
Abstract
Aims: Durable polymer drug-eluting stents (DP-DES) may contribute to persistent inflammation, delayed endothelial healing and subsequent late DES thrombosis. The aim of this optical coherence tomography (OCT) substudy was to compare healing and neointimal coverage of a novel bioabsorbable polymer sirolimus-eluting stent (Firehawk®) (BP-DES) versus the DP-DES (XIENCE) at 90 days in an all-comers patient population.
Methods and results: The TARGET All Comers study is a prospective multicentre randomised post-market trial of 1,656 patients randomised 1:1 to Firehawk or XIENCE at 21 centres in 10 European countries. The TARGET OCT substudy enrolled 36 consecutive patients with 52 lesions at six centres proficient in OCT. Follow-up OCT was performed at three months or prior to revascularisation when occurring before the three-month window. The substudy was designed for non-inferiority of the primary endpoint of neointimal thickness. At follow-up, the mean neointimal thickness by OCT (52 lesions: Firehawk, n=24; XIENCE, n=28), was not significantly different between groups (Firehawk 75.5 μm vs. XIENCE V 82.3 μm) meeting the primary endpoint of non-inferiority (pnoninferiority<0.001). The percentage of stent strut coverage was high in both groups (strut level: 99.9±0.3% vs. 100±0.1%, p=0.26), and the proportion of malapposed struts (1.0±1.6% vs. 1.2±2.0%, p=0.51) was low in both groups.
Conclusions: Based on OCT, the Firehawk BP-DES has a similar healing response three months after implantation compared to the DP-DES, with near complete strut coverage, moderate neointima formation and minimal strut malapposition.
Abbreviations
BP-DES: biodegradable polymer drug-eluting stent
CAD: coronary artery disease
DP-DES: durable polymer drug-eluting stent
ISA: incomplete stent apposition
OCT: optical coherence tomography
Introduction
Drug-eluting stents (DES) have been widely adopted as the main percutaneous revascularisation treatment for ischaemic coronary artery disease (CAD) on the basis of proven superior safety and efficacy compared to bare metal stents1,2. Despite these benefits, durable polymer (DP)-DES exhibit delayed vessel healing, hypersensitivity reactions and neoatherosclerosis formation, resulting in delayed restenosis and repeat revascularisation at an annual rate of 2-2.5%, as well as late and very late stent thrombosis3,4. Bioabsorbable polymer coatings degrade over months to years and allow delivery of an antiproliferative drug until the polymer disappears leaving behind a bare metal stent. Limiting the duration of polymer exposure to the vessel wall confines the inflammatory exposure to the duration necessary to deliver the antiproliferative drug, thus offering potential for improved late safety and efficacy in comparison with durable polymer stents5. The Firehawk® abluminal groove-filled biodegradable polymer (BP) sirolimus-eluting stent (MicroPort Medical, Shanghai, China) is a thin-strut cobalt-chromium stent platform, with a fully biodegradable PLA polymer coating applied to abluminal grooves to minimise polymer burden and reduce drug concentrations. The Firehawk DES received CE mark approval in 2015 based on extensive clinical evaluation6-9.
The TARGET All Comers study is an ongoing (enrolment complete) prospective multicentre randomised post-market trial designed to evaluate the safety and efficacy of the Firehawk BP-DES compared to the XIENCE V® DP-DES (Abbott Vascular, Santa Clara, CA, USA) in an expanded patient population. The purpose of the OCT substudy of TARGET All Comers is to assess stent healing of the stented vessel segment at three months.
Methods
STUDY DESIGN AND PATIENT POPULATION
TARGET OCT is a substudy of the ongoing TARGET All Comers clinical trial, a prospective European multicentre open-label 1:1 randomised trial, designed to demonstrate non-inferiority of the novel Firehawk BP-DES compared to the XIENCE DP-DES in an all-comers population. It is intended to evaluate the safety and effectiveness of the Firehawk BP-DES for the treatment of subjects with atherosclerotic lesion(s) in coronary arteries ≥2.25 mm to ≤4.0 mm in diameter (by visual estimation) with minimal exclusion criteria in a real-world consecutive population. The pre-specified OCT substudy was designed to enrol up to 50 consecutive subjects who consented to undergo OCT assessment and clinical follow-up at three months after the index procedure. The OCT substudy was performed at six pre-selected centres with OCT proficiency based on case volume and investigator experience. Patients were included if they had stable symptomatic CAD or silent ischaemia with objective evidence of myocardial ischaemia or acute coronary syndrome and were candidates for stent implantation. Patients presenting with ST-elevation myocardial infarction were excluded from the substudy. There were no restrictions on the number of treated lesions or stents implanted.
STUDY DEVICE
The Firehawk BP-DES is a balloon-expandable L605 cobalt-chromium stent platform with a strut thickness of 86 μm and abluminal grooves containing a PLA biodegradable polymer (10 μm within the groove only), which provides controlled release of the antiproliferative drug sirolimus (dose 0.28 μg/mm2) over time (90% at 90 days) (Figure 1). The polymer biodegrades within six to nine months, leaving only the metallic stent permanent implant. The stent is mounted on a rapid exchange delivery catheter system6. Firehawk stent lengths included in the trial were 13, 18, 23 and 29 mm for the 2.25 mm diameter stent, 13, 18, 23, 29 and 33 mm for the 2.50 mm stent, and 13, 18, 23, 29, 33, 38 mm for the 2.75, 3.00, 3.50 and 4.00 mm diameter DES.
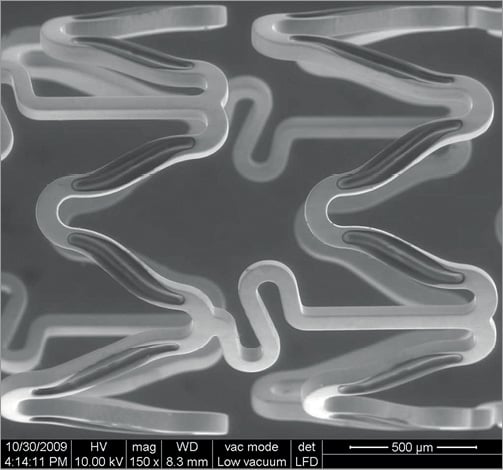
Figure 1. The Firehawk cobalt-chromium coronary stent (rapamycin target-eluting) system.
PROCEDURE AND FOLLOW-UP
Consecutive patients meeting all inclusion and no exclusion criteria and providing informed consent were enrolled in the OCT substudy and randomised by interactive web system to either the Firehawk BP-DES or the XIENCE DP-DES. Stent implantation was performed according to each device’s instructions for use and local standard practice. The same DES platform was used for all treated lesions for a given patient based on randomised assignment. Patients were preloaded with aspirin and a P2Y12 inhibitor (clopidogrel, ticagrelor or prasugrel) and continued on dual antiplatelet therapy according to guidelines and local standard of care. Procedural anticoagulation was according to local standard practice. An independent angiographic core laboratory reviewed all procedural angiograms (FuWai Hospital, Beijing, China). Quantitative coronary angiography (QCA) was performed using validated software (Medis, Leiden, the Netherlands) and standard definitions.
OCT ACQUISITION AND ANALYSIS
Follow-up OCT was performed at three months (±14 days) after the index procedure using a commercially available OCT system (LightLab C7/C8 ILUMIEN™ system [St. Jude Medical, St. Paul, MN, USA] or Lunawave® [Terumo Corp., Tokyo, Japan]). In the event of an early (≤ 3 months) target lesion revascularisation, every effort was made to perform an OCT pullback prior to any revascularisation. The antiplatelet regimen was according to the local standard of care. An automated pullback at 20 mm/s through the stented segment was performed according to guidelines from the independent OCT core lab (Cardialysis B.V., Rotterdam, the Netherlands). Analysis of the OCT images was performed with the QIvus software, version 3.0 (Medis, Leiden, the Netherlands) by analysts blinded to device allocation. Stented segments were analysed at 1 mm interval cross-sections, and mean and minimum lumen/stent areas measured for each stent. The quantitative measurements were based on endoluminal stent contour, which considers the endoluminal optical leading edge of the strut and connects by interpolation these leading edges to form a circular boundary of stent area7,8. This circular boundary and the luminal contour comprise the circumferential neointima area covering the struts. Neointimal thickness was defined as a distance, measured along the line from the centre of gravity of the lumen area, between the endoluminal leading edge of the strut and the luminal boundary (Figure 2). The 5 mm proximal and distal reference lumen areas were averaged for the reference lumen area. The stent expansion index was a ratio of minimum stent area to the reference lumen area. The distance measured between the endoluminal reflective border of a strut and the lumen contour quantified strut apposition. If this distance was greater than the strut thickness (89 μm for XIENCE and 86 μm for Firehawk), it defined incomplete stent apposition (ISA). Struts crossing over the ostium of side branches and overlapping stents were excluded from the analysis of apposition. The percentage of ISA struts in each stented lesion was calculated as (the number of ISA struts)/(total number of struts in all cross-sections of the lesion)*100. The ISA area was defined as the space between the lumen contour and stent contour at the location of ISA struts. Mean and the largest ISA areas were evaluated at lesion level.
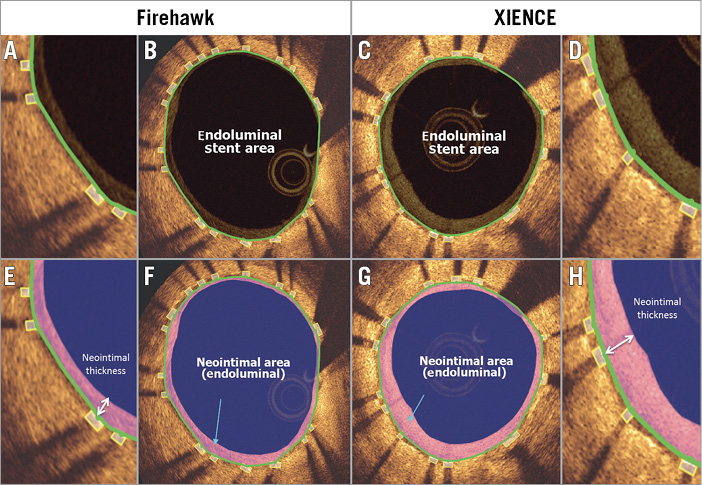
Figure 2. OCT analysis of stent area and neointimal area at follow-up. Representative follow-up OCT cross-sections of Firehawk (A, B, E & F) and XIENCE (C, D, G & H) are shown with stent area and neointimal measurements. Panels A, D, E and H are magnified views of B, C, F and G, respectively. Stent area was delineated using endoluminal stent contour (area inside the green line). Neointimal area was calculated as endoluminal stent area – lumen area. Neointimal thickness was measured per strut (white arrows in E and H).
ENDPOINTS AND STATISTICAL ANALYSIS
The primary OCT endpoint was mean neointimal thickness at three months. The substudy was powered for non-inferiority with sequential superiority testing to control for type I error. The t-test was used to test the one-sided hypothesis of non-inferiority in mean neointimal thickness. Sample size calculations were based on historical data, with an expected XIENCE mean neointimal thickness of 75 µm, no difference between treatments, a non-inferiority margin (Δ) of 35 µm, a one-sided alpha (α)=0.05, one analysable lesion per patient and a 25% expected attrition rate at follow-up. Based on these assumptions, a total of 36 evaluable patients would provide 80% power (1–ß) to meet non-inferiority. Secondary exploratory OCT endpoints at three months were compared between groups and included mean and minimal stent and lumen diameter (mm) and area (mm2), the lumen and stent volumes (mm3), mean neointimal hyperplasia area (mm2) and volume (mm3), in-stent neointimal hyperplasia volume obstruction (%), the percentage of incompletely apposed struts (%), and the uncovered and malapposed strut rates.
Results
SUBJECT AND PROCEDURAL CHARACTERISTICS
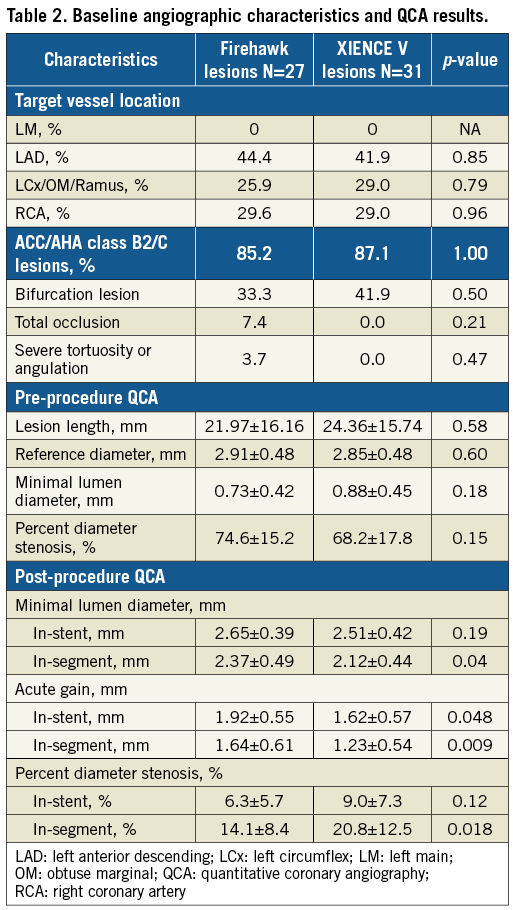
A total of 36 subjects (18 per group) with a total of 58 lesions (31 XIENCE and 27 Firehawk) were enrolled at six sites in Europe (Figure 3). Baseline patient and procedural characteristics were matched and are displayed in Table 1. Mean age was 62 years, 89% were male, 25% presented with NSTEMI and on average 1.3 stents were implanted per lesion. Lesion complexity was similar in both groups with AHA/ACC B2/C lesion complexity in 86% of patients (Table 2). Firehawk resulted in a larger in-segment MLD, a larger in-stent and in-segment acute gain post procedure and a significantly lower in-segment residual diameter stenosis (Table 2).
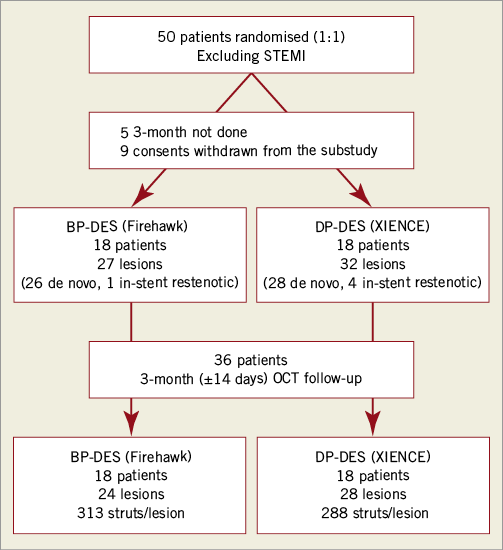
Figure 3. Study flow chart. BP-DES: biodegradable polymer drug-eluting stent; DP-DES: durable polymer drug-eluting stent; OCT: optical coherence tomography
OCT RESULTS
On average 300 struts were analysed (313 for Firehawk and 288 for XIENCE) over a stent length of 28.9±21 mm for Firehawk and 27.3±12 mm for XIENCE (p=0.75). Mean neointimal thickness was not significantly different between groups (Firehawk 75.5±25.8 μm vs. XIENCE 82.3±31.1 μm), meeting the primary endpoint of non-inferiority (mean difference 6.8%, 95% CI: –22.9, 9.3; pnoninferiority<0.001) (Figure 4). The primary analysis was performed without considering the cluster effect of multiple lesions in a single patient, assuming that neointimal growth occurred independently among multiple lesions within the same patient9. However, 13 patients in the current cohort had more than one lesion. To address the possible effect of clustering, an exploratory analysis of the primary endpoint was conducted using a generalised mixed-effect model analysis. The mean difference of neointimal thickness between Firehawk and XIENCE was –6.6 µm with 7.3 µm of a one-sided 95% CI, which is similar to the primary analysis of –6.8 µm with 4.0 µm of a one-sided 95% CI. The non-inferiority criterion was achieved using both methods.
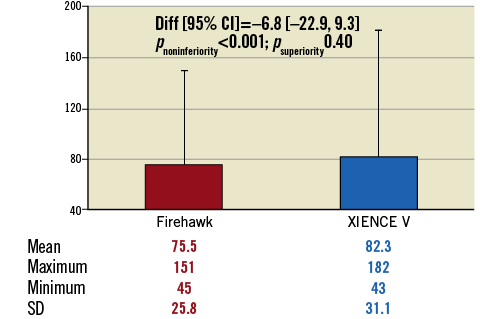
Figure 4. Primary endpoint: mean neointimal thickness/strut. Comparison of mean neointinal thickness/strut (μm) at three months of Firehawk vs. XIENCE V.
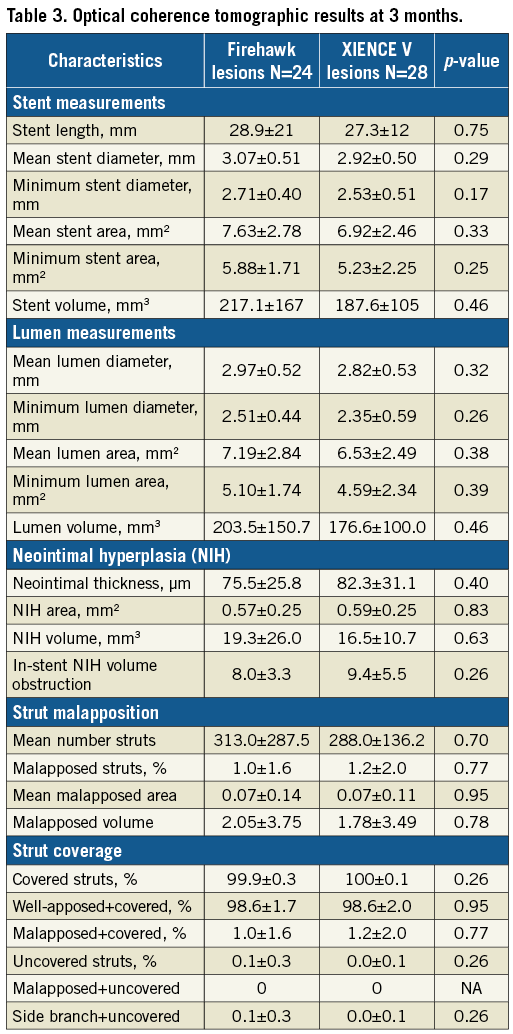
The percent of covered struts was high and similar with both stents (99.9% Firehawk and 100% XIENCE, p=0.26). Malapposed struts were rare (<1.2%) with a corresponding malapposed area of <0.1 mm2 in both groups. There were 10 lesions with overlapping stents (three in the Firehawk and seven in the XIENCE arm). There were no differences in mean neointimal hyperplasia between overlap and non-overlap areas for Firehawk (1.47±0.58 mm2 vs. 0.97±0.50 mm2, p<0.098) and for XIENCE (0.88±0.51 mm2 vs. 0.52±0.88 mm2, p<0.114). All other measures of stent, lumen and NIH areas and volumes were similar between groups (Table 3).
Discussion
This OCT substudy demonstrates complete healing of the Firehawk BP-DES at three months comparable to the benchmarked XIENCE DP-DES.
FIREHAWK MECHANISM OF ACTION AND DRUG AND POLYMER KINETICS
BP-DES transform to a bare metal stent once the drug-polymer combination has completely degraded. The duration of polymer degradation, and thus its exposure to the vessel wall, varies among BP-DES and modulates the duration of drug delivery as well as the inflammatory effects of the polymer. However, differences in stent platform and strut thickness, as well as polymer degradation kinetics, can all impact on healing, which has been shown to be a strong correlate of safety and future stent thrombosis after DES implantation. The Firehawk DES (Figure 1) is uniquely designed with abluminal grooves within which the bioresorbable PLA polymer and sirolimus is applied, thus limiting exposure to the vessel wall. Ninety percent (90%) of the drug elutes over 90 days, and drug elution is complete by six months, with simultaneous degradation and complete resorption of polymer by nine months. This design limits polymer exposure to the vessel wall and allows a timed drug release, intended to inhibit smooth muscle cell proliferation while maintaining early healing, endothelial cell recovery, and minimal inflammatory response. A number of approval studies performed in China have demonstrated good performance of the Firehawk DES with a low angiographic late lumen loss (0.13±0.24 mm) at nine months comparable to the XIENCE V DP-DES (TARGET I trial)10 and low rates of target vessel failure at two and three years10-12.
LESSONS FROM OCT AND EARLY HEALING OF BIODEGRADABLE DES
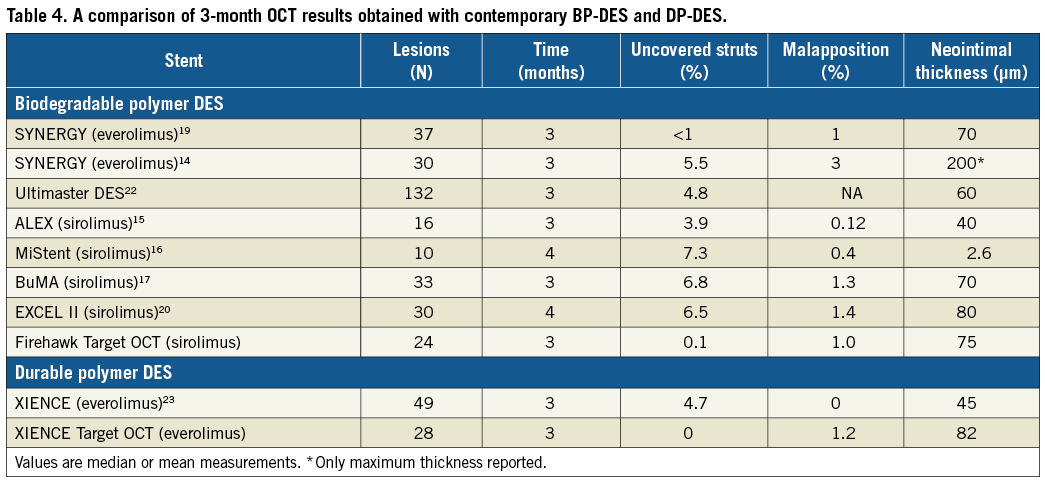
OCT has become the gold standard surrogate to evaluate stent healing after DES implantation. Early healing within three months of BP-DES implantation has recently been evaluated in a number of different DES platforms using OCT, with the aim of reducing the duration of dual antiplatelet therapy (DAPT)13-20. Table 4 summarises these results along with results of the Firehawk and XIENCE from our study. In general, healing demonstrated with the Firehawk compares favourably with other BP-DES at three months, with high strut coverage, low rates of strut malapposition (1% and 1.2%), and average neointima thickness (75.5 µm) and neointima area (0.57 mm2) within the range seen with other devices at this time point. Near complete coverage and moderate neointimal proliferation indicate a good early healing response. These data complement a prior study that established that late healing of the Firehawk DES by OCT was comparable to the XIENCE DP-DES three years after implant. Mean stent strut neointimal thickness (SES: 0.13 ±0.02 mm vs. EES: 0.13±0.02 mm, p=0.80) and mean percentage of covered struts (SES: 99.2% vs. EES: 99.3%, p=0.53) were similar between groups at three years21.
Whether these results will translate into increased safety and lower rates of untoward thrombotic events at long-term follow-up will be evaluated in the TARGET-AC trial with completion of the five-year follow-up.
Limitations
This OCT study has several limitations. This OCT substudy represents a pre-specified nested consecutive series of patients within the randomised clinical trial, but is not itself randomised, which may introduce bias; the OCT results should be interpreted in this context. Though anticipated in the statistical assumptions, the 25% loss to follow-up rate and lack of adjustment of lesion to lesion clustering may have introduced bias to the results. The study is not designed to evaluate the long-term clinical implications of OCT endpoints. Finally, accuracy of the OCT measurements is impacted by the inherent variability of OCT acquisition, resolution, measurement methodology and error.
Conclusions
This OCT substudy of the post-market randomised TARGET-AC trial demonstrates that the Firehawk BP-DES has a similar healing response three months after implantation compared to the DP-DES. After three months, the Firehawk had near complete strut coverage, moderate neointima formation and minimal strut malapposition. Implications on safety outcomes will be evaluated in the overall clinical trial.
| Impact on daily practice The findings of the Firehawk OCT substudy suggest that complete stent coverage is reliably achieved at three months following stent implantation. The healing process with the Firehawk is similar to that found following implantation of the XIENCE stent. This would suggest that dual antiplatelet therapy could be safely limited to three months if clinically indicated after implantation of the Firehawk. |
Guest Editors
This paper was guest edited by Adnan Kastrati, MD; Deutsches Herzzentrum, Munich, Germany, and Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Funding
This study was sponsored by Shanghai MicroPort Medical Co., Ltd., Shanghai, China.
Conflict of Interest statement
A. Baumbach has received speaker fees from Abbott Vascular, MicroPort, and AstraZeneca. A. Lansky has a research grant from MicroPort and speaker fees from AstraZeneca and MicroPort. Y. Onuma is an advisor to Abbott Vascular. T. Johnson has received speaker fees from Abbott Vascular and Terumo. M. Zheng is an employee of MicroPort. W. Wijns has received research grants and speaker fees from Biotronik, MicroPort, Micell and Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor Adnan Kastrati has no conflicts of interest to declare. The Guest Editor Alec Vahanian is a consultant for Edwards Lifesciences.

