Abstract
Background: In the prospective, multicentre, randomised TARGET All Comers study, percutaneous coronary intervention (PCI) with the FIREHAWK biodegradable-polymer sirolimus-eluting stent (BP-SES) was non-inferior to the durable-polymer everolimus-eluting stent (DP-EES) for the primary endpoint of target lesion failure (TLF) at 12 months.
Aims: We aimed to report the final study outcomes at 5 years.
Methods: Patients referred for PCI were randomised to receive either a BP-SES or DP-EES in a 1:1 ratio in 10 European countries. Randomisation was stratified by centre and ST-elevation myocardial infarction (STEMI) presentation, and clinical follow-up extended to 5 years. The primary endpoint was TLF (composite of cardiac death, target vessel myocardial infarction [MI], or ischaemia-driven target lesion revascularisation). Secondary endpoints included patient-oriented composite events (POCE; composite of all-cause death, all MI, or any revascularisation and its components).
Results: From December 2015 to October 2016, 1,653 patients were randomly assigned to the BP-SES or DP-EES groups, of which 93.8% completed 5-year clinical follow-up or were deceased. At 5 years, TLF occurred in 17.1% of the BP-SES group and in 16.3% of the DP-EES group (p=0.68). POCE occurred in 34.0% of the BP-SES group and 32.7% of the DP-EES group (p=0.58). Revascularisation was the most common POCE, occurring in 19.3% of patients receiving BP-SES and 19.2% receiving DP-EES, of which less than one-third was ischaemia-driven target lesion-related. In the landmark analysis, there were no differences in the rates of TLF and POCE between groups from 1 to 5 years, and these results were consistent across all subgroups.
Conclusions: In an all-comers population requiring stent implantation for myocardial ischaemia, the BP-SES was non-inferior to the DP-EES for the primary endpoint of TLF at 12 months, and results were sustained at 5 years, confirming the long-term safety and efficacy of the FIREHAWK BP-SES.
Introduction
Drug-eluting stents (DES) are the standard of care in the percutaneous treatment of ischaemic coronary artery disease (CAD)12. The promises of improved safety and efficacy of DES with biodegradable- compared to durable-polymer coatings have not born out in the short term. Failure of durable-polymer DES occurs over time and has been characterised by delayed vessel healing, neoatherosclerosis formation, and associated late repeat revascularisation and late and very late stent thrombosis345. Whether modifying the drug/biodegradable-polymer kinetics of a DES can improve clinical outcomes in the long term is controversial6789.
The FIREHAWK stent (MicroPort) is a thin-strut cobalt-chromium platform with a biodegradable, sirolimus-eluting polymer (BP-SES) that is applied to recessed grooves on the stent surface to minimise the inflammatory vascular response1011121314. The TARGET All Comers (TARGET-AC) study confirmed the safety and efficacy of this low-dose BP-SES by demonstrating non-inferiority for the primary endpoint of target lesion failure (TLF) at 12 months compared with the benchmark durable-polymer XIENCE everolimus-eluting stent (DP-EES; Abbott) in a randomised all-comers patient population15. The long-term results for this BP-SES compared to the DP-EES are not known. In addition, non-target lesion-related events in patients with CAD continue to accrue over time but are unpredictable and poorly defined in an all-comers population. We report target- and non-target-related outcomes at 5 years in the TARGET All Comers trial.
Methods
STUDY DESIGN AND PATIENT POPULATION
TARGET All Comers is a prospective, multicentre, open-label, randomised trial conducted at 21 centres in 10 European countries (Supplementary Table 1). The trial design and 1-year outcomes were reported previously15. In brief, patients referred for percutaneous coronary intervention (PCI) were eligible if they had at least 1 epicardial coronary artery target lesion of ≥50% diameter stenosis in a vessel ≥2.25 mm and ≤4.0 mm in diameter by visual estimation. The study was broadly inclusive to reflect routine clinical practice, without restrictions on the total number of treated lesions or vessels, lesion length, number of stents implanted, or the patient’s clinical presentation15. The ethics committee of each participating centre approved the protocol, and all patients provided informed consent. Data were monitored, collected, validated, and analysed by Cardialysis (Rotterdam, the Netherlands). The executive committee together with the sponsor designed the TARGET All Comers clinical trial.
RANDOMISATION AND MASKING
Patients meeting the entry criteria were randomly assigned 1:1 in an open-label manner to receive the BP-SES or the DP-EES. Randomisation was stratified by clinical site and ST-elevation myocardial infarction (STEMI) presentation. Patients were followed up at 12 months for the primary endpoint and annually thereafter until all patients had completed a 5-year visit. Treatment assignment was available to treating physicians and patients; the clinical event committee remained blinded to the allocated stent type throughout the study.
PROCEDURES
The FIREHAWK DES is a balloon-expandable, L605 cobalt-chromium stent with a strut thickness of 86 μm and cell area of 4.73 mm2 for the 3.5 mm stent. Recessed abluminal grooves contain a D,L-polylactic acid biodegradable polymer of 10-μm thickness, providing controlled release of the antiproliferative drug, sirolimus, at a density of 0.3 μg/mm2, with 90% released by 90 days. The polymer biodegrades within 6-9 months, leaving a permanent bare metal stent implant. The everolimus-eluting durable-polymer XIENCE stent, used as the control, is a laser-cut cobalt-chromium stent with a strut thickness of 81 μm, coated with a 7.7-μm durable fluoride-hexafluoropropylene polymer. The everolimus drug density is 1 μg/mm2 and is released by 120 days.
Stent implantation was performed according to the manufacturer’s instruction for use and based on local standard practice. The same DES platform was used for all treated lesions for a given patient, based on the randomised assignment. Dual antiplatelet therapy was recommended according to current guidelines16. Patients were followed up at 1, 6, and 12 months and annually up to 5 years for adverse events.
OUTCOMES
The primary endpoint of the trial was the device-oriented outcome of TLF – a composite of cardiovascular death, target vessel myocardial infarction, or ischaemia-driven (ID) target lesion revascularisation (TLR). Secondary endpoints included components of the primary endpoint and patient-oriented composite events (POCE) – a composite of all-cause death, all myocardial infarction, or any revascularisation. Other secondary endpoints included all-cause death, target vessel revascularisation (TVR), periprocedural myocardial infarction (MI) defined by the extended World Health Organization definition17, spontaneous MI defined by the third universal definition18, and stent thrombosis defined by the Academic Research Consortium at all timepoints19. An independent clinical event committee (Baim Institute for Clinical Research, Boston, MA, USA) adjudicated all protocol-defined endpoints. An independent angiographic core laboratory (China Cardiovascular Research Foundation, Beijing, China) reviewed all baseline and procedural angiograms.
STATISTICAL ANALYSIS
TARGET-AC was designed to demonstrate the non-inferiority of the FIREHAWK stent for the primary endpoint TLF at 12 months. Details of the statistical assumptions and results have been reported previously15. We have reported all secondary endpoints at 5 years in the intention-to-treat population. Prespecified subgroups for the primary endpoint of TLF, extended to 5 years, included age (≥65 vs <65 years), sex, patients with diabetes, ST-segment elevation MI, small vessels (≤3.0 mm), multivessel treatment, long lesions (>15 mm), in-stent restenosis, total coronary occlusion >72 hours, left main treatment, bifurcation treatment, and overlapping stents. Categorical variables are reported as counts and percentages. Categorical variables with more than 2 categories were assessed by the Mantel-Haenszel rank score test, and dichotomous variables were assessed by Fisher’s exact test. Continuous variables are presented as mean±standard deviation and were compared with t-tests. Kaplan-Meier methods were used to calculate the time-to-event outcomes, and the log-rank test was used to compare between-group differences. Kaplan-Meier estimates censored incomplete data at the last date of available follow-up, assuming complete reporting of all events up to that date and unknown event status after that date. Landmark analyses at 1 year were performed for TLF and POCE and their components. Cox proportional hazards analyses were used to calculate hazard ratios with 95% confidence intervals (CI) and p-values. Unless otherwise specified, a 2-sided p-value<0.05 was considered to indicate statistical significance. Multivariable analysis was performed to identify predictors of TLF and POCE at 5 years, evaluating the following baseline characteristics: age, sex, acute coronary syndrome presentation, diabetes mellitus, hypertension, hypercholesterolaemia, prior MI or PCI, chronic kidney disease, multivessel treatment, number of lesions treated, and lesion length and complexity. All statistical analyses were performed using SAS version 9.3 (SAS Institute). This trial was registered at ClinicalTrials.gov: NCT02520180.
ROLE OF THE FUNDING SOURCE
The sponsor of the study had no role in data collection, data analysis, data interpretation, writing the manuscript, or the decision to submit the manuscript for publication. The executive committee members had full access to the data in the study and had the final responsibility for the submission for publication.
Results
From December 2015 to October 2016, a total of 1,653 subjects with 2,400 lesions were randomly assigned to BP-SES (n=823 patients, 1,221 lesions) or DP-EES (n=830 patients, 1,179 lesions) implantation. At 5 years 1,551/1,653 (93.8%) patients completed clinical follow-up or had died, including 93.3% in the BP-SES group and 94.3% in the DP-EES group (Figure 1). Baseline patient characteristics were matched between the groups; a total of 43.9% of BP-SES patients and 44.4% of DP-EES patients presented with acute coronary syndromes (Table 1). Lesion and procedural characteristics were also matched between the groups (Table 2). At 5 years, TLF occurred in 133/777 (17.1%) patients in the BP-SES group and 129/790 (16.3%) patients in the DP-EES group (difference 0.8%, 95% CI: −2.9% to 4.5%). For BP-SES versus DP-EES in the intention-to-treat population, cardiac death (4.0% vs 4.2%; difference −0.2%; 95% CI: −2.1% to 1.8%; p=0.85), target vessel myocardial infarction (10.6% vs 10.3%; difference 0.3%, 95% CI: −2.7% to 3.3%; p=0.85), ischaemia-driven TLR (6.0% vs 6.5%; difference −0.4%; 95% CI: −2.8% to 2.0%; p=0.74), definite and probable stent thrombosis (2.8% vs 3.0%; difference −0.2%, 95% CI: −1.9% to 1.5%; p=0.81) and other secondary outcome measures were similar between groups (Table 3, Figure 2).
At 5 years, the patient-oriented composite events had occurred in 264/777 (34.0%) patients in the BP-SES group and 258/790 (32.7%) patients in the DP-EES group (difference 1.3%, 95% CI: −3.3% to 6.0%; p=0.58). For BP-SES versus DP-EES in the intention-to-treat population, the occurrence of all-cause death (10.7% vs 11.1%; difference −0.5%, 95% CI: −3.5% to 2.6%; p=0.77), any myocardial infarction (14.8% vs 13.9%; difference 0.9%, 95% CI: −2.6% to 4.3%; p=0.62), and any revascularisation (19.3% vs 19.2%; difference 0.1%, 95% CI: −3.8% to 4.0%; p=0.97) were similar between groups (Figure 3).
For BP-SES versus DP-EES, non-target vessel-related MI (5.4% vs 4.6%; difference 0.8%, 95% CI: −1.3% to 3.0%; p=0.44), and non-target vessel revascularisation (12.1% vs 11.1%; difference 1.0%, 95% CI: −2.2% to 4.1%; p=0.55) accounted for half of the recurrent events in this population (Table 3). Any revascularisation (19%) was the most common POCE, two-thirds of which were non-target lesion-related (Table 3, Figure 3). The annual rate of accrual from 0-1,825 days for TLF was 3.5% and for POCE was 6.6% for BP-SES and 3.2% and 6.4% for DP-EES, respectively, and these were similar between groups. Guideline-recommended medications from 12 to 60 months are presented in Supplementary Table 2.
In the landmark analysis, there were no differences in overall TLF or POCE rates within the first year or beyond (Central illustration) or for any of the components of TLF or definite or probable stent thrombosis (Supplementary Figure 1). There were no differences in the 5-year TLF or POCE rates in any of the prespecified subgroups (Figure 4, Supplementary Figure 2). In multivariable analyses, the predictors of TLF at 5 years included diabetes (odds ratio [OR] 1.9, 95% CI: 1.38 to 2.62; p<0.0001) and age (OR 1.02, 95% CI: 1.00 to 1.04; p<0.0001), and the predictors of POCE at 5 years included diabetes (OR 1.7, 95% CI: 1.27 to 2.18; p=0.0002), prior PCI (OR 1.49, 95% CI: 1.14 to 1.96; p=0.004), male sex (OR 1.48, 95% CI: 1.11 to 1.99; p=0.008), and age (OR 1.03, 95% CI: 1.01 to 1.04; p=0.0001). Acute coronary syndrome presentation or treatment of complex lesions, multivessel disease, left main disease, and left anterior descending artery disease were not predictive of future events.
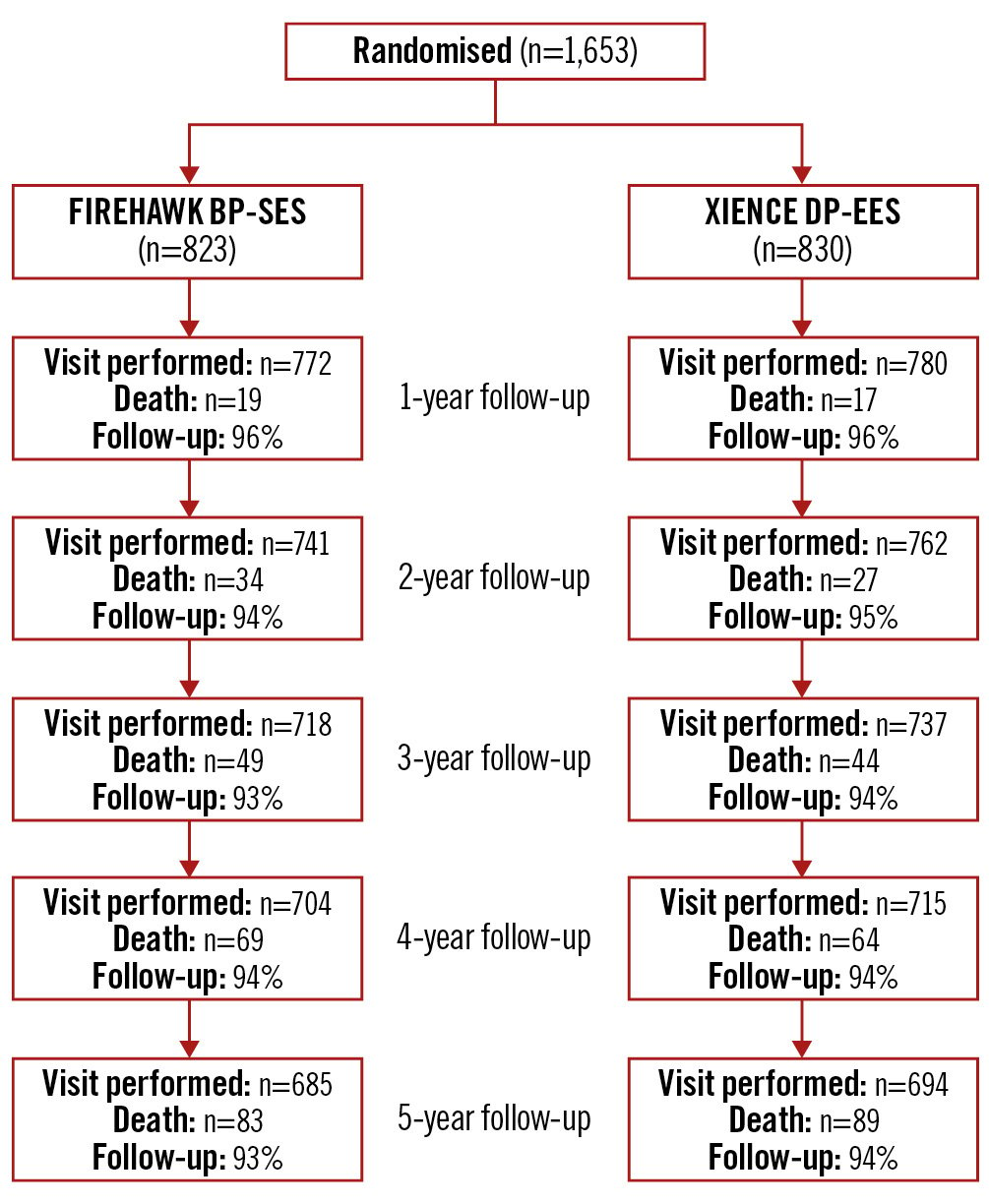
Figure 1. Patient flow according to the CONSORT statement. BP-SES: biodegradable-polymer sirolimus-eluting stent; DP-EES: durable-polymer everolimus-eluting stent
Table 1. Baseline characteristics in the intention-to-treat population.
| Characteristics | BP-SES n=823 patients |
DP-EES n=830 patients |
|---|---|---|
| Age, years | 64.9±9.8 | 65.3±10.5 |
| Male | 78.1 | 76.4 |
| Smoker (current/previous) | 59.5 | 64.2 |
| Diabetes mellitus | 24.0 | 23.1 |
| Non-insulin dependent | 15.2 | 14.5 |
| Insulin dependent | 8.8 | 8.6 |
| Hypertension | 59.9 | 62.5 |
| Hypercholesterolaemia | 53.0 | 51.2 |
| Family history of CAD | 42.8 | 43.4 |
| Previous MI | 21.7 | 24.8 |
| Previous PCI | 28.7 | 31.6 |
| Previous CABG | 8.4 | 7.5 |
| Previous neurological events | 8.1 | 7.7 |
| Renal insufficiency (site reported) | 5.5 | 7.0 |
| Peripheral arterial disease | 5.4 | 5.7 |
| Clinical presentation | ||
| Stable angina | 46.7 | 46.1 |
| Silent ischaemia | 9.4 | 9.5 |
| Unstable angina | 12.8 | 15.7 |
| NSTEMI | 22.7 | 19.8 |
| STEMI | 8.4 | 8.9 |
| Lesions treated per patient | ||
| Number of lesions per patient | 1.5±0.8 | 1.4±0.7 |
| Range (min-max) | 1-6 | 1-5 |
| Number of stents per patient | 1.7±1.0 | 1.7±1.0 |
| Range (min-max) | 0-8 | 0-8 |
| Number of vessels per patient | 1.2±0.5 | 1.2±0.5 |
| Range (min-max) | 1-4 | 1-3 |
| Multiple vessels treated | 21.6 | 18.6 |
| Values are mean±standard deviation, range (min-max), or %. BP-SES: biodegradable-polymer sirolimus-eluting stent; CABG: coronary artery bypass grafting; CAD: coronary artery disease; DP-EES: durable-polymer everolimus-eluting stent; MI: myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction | ||
Table 2. Angiographic and procedural characteristics.
| BP-SES n=1,221 lesions |
DP-EES n=1,179 lesions |
p-value | |
|---|---|---|---|
| Target vessel location* | |||
| Left anterior descending | 453 (42.2) | 463 (43.8) | 0.46 |
| Left circumflex | 272 (25.3) | 269 (25.4) | 0.96 |
| Right | 313 (29.1) | 288 (27.2) | 0.32 |
| Left main | 19 (1.8) | 18 (1.7) | 0.90 |
| Bypass graft | 17 (1.6) | 20 (1.9) | 0.59 |
| ACC/AHA lesion class* | 0.80 | ||
| A | 24 (2.2) | 30 (2.8) | |
| B1 | 157 (14.6) | 155 (14.7) | |
| B2 | 432 (40.2) | 432 (40.8) | |
| C | 461 (42.9) | 441 (41.7) | |
| Total occlusion (TIMI 0/1) | 102 (9.5) | 86 (8.1) | 0.27 |
| Calcification (moderate/severe) | 65 (6.1) | 65 (6.2) | 0.41 |
| Thrombus | 25 (2.3) | 18 (1.7) | 0.30 |
| In-stent restenosis | 47 (3.9) | 55 (4.7) | 0.47 |
| Bifurcation | 359 (33.4) | 344 (32.5) | 0.65 |
| Bifurcation side branch treatment | |||
| Side branch stent | 79 (22.5) | 73 (21.7) | |
| Side branch balloon only | 23 (6.6) | 22 (6.5) | |
| Stent implantation characteristics | |||
| Number of stents per lesion | 1.1±0.5 | 1.2±0.6 | 0.10 |
| Range (min-max) | 0-6 | 0-4 | |
| Stent length per lesion, mm | 26.7±15.3 | 27.1±16.9 | 0.46 |
| Range (min-max) | 8-149 | 8-134 | |
| Stent diameter, mm | 3.07±0.47 | 3.07±0.50 | 0.88 |
| Baseline QCA results* | |||
| Reference diameter, mm | 2.77±0.49 | 2.77±0.52 | 0.77 |
| Minimal lumen diameter, mm | 0.78±0.47 | 0.79±0.48 | 0.83 |
| Percentage diameter stenosis, % | 71.7±15.9 | 71.5±16.1 | 0.76 |
| Lesion length, mm | 19.0±11.8 | 18.8±12.4 | 0.76 |
| Final QCA results | |||
| In-stent MLD, mm | 2.56±0.45 | 2.55±0.47 | 0.54 |
| In-stent percentage diameter stenosis, % | 7.4±6.9 | 7.6±6.5 | 0.54 |
| In-stent acute gain, mm | 1.77±0.55 | 1.76±0.57 | 0.50 |
| Segment MLD, mm | 2.23±0.49 | 2.24±0.51 | 0.64 |
| Segment percentage diameter stenosis, % | 16.2±11.5 | 15.7±10.7 | 0.31 |
| Segment acute gain, mm | 1.45±0.57 | 1.45±0.59 | 0.82 |
| Values are n (%), mean±SD or range (min-max). *Results reported are based on angiographic core laboratory analysis.ACC: American College of Cardiology; AHA: American Heart Association; BP-SES: biodegradable-polymer sirolimus-eluting stent; DP-EES: durable-polymer everolimus-eluting stent; MLD: minimal lumen diameter; QCA: quantitative coronary angiography; SD: standard deviation; TIMI: Thrombolysis in Myocardial Infarction | |||
Table 3. Clinical outcomes at 5 years after stent implantation (intention-to-treat population).
| BP-SES n=823 patients |
DP-EES n=830 patients |
Percentage difference (95% CI) |
p-value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Target lesion failure | 17.1 (133/777) | 16.3 (129/790) | 0.8 [−2.9, 4.5] | 0.68 |
| Components of primary outcome | ||||
| Cardiac death | 4.0 (31/777) | 4.2 (33/790) | −0.2 [−2.1, 1.8] | 0.85 |
| Target vessel MI | 10.6 (82/777) | 10.3 (81/790) | 0.3 [−2.7, 3.3] | 0.85 |
| Ischaemia-driven TLR | 6.0 (47/777) | 6.5 (51/790) | −0.4 [−2.8, 2.0] | 0.74 |
| Secondary outcomes | ||||
| POCE | 34.0 (264/777) | 32.7 (258/790) | 1.3 [−3.3, 6.0] | 0.58 |
| Target vessel failure | 19.4 (151/777) | 18.2 (144/790) | 1.2 [−2.7, 5.1] | 0.54 |
| Cardiac death and MI | 17.5 (136/777) | 16.8 (133/790) | 0.7 [−3.1, 4.4] | 0.73 |
| Any death | 10.7 (83/777) | 11.1 (88/790) | −0.5 [−3.5, 2.6] | 0.77 |
| Cardiac death | 4.0 (31/777) | 4.2 (33/790) | −0.2 [−2.1, 1.8] | 0.85 |
| Any MI | 14.8 (115/777) | 13.9 (110/790) | 0.9 [-2.6, 4.3] | 0.62 |
| Q wave | 1.2 (9/777) | 2.5 (20/790) | −1.4 [−2.7, 0.0] | 0.044 |
| Non-Q wave | 13.8 (107/777) | 12.0 (95/790) | 1.7 [−1.6, 5.1] | 0.30 |
| Target vessel MI | 10.6 (82/777) | 10.3 (81/790) | 0.3% [−2.7, 3.3] | 0.85 |
| Q wave | 0.9 (7/777) | 1.5 (12/790) | −0.6 [−1.7, 0.5] | 0.26 |
| Non-Q wave | 9.8 (76/777) | 8.7 (69/790) | 1.0 [−1.8, 3.9] | 0.47 |
| Non-target vessel MI | 5.4 (42/777) | 4.6 (36/790) | 0.8 [−1.3, 3.0] | 0.44 |
| Q wave | 0.3 (2/777) | 1.0 (8/790) | −0.8 [−1.5, 0.0] | 0.11 |
| Non-Q wave | 5.1 (40/777) | 3.7 (29/790) | 1.5% [−0.6, 3.5] | 0.15 |
| Any revascularisation | 19.3 (150/777) | 19.2 (152/790) | 0.1% [−3.8, 4.0] | 0.97 |
| Target lesion revascularisation | 7.6 (59/777) | 7.7 (61/790) | −0.1 [−2.8, 2.5] | 0.92 |
| Ischaemia-driven | 6.0 (47/777) | 6.5 (51/790) | −0.4 [−2.8, 2.0] | 0.74 |
| Non-ischaemia-driven | 2.2 (17/777) | 2.0 (16/790) | 0.2 [−1.3, 1.6] | 0.82 |
| Target vessel revascularisation | 11.8 (92/777) | 11.1 (88/790) | 0.7 [−2.5, 3.9] | 0.66 |
| Ischaemia-driven | 9.8 (76/777) | 9.2 (73/790) | 0.5 [−2.4, 3.4] | 0.72 |
| Non-ischaemia-driven | 3.7 (29/777) | 3.4 (27/790) | 0.3 [−1.5, 2.2] | 0.74 |
| Non-target vessel revascularisation | 12.1 (94/777) | 11.1 (88/790) | 1.0 [−2.2, 4.1] | 0.55 |
| Thrombosis endpoints | ||||
| Definite stent thrombosis | 2.6 (20/777) | 2.9 (23/790) | −0.3 [−2.0, 1.3] | 0.68 |
| Acute (0-30 days) | 0.5 (4/777) | 0.9 (7/790) | −0.4 [−1.2, 0.5] | 0.38 |
| Late (31-365 days) | 0.8 (6/777) | 0.5 (4/790) | 0.3 [-0.5, 1.1] | 0.54 |
| Very late (after 365 days) | 1.3 (10/777) | 1.9 (15/790) | −0.6 [−1.9, 0.6] | 0.33 |
| Definite/probable stent thrombosis | 2.8 (22/777) | 3.0 (24/790) | −0.2 [−1.9, 1.5] | 0.81 |
| Acute (0-30 days) | 0.6 (5/777) | 1.0 (8/790) | −0.4 [−1.3, 0.5] | 0.42 |
| Late (31-360 days) | 0.8 (6/777) | 0.5 (4/790) | 0.3 [−0.5, 1.1] | 0.54 |
| Very late (after 365 days) | 1.4 (11/777) | 1.9 (15/790) | −0.5 [−1.7, 0.8] | 0.45 |
| Values are % (n/N). BP-SES: biodegradable-polymer sirolimus-eluting stent; CI: confidence interval; DP-EES: durable-polymer everolimus-eluting stent; MACE: major adverse cardiac events (any death, any MI, ischaemia-driven target vessel revascularisation); MI: myocardial infarction; POCE: patient-oriented composite endpoints; TLR: target lesion revascularisation | ||||
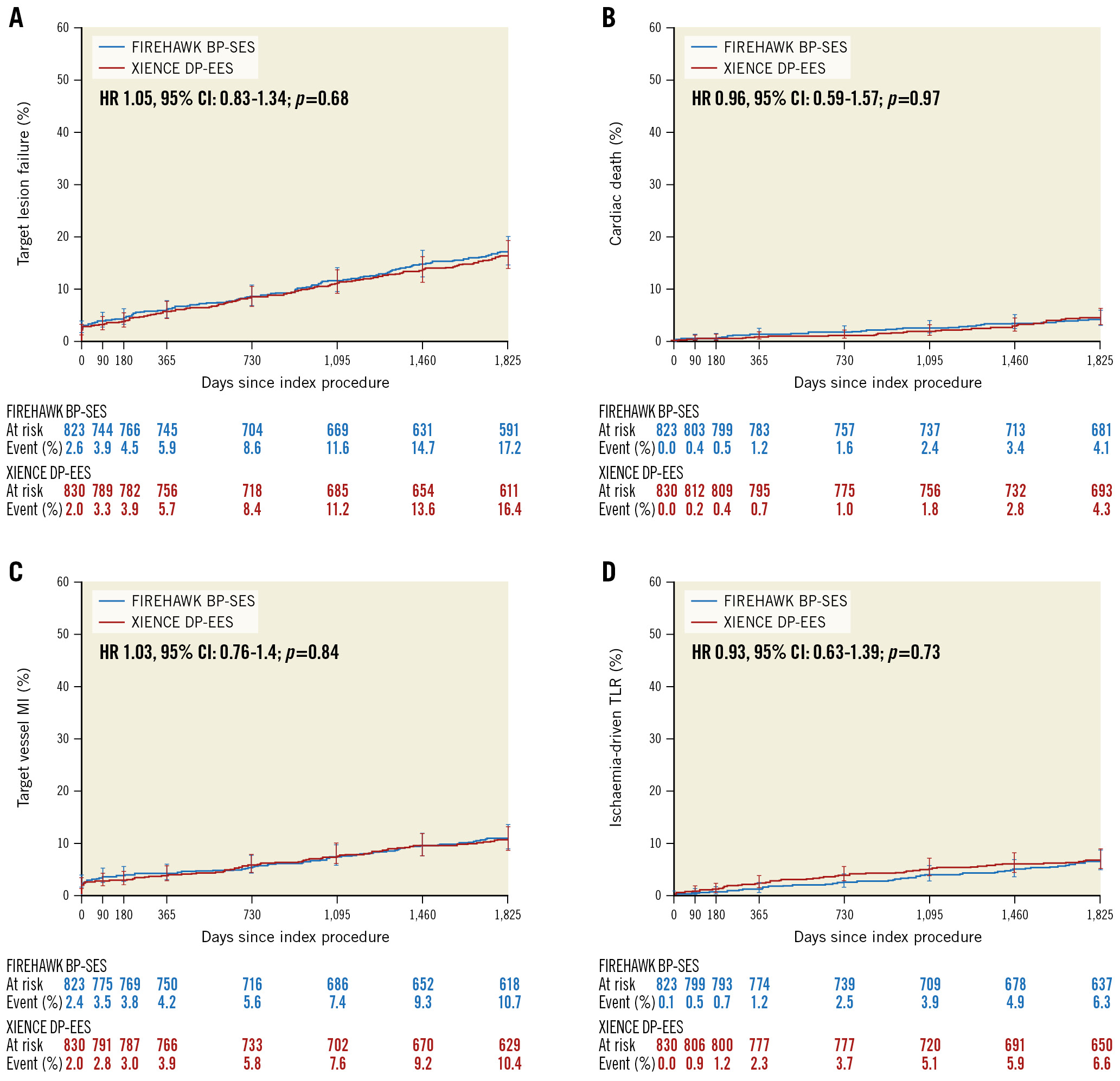
Figure 2. Time-to-event curves for the primary endpoint (target lesion failure) and the individual components of the primary endpoint up to 5 years of follow-up. A) Target lesion failure (composite of cardiac death, target vessel myocardial infarction [MI], or ischaemia-driven target lesion revascularisation [TLR]). B) Cardiac death. C) Target vessel MI (spontaneous MI according to the Third Universal Definition, periprocedural MI according to the World Health Organization Extended Definition). D) Ischaemia-driven TLR. BP-SES: biodegradable-polymer sirolimus-eluting stent; CI: confidence interval; DP-EES: durable-polymer everolimus-eluting stent; HR: hazard ratio
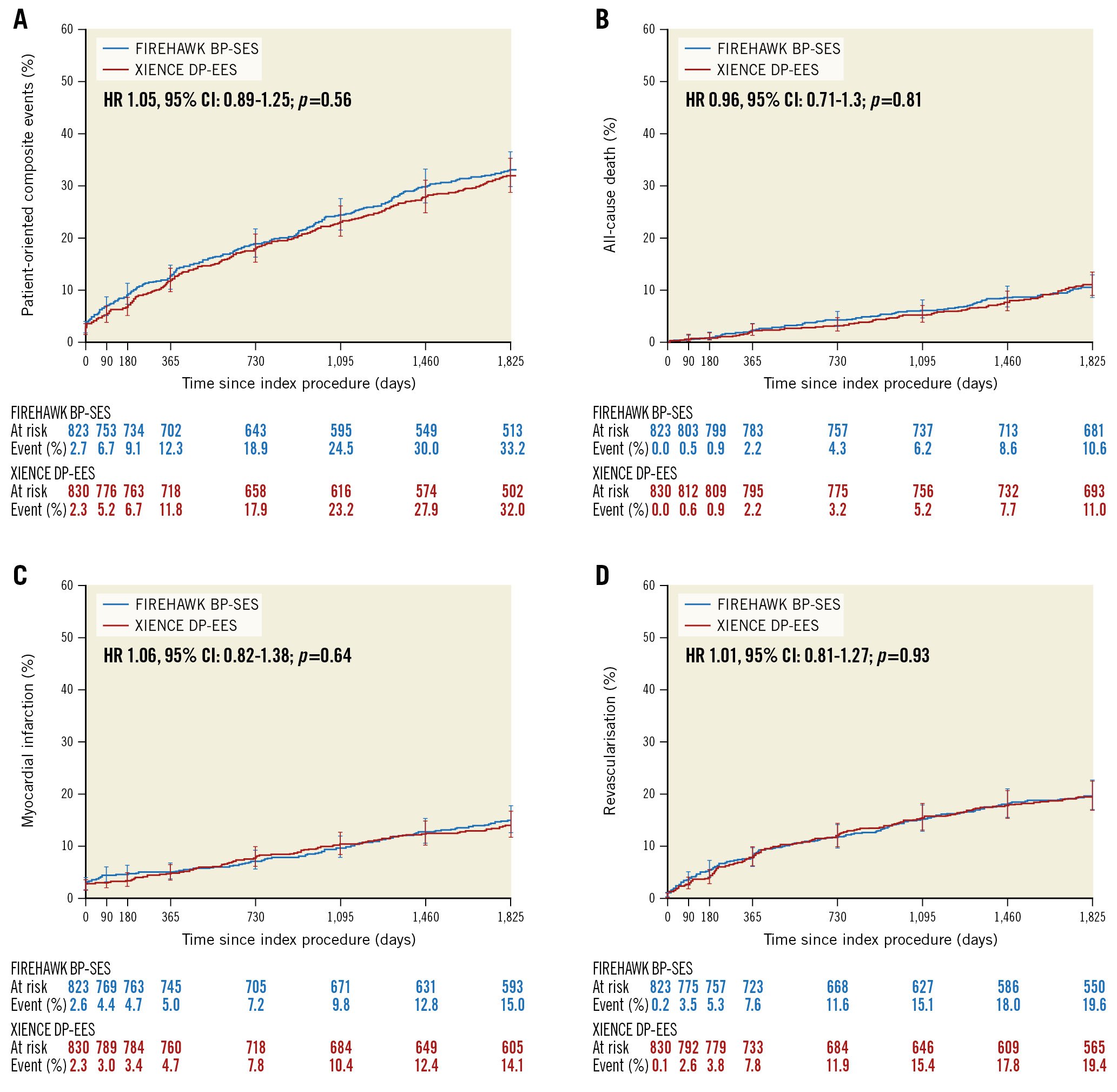
Figure 3. Patient-oriented composite events up to 5 years of follow-up. A) Patient-oriented composite events. B) All-cause death. C) All myocardial infarction (MI) (spontaneous MI according to Third Universal Definition, periprocedural MI according to World Health Organization Extended Definition). D) All revascularisation. BP-SES: biodegradable-polymer sirolimus-eluting stent; CI: confidence interval; DP-EES: durable-polymer everolimus-eluting stent; HR: hazard ratio
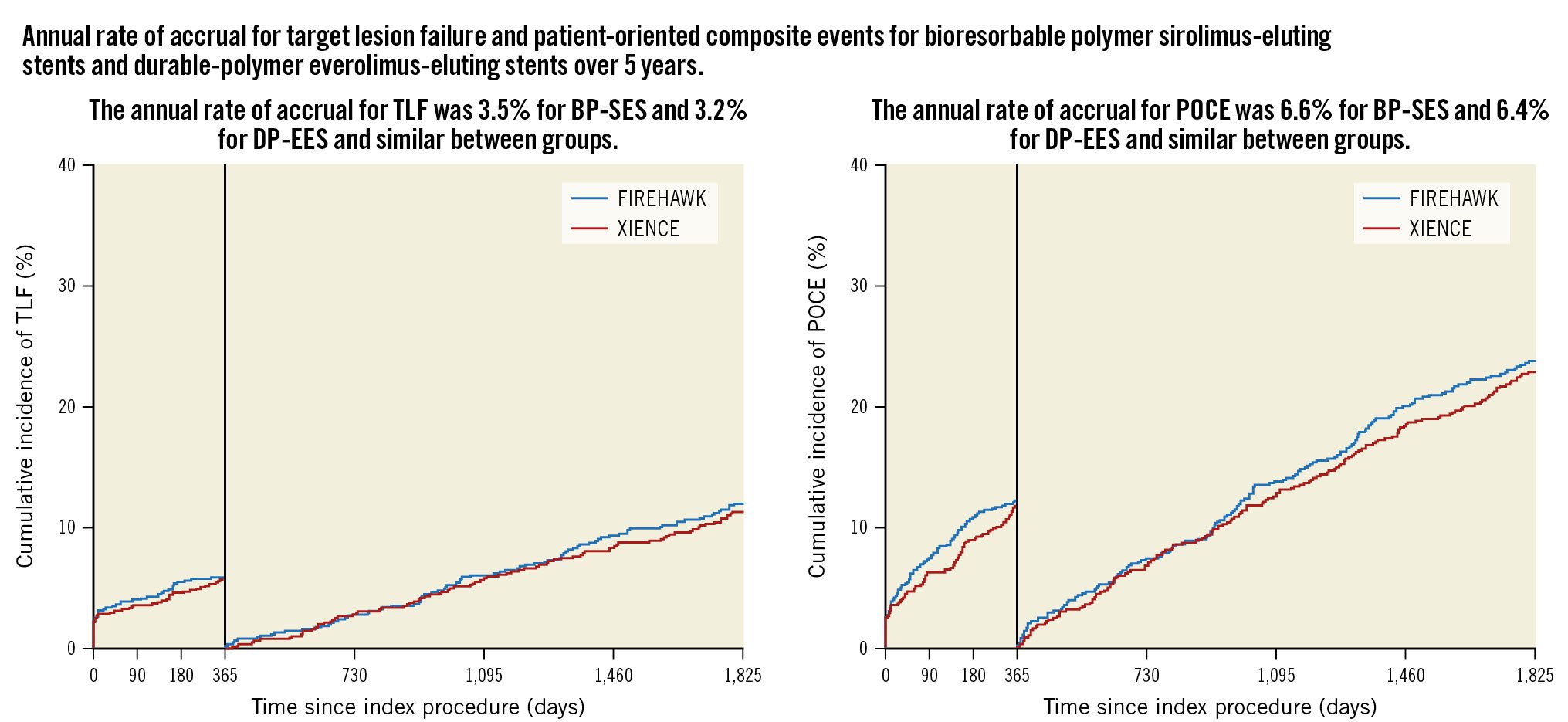
Central illustration. Time-to-event analysis for endpoints up to 5 years with a landmark set at 1 year. A) Target lesion failure (TLF; composite of cardiac death, target vessel myocardial infarction [MI], or ischaemia-driven target lesion revascularisation). B) Patient-oriented composite events (POCE; composite of all death, all MI, all revascularisation). BP-SES: biodegradable-polymer sirolimus-eluting stent; DP-EES: durable-polymer everolimus-eluting stent
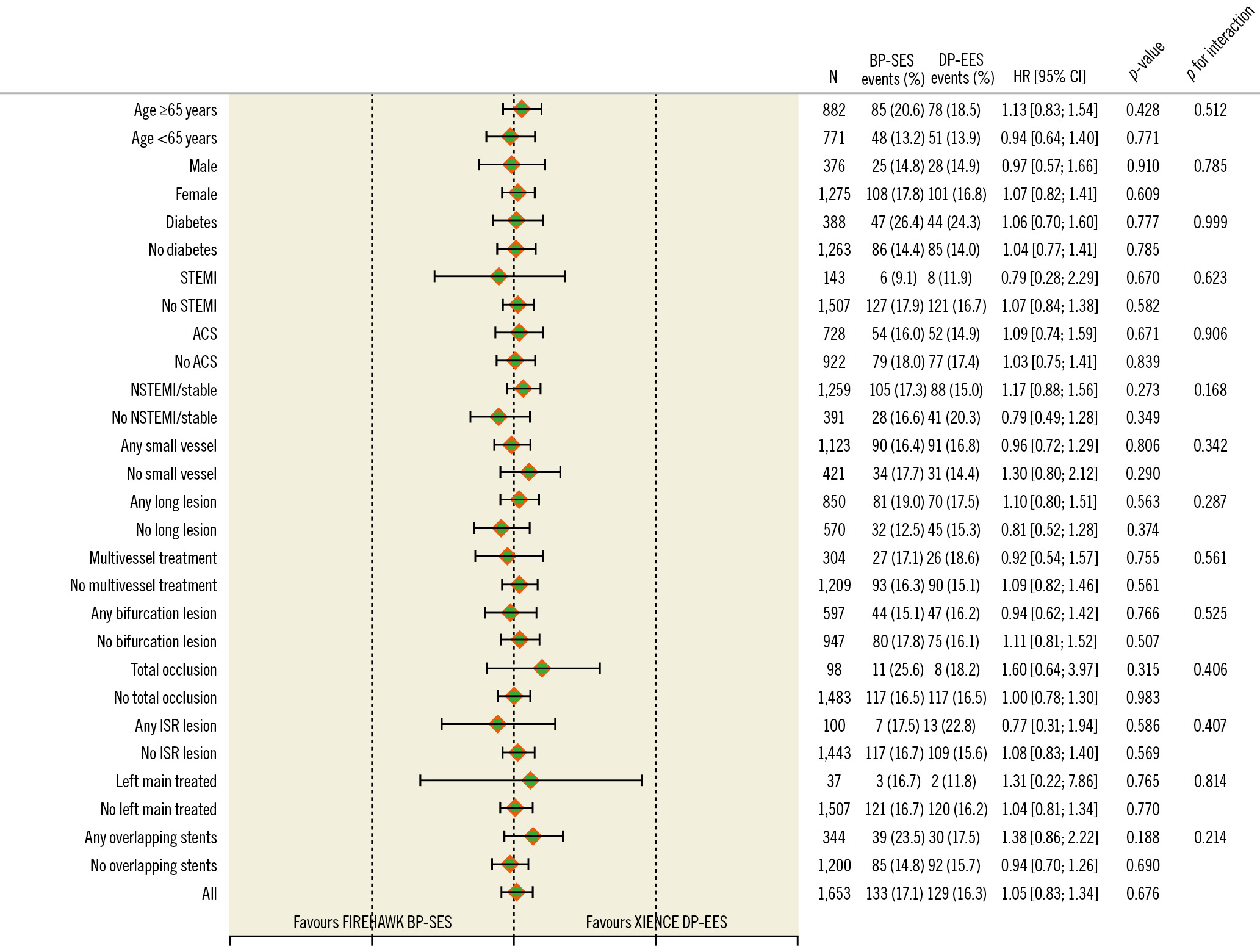
Figure 4. Subgroup analysis for target lesion failure at 5 years. ACS: acute coronary syndromes; BP-SES: biodegradable-polymer sirolimus-eluting stent; CI: confidence interval; DP-EES: durable-polymer everolimus-eluting stent; HR: hazard ratio; ISR: in-stent restenosis; NSTEMI: non-ST-elevation myocardial infarction; STEMI: ST-elevation myocardial infarction
Discussion
This large-scale, prospective, multicentre, randomised, all-comers PCI trial demonstrates several important findings. First, the FIREHAWK BP-SES was confirmed to be as safe and effective as the control XIENCE DP-EES for up to 5 years of follow-up across all tested subgroups. Second, in landmark analyses, TLF and POCE rates were similar up to 1 year and beyond, showing a lack of clinical event differentiation from the bioresorbable- compared with the durable-polymer DES. Finally, 1 in 3 patients had a recurrent event at 5 years, of which two-thirds were non-target lesion-related, calling for improved prediction and prevention of subsequent non-target-related events in this population.
TARGET-AC confirms that up to 5 years, the FIREHAWK BP-SES is as safe and effective as the well-established DP-EES in an all-comers population, as suggested in prior studies1112131420. Based on the broadly inclusive TARGET-AC population, these results can reliably be generalised to the full spectrum of patients with acute and chronic coronary syndromes and lesion interventions encountered in routine contemporary clinical practice.
While the BP-SES demonstrated outstanding and comparable outcomes to the benchmarked control DP-EES, the landmark analysis of TARGET-AC (although not powered to do so) failed to demonstrate any clinical benefit of the BP-SES compared with the DP-EES. This suggests that the reduction in long-term inflammatory response observed in histological series with BP-DES2122 is not sufficient to overcome other patient-, lesion- and DES implant-related aspects that contribute to recurrent events at the target lesion. By breaking down the polymer to biostable water and carbon dioxide after antiproliferative drug delivery, BP-DES were anticipated to reduce the ≈2-3% annual accrual of target lesion-related clinical events observed with second-generation DP-DES23; however, the annual TLF accrual rates in TARGET-AC up to 5 years are consistent with prior reports (3.5% for BP-SES and 3.2% for DP-EES). This suggests that other biological and DES-related factors, such as strut thickness rather than polymer alone, are stronger contributors to target site stent failure.
Long-term results for BP-DES compared with DP-DES have been controversial. A clinical benefit of BP-DES has been suggested in some large-scale pooled analyses of all-comer patients8 and in acute coronary syndromes24, whereas an absence of benefit has been demonstrated in large-scale randomised trials6925 and in other meta-analyses26. TARGET-AC contributes to the growing number of trials that demonstrate no differentiation in clinical outcomes based on polymer when newer-generation coronary stents are compared. One notable exception is the ultrathin 60-μm strut BP-DES (Orsiro; BIOTRONIK) evaluated in the BIOFLOW-V trial, which demonstrated a reduced rate of TLF at 3 years compared with the DP-EES (8.2% vs 13.6%; p=0.0002) and lower rates of target vessel MI (5.0% vs 9.2%; p=0.0003), clinically driven TLR (3.2% vs 6.7%; p=0.006), and definite and probable stent thrombosis (0.1% vs 1.2%; p=0.018)27. At 5 years, the difference in TLF rates (12.3% Orsiro vs 15.3% XIENCE; p=0.108) was no longer significant, although the difference in the incidence of target vessel MI (6.6% vs 10.3%; p=0.015) remained significant28. These benefits suggest that beyond the biodegradable polymer, lower strut thickness has an important role in reducing target lesion-related events in the near and longer term. The BIOSCIENCE randomised trial, evaluating the same ultrathin BP-SES as in BIOFLOW-V, demonstrated higher all-cause mortality related to a higher rate of cancer deaths for the investigational BP-SES compared with the DP-EES29. This finding raised speculative concern about the stent design and drug dose as possible explanations; however, TARGET-AC did not show any difference in all-cause or cardiac mortality between treatment groups. Lastly, while the antiproliferative drug was different between the BP-DES (sirolimus) and DP-DES (everolimus), these have not previously demonstrated differences in DES treatment effects.
While much of the focus of comparative DES trials is on device-specific target lesion-related outcomes, overall long-term POCE results warrant attention. One-third of patients in our all-comers study had recurrent events at 5 years, including 11% all-cause death, 17% cardiac death or MI, and 19% revascularisation, with non-cardiac death rates accruing the longer the follow-up time frame30. These rates are in line with previously reported all-comer trials at 5 years313233 and represent a 6.5% annual accrual rate of major adverse events. The observed rates and difference between all-cause death (11%) and cardiac death (4%) in the present study appear to be representative of all-comers populations reported in other randomised trials and warrants a better understanding of preventable triggers, such as those related to stent thrombosis, dual antiplatelet therapy non-adherence, and inadequate guideline-directed medical therapy, in addition to a focus on non-cardiac causes34.
Differentiating whether POCE events are target lesion-related or not is readily assessable for revascularisation but not reliably accomplished for the safety endpoints of cardiac death and MI. Two out of every 3 repeat revascularisations were not related to the target lesion (11.5% non-target vessel and 6.5% target vessel but non-target lesion-related) (Supplementary Figure 3), emphasising the aggressive nature of disease progression in this population. Detailed non-target lesion assessment by invasive physiology and an imaging or angiographic core laboratory was not recorded in our study. As the majority of patients treated in TARGET-AC had single-lesion and single-vessel treatment, it is possible that residual ischaemic lesions were not revascularised and may account for the non-target revascularisation rates observed over time. Not surprisingly, diabetes was the strongest predictor of both TLF and POCE at 5 years.
Compliance with guideline-directed medical therapy in our patient population was suboptimal at 5 years, with only 18.5% of patients on a P2Y12 inhibitor, despite the known benefits and reduced ischaemic events with extended therapy that must be balanced against the risk of bleeding3536. Furthermore, 30% of patients were not on statin therapy, 45% were not on angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker therapy, and 47% were not on beta-blocker therapy at 5-year follow-up. Since trial monitoring of medication probably represents the best possible values, methods to improve prescription and compliance with guideline-directed medical therapy are needed in daily practice to reduce morbidity and mortality in unselected patients with coronary artery disease.
Limitations
This randomised, open-label, clinical trial was designed to demonstrate the non-inferiority of the FIREHAWK stent for TLF at 12 months and was not powered to detect between-group differences at 5 years, overall or in the landmark analysis. There was no adjustment for multiplicity testing in our study, and the results should be interpreted in that context. Non-target lesion assessment by invasive physiology (fractional flow reserve, instantaneous wave-free ratio), invasive imaging (intravascular ultrasound, optical coherence tomography), or quantitative angiography at index procedure was not recorded; these would have provided important insights into rates of complete revascularisation and overall clinical outcomes. A 3-vessel quantitative angiographic analysis is ongoing. Medical therapy was left to the discretion of site investigators, based on local standards.
Conclusions
In an all-comers population requiring stent implantation for myocardial ischaemia, the BP-SES was non-inferior to the DP-EES based on the primary endpoint of TLF at 12 months, and results were sustained at 5 years, confirming the long-term safety and efficacy of the FIREHAWK BP-SES. One in 3 patients had POCE at 5 years, and two-thirds of the subsequent revascularisations were not related to the target lesion. Additional measures are needed to predict and reduce non-target lesion-related and non-cardiac events in this all-comers population.
Impact on daily practice
In a population of patients requiring stent implantation for myocardial ischaemia, the FIREHAWK BP-SES was non-inferior to the XIENCE DP-EES for the primary endpoint of target lesion failure at 12 months, and results were sustained at 5 years, confirming the long-term safety and efficacy of the FIREHAWK BP-SES. One in 3 patients had patient-oriented composite events at 5 years, and 2 of 3 subsequent revascularisations were not related to the target vessel. Additional measures are needed to predict and reduce non-target lesion-related events in this high-risk population.
Acknowledgements
We acknowledge Laure Artus-Jacenko for her outstanding support in coordinating the trial.
Funding
Funding for our study was provided by Shanghai MicroPort Medical (Group) Co., Ltd.
Conflict of interest statement
A.J. Lansky reports research grants from MicroPort, Sinomed, Abbott Vascular, and Abiomed (Johnson & Johnson); and speaker fees from MicroPort and Shockwave Medical. A. Baumbach reports research support from Abbott Vascular; and speaker fees from Abbott Vascular, MicroPort, AstraZeneca, and Sinomed. N. van Royen reports speaker fees from Medtronic, Abbott Vascular, and Medtronic; research grants from Abbott, Biotronik, Philips/Volcano, and AstraZeneca. M. Zheng is an employee of MicroPort. T.W. Johnson reports research grants from Abbott Vascular; speaker fees from Abbott Vascular, Boston Scientific, MicroPort, Shockwave Medical, and Terumo. S. Brugaletta is on the advisory board at Boston Scientific; and reports speaker fees and research grants from AstraZeneca. W. Wijns reports research grants and speaker fees from Biotronik, MicroPort, Micell Technologies, and Abbott Vascular. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

