Introduction
New-generation drug-eluting stents (DES), including both biodegradable and durable polymer stents, are currently recommended as a default strategy in percutaneous coronary intervention (PCI). A meta-analysis of 16 contemporary randomised DES trials demonstrated similar safety and efficacy of biodegradable polymer DES with respect to stent-related coronary events up to a mean follow-up period of 26 months compared to current-generation durable polymer DES, suggesting no clinical benefit of biodegradable polymer at least at two-year follow-up1. The Firehawk® (Shanghai MicroPort® Medical (Group), Co., Ltd., Shanghai, China) is a thin-strut coronary stent with sirolimus and biodegradable polymer complex localised in abluminal grooves on the strut surface. The TARGET All Comers study recently reported the non-inferiority of target lesion failure (TLF) at one and two years with the Firehawk stent compared to the XIENCE, a durable polymer everolimus-eluting stent (Abbott Vascular, Santa Clara, CA, USA)2,3. Nevertheless, the clinical outcomes of the Firehawk stent might further improve at a later stage. The present paper reports the three-year results of the TARGET All Comers study.
Methods
STUDY POPULATION AND PROCEDURES
TARGET All Comers was a prospective, multicentre, open-label, non-inferiority trial with 1:1 randomisation conducted at 21 centres in 10 European countries (NCT02520180). The study protocol was approved by the ethics committee at each participating site, and written informed consent was obtained from all participants. The detailed study design has been reported previously2,3.
CLINICAL OUTCOMES
The primary endpoint of the TARGET All Comers study was TLF, a composite of cardiac death, target vessel myocardial infarction (MI), or ischaemia-driven target lesion revascularisation at 12 months. The patient-oriented composite endpoint (PoCE) was defined as a composite of all-cause death, any MI, and any revascularisation. Clinical follow-up was scheduled at 1, 6, and 12 months, and annually thereafter up to 5 years by the study protocol. We report the main endpoints of interest including TLF, PoCE, and stent thrombosis at three-year follow-up.
STATISTICAL ANALYSIS
The Kaplan-Meier analysis was used to assess time to clinical events, and the log-rank test was applied to compare between-group differences. Cox proportional hazards regression analysis was used for hazard ratio calculations with 95% confidence intervals. Landmark analyses were performed using the one-year landmark.
Results
A total of 1,653 patients with 2,400 lesions were randomised 1:1 to receive either Firehawk or XIENCE stent implantation, of whom 1,549 (93.7%) patients completed three-year follow-up or had died (Supplementary Figure 1). Baseline patient and lesion characteristics were matched; post-PCI angiographic results were not significantly different between the two groups, as shown previously.
The primary endpoint of TLF occurred similarly in the Firehawk and XIENCE groups at three years (Figure 1). The rates of PoCE, stent thrombosis, individual components of the primary endpoint and other secondary endpoints were comparable between the groups (Supplementary Table 1). Landmark analyses between one and three years demonstrated no significant differences in TLF, PoCE, and definite or probable stent thrombosis (Figure 2). Very late definite or probable stent thrombosis beyond one year occurred in 6 (0.8%) Firehawk-treated patients and in 11 (1.4%) XIENCE-treated patients (p=0.24).
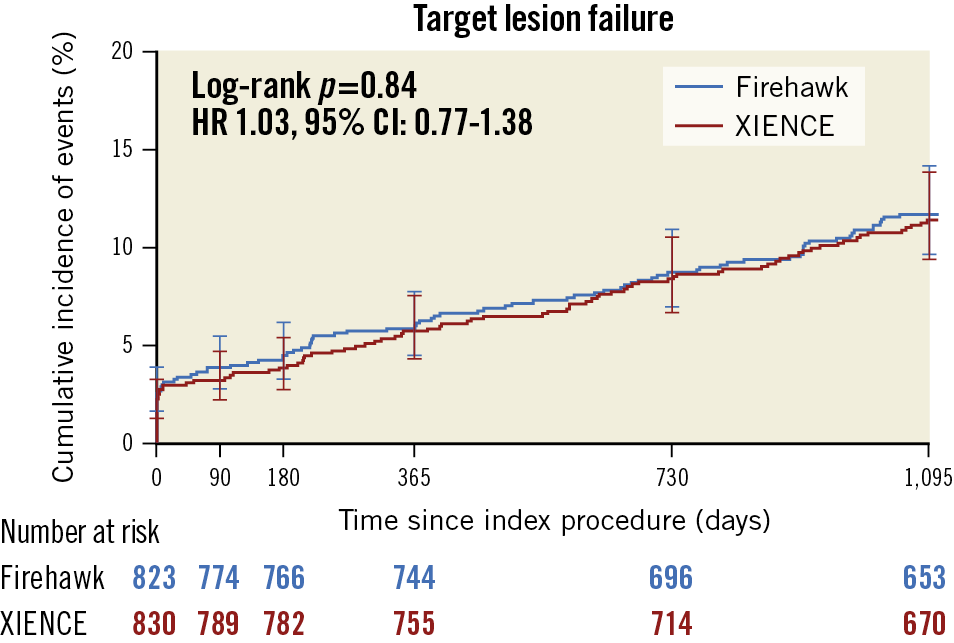
Figure 1. Target lesion failure up to three years.
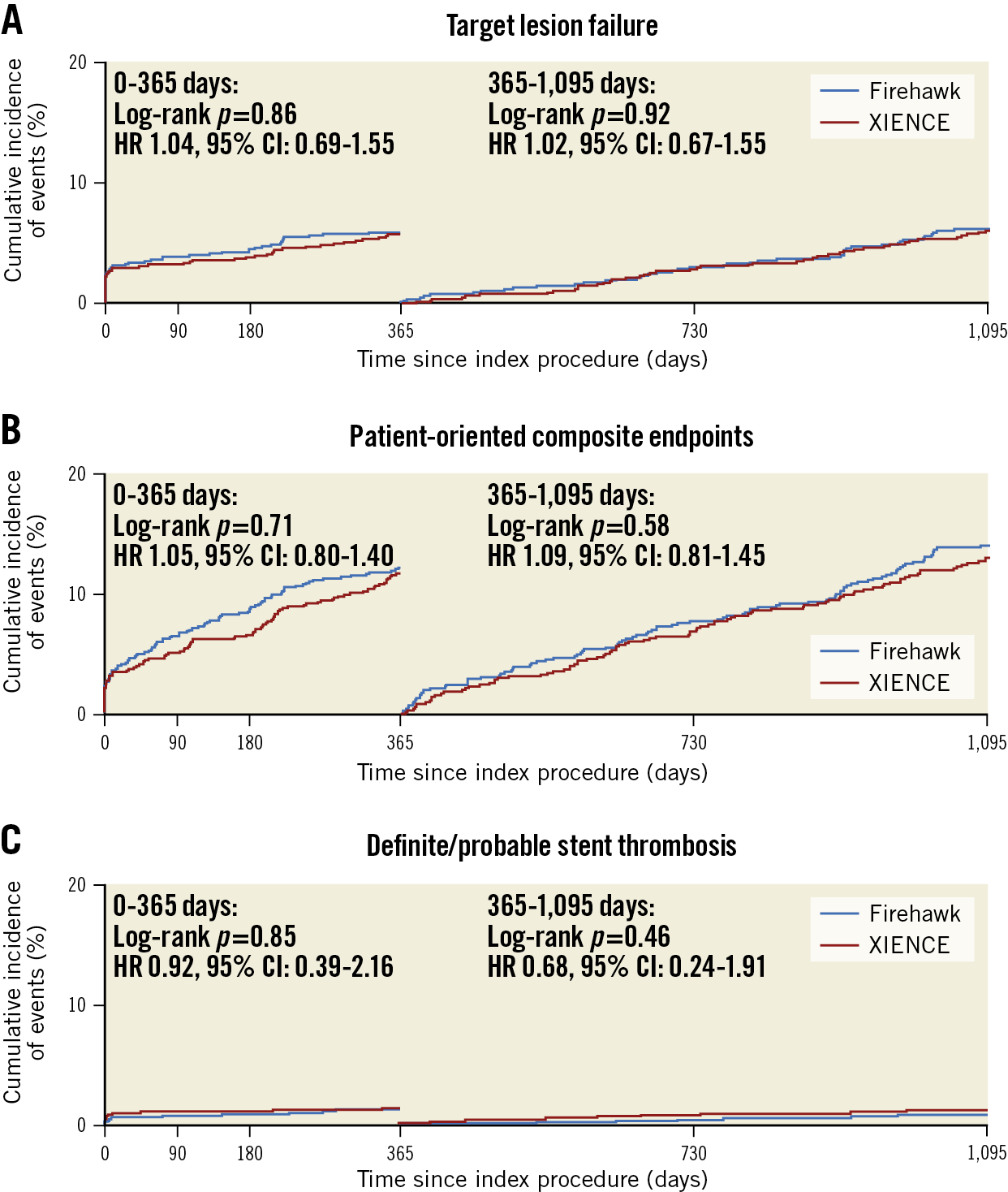
Figure 2. Time-to-event curve for endpoints up to three years. A landmark was set at one year. A) Target lesion failure. B) Patient-oriented composite endpoints. C) Definite/probable stent thrombosis.
Discussion
At three-year follow-up, the TARGET All Comers study demonstrated that the abluminal groove-filled biodegradable polymer sirolimus-eluting Firehawk stent had no significant difference in the major clinical endpoints compared with the benchmark durable polymer everolimus-eluting XIENCE stent.
Very late stent-related adverse events continue to accrue even in PCI with contemporary DES. In a recent, large-scale, individual patient data pooled study (n=25,032), very late stent-related events (defined as TLF) occurred at an annual rate of around 2% with all stent types between one and five years after PCI. The only difference was a decrease in the rate of TLF within one year as coronary stents evolved from bare metal stents to first-generation DES, and from first- to second-generation DES (17.8% vs 8.1% vs 5.0%, p<0.0001)4. Therefore, novel approaches are required to improve long-term outcomes after current-generation DES implantation.
The Firehawk stent has been established as being as safe and effective as the XIENCE stent with similar TLF rates at one and two years2,3. In addition, a sub-analysis of the TARGET All Comers study showed that outcomes with the Firehawk were comparable to the XIENCE stent across the spectrum of patient and lesion risk profiles5. The present study demonstrated that, during the third year of follow-up, the Firehawk continued to perform as well as the XIENCE stent but has not yet demonstrated an advantage of the low polymer density and drug concentration. Continued longer-term observations are needed and are planned up to five years.
Limitations
This study was powered for the primary composite endpoint of TLF at one year. The analysis therefore remained underpowered to detect differences in the individual components of the primary endpoint and stent thrombosis. The TARGET All Comers study will continue to evaluate clinical outcomes up to five years to address the potential benefits of the unique technology, although this follow-up period may not be sufficient.
Conclusion
The TARGET All Comers three-year outcomes confirm the continued safety and efficacy of the Firehawk compared to the XIENCE stent.
|
Impact on daily practice Long-term data with the abluminal groove-filled biodegradable polymer sirolimus-eluting Firehawk stent have not been reported in a randomised all-comers population. The present three-year results of the TARGET All Comers study confirm similar safety and efficacy profiles of the Firehawk stent compared with the XIENCE stent. Further long-term follow-up and real-world data are needed to address potential benefits of the Firehawk stent. |
Funding
This research was funded by Shanghai MicroPort Medical.
Conflict of interest statement
M. Zheng is a MicroPort employee. W. Wijns has received research grant and speaker fees from MicroPort. A. Baumbach has received speaker fees from MicroPort. A. Lansky has received research grant and speaker fees from MicroPort. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

