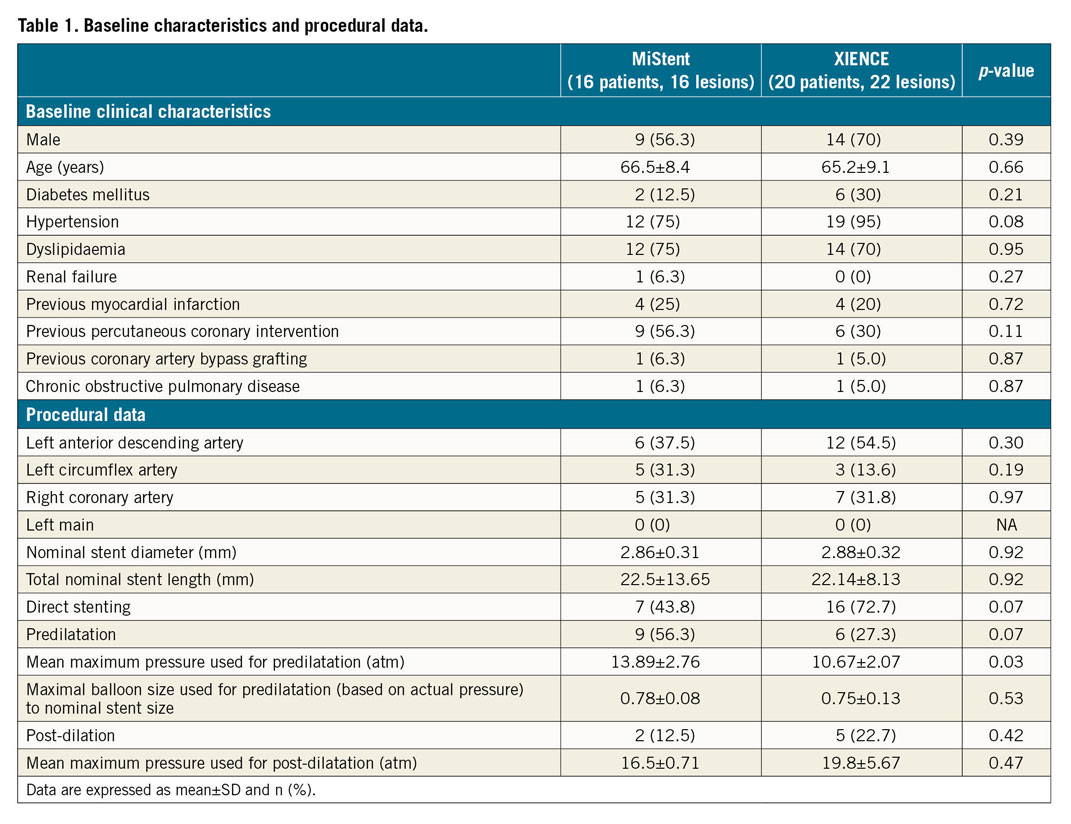
Preprocedural QCA parameters were also comparable between the two groups. However, lesions in the MiStent group were better and more aggressively prepared in terms of balloon predilatation. These lesions were numerically more often predilated, and significantly higher predilatation pressures were applied in this group (p=0.03). However, the maximal size of the expected balloon diameter according to the charts provided by the manufacturers was not significantly different between the two groups (p=0.53). Two and five lesions were post-dilated in the MiStent and XIENCE group, respectively. In post-procedural QCA, in-stent MLD was comparable between the two groups (p=0.25).
OCT RESULTS
Abluminal quantitative OCT results are presented in Table 2. The endoluminal OCT results are presented in Supplementary Table 1. The mean stent area was comparable between the two stents at both six and 24 months. Compared with the XIENCE, the mean %VO was significantly lower in the MiStent group at six months (14.54±3.70 vs 19.11±6.70; p=0.011), whereas this difference decreased at 24 months (20.88±5.72 vs 23.50±7.33; p=0.24). The late neointimal hyperplasia of the MiStent between six and 24 months was comparable to that of the XIENCE (6.35±4.17 vs 4.39±4.59; p=0.19).
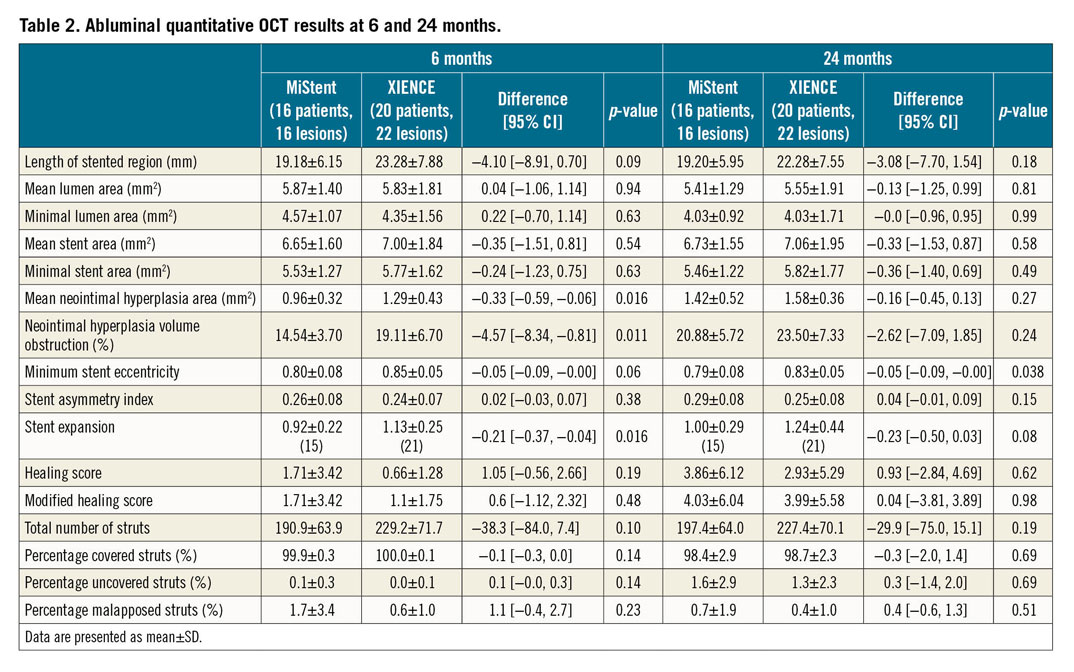
Minimum lumen area was comparable between the two stents at six months (p=0.63) and 24 months (p=0.99). The MiStent was comparable to the XIENCE in terms of mean lumen area at six months (p=0.94) and 24 months (p=0.81).
In both study groups, there was only a minimal number of malapposed struts (MiStent: 1.7±3.4 vs XIENCE: 0.6±1.0; p=0.23, at six months; 0.7±1.9 vs 0.4±1.0; p=0.51, at 24 months) and practically all analysed struts were completely covered at both time points. There was also no significant difference in abluminal healing score between the two groups at six and 24 months.
Representative cases of the MiStent and the XIENCE are shown in Figure 3.
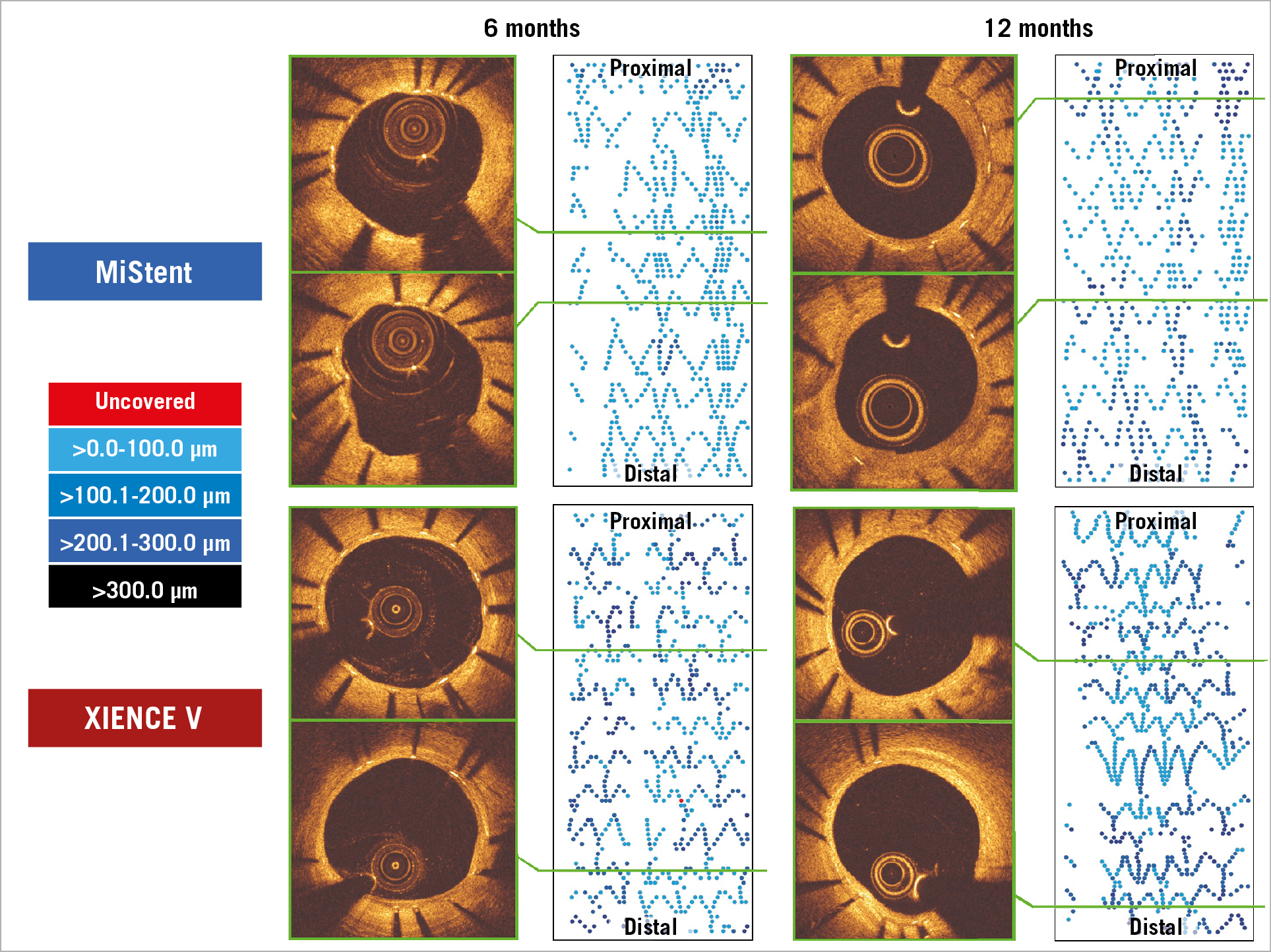
Figure 3. Representative cases in the DESSOLVE III OCT study involving the MiStent (upper) and the XIENCE stent (lower). Cross-sectional images and 2D fold-out views of strut coverage with colour coding of the neointimal thickness on struts.
QCA ANALYSIS
Serial QCA was available in 16 patients and 16 lesions in the MiStent group and in 20 patients and 22 lesions in the XIENCE group. The MLD was numerically higher in the MiStent group than in the XIENCE group at six months (MiStent: 2.28±0.24 mm, XIENCE: 2.07±0.36 mm; p=0.07) and 24 months (2.06±0.27 mm vs 1.97±0.40 mm; p=0.45). Angiographic late loss was numerically lower in the MiStent group than in the XIENCE group at six months (0.02±0.31 mm vs 0.18±0.24 mm; p=0.10); however, this difference was not observed at 24 months (0.26±0.32 mm vs 0.23±0.32 mm; p=0.76). No binary restenosis was observed in either group (Supplementary Table 2).
Discussion
The main findings of the current study can be summarised as follows.
i) Abluminal %VO (efficacy endpoint), so-called histomorphometric neointima, was significantly lower in the MiStent than in the XIENCE at six months. This difference was partially maintained at 24 months.
ii) Strut coverage and malapposition (safety endpoint) were comparable between the two groups at both time points.
iii) The minimal and mean lumen area were comparable between the two groups at six and 24 months.
NEOINTIMAL GROWTH UP TO SIX MONTHS
The current serial OCT analysis, in the context of a randomised trial, demonstrated that the MiStent resulted in a significantly better suppression of neointimal hyperplasia than the XIENCE at six-month follow-up. One potential explanation of this result in favour of the MiStent may reside in the stent expansion index, which was significantly higher in the XIENCE group than in the MiStent group. A higher stent expansion index may affect the amount of neointimal formation by creating larger injury15; however, this effect should be counterbalanced by the antiproliferative drug. Furthermore, due to the absence of baseline OCT, it is not possible to assess the impact of embedment of struts on the subsequent neointimal hyperplasia.
The favourable results of the MiStent might also be attributed to the early elimination of the polymer coating (three months) in conjunction with sustained drug release from the sirolimus microcrystals embedded in the vessel wall (nine months) (Supplementary Figure 2). Additionally, the thin stent platform of the MiStent could have played a role in the favourable findings of this study. The importance of prolonged drug delivery was originally demonstrated in the PISCES trial in which paclitaxel, a hydrophilic drug, was effective only if its release kinetics were prolonged for at least 10 or 30 days (as opposed to the shorter time points)16. In addition, that study showed that an increase in the drug dose did not play an important role in efficacy, as its concentration may easily exceed receptor capacity, leading to toxic levels with subsequent adverse clinical events17. Thus, prolonged delivery of the optimal drug dose adjusted to the binding capacity of the tissues is of paramount importance in the efficacy of tested drug and stent technologies. Furthermore, low levels of drug release with prolonged retention of a drug in the tissue minimise toxic levels, thereby improving the safety of a device16.
Currently, technological advances allow optimal stent design with controlled and predictable systems of drug elution, avoiding an uncontrolled initial burst. In contrast to the amorphous or aqueous forms of drugs used in previous-generation DES, the currently available crystalline forms of antiproliferative compounds (such as crystalline sirolimus on the MiStent) can provide additional control over drug elution for an extended period of time. The drug locked in crystalline form does not diffuse within the coating after implantation and allows a more desirable drug elution pattern18. This system of antiproliferative drug delivery assures not only long-term antiproliferative effects, but, in the case of sirolimus with its anti-inflammatory properties, may also minimise potential inflammatory reactions caused by polymers19.
NEOINTIMAL GROWTH BETWEEN SIX AND 24 MONTHS
In the current OCT substudy, the significant inhibition of %VO at six months in the MiStent group was no longer observed at 24 months; the delta %VO between six and 24 months was also not significantly different between the two stents. Although we expected that the potential benefit of prolonged drug release in the absence of polymer would result in less neointimal proliferation, the expected effect was not observed in this series. Late neointimal growth has also been observed in other DES types including the XIENCE stent20. The mechanisms for this phenomenon are still unclear. The longer duration of drug elution even at a low level might be associated with a delayed late catch-up of neointimal hyperplasia of the MiStent once the upregulation of p27 is eliminated21. Late neointimal growth might be an adaptive biological reaction of the vessel, aimed at homogenising shear stress towards physiological values22. Alternatively, that might be related to a delayed healing response of the vessel or to a hypersensitivity reaction to durable polymers23.
The current serial OCT analysis found that the MiStent group had low %VO of 14.50 and 20.88 on OCT at six and 24 months, respectively, which was corroborated with the late loss and in-segment diameter stenosis on QCA at six and 24 months. Asano et al reported that angiographic late loss (usually driven by neointimal growth) below the threshold of 0.50 mm was not associated with an increased risk for TLR and may be considered as a subclinical level of neointimal hyperplasia. Beyond the threshold value of 0.50 mm, there was an exponential increase in TLR24. In the current serial QCA assessment, the MiStent had late loss of 0.02 and 0.26 mm and in-segment percent diameter stenosis of 15.1% and 18.4% on QCA at six and 24 months, respectively. These values of late loss and in-segment percent diameter stenosis corresponded with those of new DES approved by the Food and Drug Administration according to a report of the European Society of Cardiology and the European Association of Percutaneous Cardiovascular Interventions Task Force25. In the DESSOLVE III main study, the rate of clinically indicated TLR was comparable between the two groups at 24 months (MiStent 4.6% vs XIENCE 5.4%, difference -0.9% [95% CI: −3.2 to 1.4], p=0.447).
Limitations
The current OCT analysis should be interpreted in the context of the following limitations. First, although the current study is a substudy of the DESSOLVE III RCT, the randomisation was not implemented for the two device groups. Second, this study included a small number of patients without formal sample size calculation. Therefore, the results are regarded as exploratory. Third, the current OCT results were reported using the abluminal stent contour. The abluminal contour could indicate the original lumen border on the assumption that all struts are apposed. Thus, in case of embedded or buried struts at baseline, we might have overestimated neointimal growth in the XIENCE group, since the XIENCE has a thicker strut thickness than the MiStent.
Conclusions
In the serial comparative OCT analysis of the MiStent versus the XIENCE stent, the MiStent showed a more favourable efficacy for preventing neointimal formation with comparable strut tissue coverage, as compared with the XIENCE stent at six months. However, this difference in %VO decreased at 24 months so that the difference in neointima at 24 months was no longer significant.
|
Impact on daily practice The current serial OCT assessment demonstrated that the MiStent with early polymer elimination and nine-month sustained drug release had not only a more potent efficacy of neointimal inhibition but also a comparable strut coverage at six months, compared with the XIENCE stent and that this intergroup difference was partially maintained at 24 months. Although the study was not powered to detect differences in clinical outcomes, the MiStent may become an option as a contemporary drug-eluting stent with a potentially low revascularisation rate in short-term follow-up and a similar safety profile to the XIENCE stent. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Funding
The trial was sponsored by the European Cardiovascular Research Institute (ECRI) and supported with unrestricted grants from Micell Technologies, Durham, NC, USA, and Stentys, Paris, France.
Conflict of interest statement
W. Wijns reports grants from Micell during the conduct of the study, grants from MicroPort, personal fees from Biotronik and Abbott Vascular, outside the submitted work, and being a co-founder of Argonauts, an innovation facilitator. P.W. Serruys reports personal fees from Abbott Laboratories, AstraZeneca, Biotronik, Cardialysis, GLG Research, Medtronic, Sino Medical Sciences Technology, Société Europa Digital & Publishing, Stentys France, Svelte Medical Systems, Philips/Volcano, St. Jude Medical, Qualimed, and Xeltis, outside the submitted work. The Guest Editor is a consultant for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

