Abstract
Aims: This analysis presents the final five-year results of the I-LOVE-IT 2 trial, a non-inferiority study comparing a biodegradable polymer (BP) sirolimus-eluting stent (SES) with a durable polymer (DP) SES in patients with coronary artery disease.
Methods and results: Overall, 2,737 Chinese patients eligible for coronary stenting were treated with BP-SES or DP-SES in a 2:1 ratio. Patients who were randomised to the BP-SES group were additionally re-randomised to receive either six-month or 12-month dual antiplatelet therapy (DAPT) in a 1:1 ratio. The primary endpoint was 12-month target lesion failure (TLF: cardiac death, target vessel myocardial infarction (MI), or clinically indicated target lesion revascularisation). At five years, the overall follow-up rate was 90.8%, and the cumulative incidence of TLF as the primary endpoint was similar between BP-SES and DP-SES (hazard ratio [HR] 1.01, 95% confidence interval [CI]: 0.79 to 1.28), as was that for the patient-oriented composite endpoint (PoCE: all-cause death, all MI and any revascularisation) (HR 1.03, 95% CI: 0.86 to 1.23), or definite/probable stent thrombosis (ST) (HR 0.91, 95% CI: 0.70 to 1.77). Cumulative events were also similar between the six-month DAPT and 12-month DAPT groups after BP-SES implantation.
Conclusions: I-LOVE-IT 2 showed that the five-year safety and efficacy of BP-SES and DP-SES were similar, as were those between six months and 12 months of DAPT after BP-SES implantation.
Introduction
Biodegradable polymers (BP) were developed to optimise the vascular healing response to drug-eluting stents (DES) by eliminating the detrimental effects of durable polymers (DP)1,2. By virtue of the transient nature of the carrier, BP-DES were expected to elicit a more physiological vascular response in comparison to DP drug-eluting stents (DP-DES)3, as appeared to be the case in clinical investigations reporting improved healing and lower thrombogenicity with BP-DES compared with early-generation DP-DES4. However, this was not the case in a few studies comparing long-term results between BP and DP on similar stent platforms eluting the same drug. In the previously reported one-year and three-year results of the I-LOVE-IT 2 (Evaluate Safety and Effectiveness of the Tivoli DES and the Firebird DES for Treatment of Coronary Revascularization) trial, the BP sirolimus-eluting stent (BP-SES) (TIVOLI™; Essen Tech, Beijing, China) was non-inferior to the DP sirolimus-eluting stent (DP-SES) (Firebird2™; MicroPort, Shanghai, China) in the primary composite safety and efficacy endpoint of target lesion failure (TLF) at 12 months5. The BP-SES yielded similar safety and efficacy outcomes at three years versus the DP-SES6. The present analysis presents the final five-year results of the I-LOVE-IT 2 trial.
Methods
STUDY DESIGN
The design and methods of the I-LOVE-IT 2 trial (NCT01681381) have been described previously and are summarised in the following text5. In brief, this was a prospective, multicentre, randomised, assessor-blinded, non-inferiority study comparing BP-SES with DP-SES, conducted in 32 centres in China (N=2,737). Patients were eligible if they were over 18 years old and had at least one coronary lesion with stenosis of >70% in a vessel with a reference diameter of 2.5 to 4.0 mm. No restriction was placed on the total number of treated lesions, treated vessels, lesion length, or number of stents implanted. Patients with multivessel disease had to receive complete revascularisation within 30 days using the same study stents if needed. Exclusion criteria were known intolerance to a study drug, metal alloys, or contrast media; life expectancy less than one year; restenosis lesions; stent implantation within one year; left ventricular ejection fraction <40%; severe renal or hepatic dysfunction; haemodynamic instability; planned surgery within six months after the index procedure; childbearing potential within one year; clinical indications of inability to tolerate dual antiplatelet therapy (DAPT) for 12 months; inability to provide written informed consent; and participation in another trial before reaching the primary endpoint. Patients scheduled for percutaneous coronary intervention (PCI) using DES were to be enrolled with fewer exclusion criteria. A screen log was required in all centres.
RANDOMISATION AND PROCEDURES
Patients were randomly assigned to undergo PCI with either BP-SES or DP-SES in a 2:1 ratio. Patients who were randomised to the BP-SES group were additionally re-randomised to a six-month DAPT group or 12-month DAPT group in a 1:1 ratio. Randomisation was performed just after the angiogram by a web-based allocation system and was stratified by centre. After completing the required information, patients were then randomised into one of the three groups: DP-SES, BP-SES with six-month DAPT group, or BP-SES with 12-month DAPT group. Angiograms were reviewed by a blinded independent core laboratory (CCRF, Beijing, China), and all adverse events qualifying as primary and secondary endpoints were adjudicated by a blinded clinical events committee.
Balloon angioplasty and stent implantation were performed according to standard techniques; direct stenting (without previous balloon dilatation) was allowed. No mixture of types of stent was permitted for a given patient unless the operator was unable to insert the study stent, in which case crossover to another non-study device of the operator’s choice was possible. Staged procedures were permitted - defined as procedures planned at the time of the index procedure and performed within 30 days with the same type of study stent. In the case of unplanned revascularisation procedures requiring stent implantation, it was recommended that physicians use the same type of study stent.
Procedural anticoagulation was achieved with unfractionated heparin at a dose of 70 to 100 IU per kilogram of body weight, and activated clotting time was maintained at 250 seconds or above; the use of glycoprotein IIb/IIIa inhibitors was left to the operator’s discretion. A loading dose of 300 mg of aspirin and 300 mg of clopidogrel was administered before all procedures. All patients were discharged with a prescription for at least 100 mg of aspirin daily indefinitely and 75 mg of clopidogrel daily for a minimum of six months after the index procedure.
Qualitative and quantitative coronary angiography (including SYNTAX score and residual SYNTAX score) was centrally evaluated at CCRF using QAngio XA Version 7.3 Analysis Software (Medis Medical Imaging, Leiden, the Netherlands).
STUDY ENDPOINTS AND FOLLOW-UP
The primary endpoint was 12-month TLF, a composite of cardiac death, target vessel myocardial infarction (TV-MI), or clinically indicated target lesion revascularisation (TLR). Secondary endpoints included TLF components, device/lesion/procedure success rates, definite/probable stent thrombosis (ST), and a patient-oriented composite endpoint (PoCE) (including all-cause death, all MI and any revascularisation).
We defined cardiac death as any death due to an evident cardiac cause, any death related to PCI, unwitnessed death, or death from unknown causes. Spontaneous infarction was defined as a typical rise and fall of troponin or creatine kinase-myocardial band (CK-MB) fraction with at least one of the following: ischaemic symptoms, development of pathological Q-waves, ischaemic electrocardiographic changes, or pathological findings of an acute MI. For comparison with other trials using Academic Research Consortium (ARC) MI definitions, we also adjudicated MI data according to ARC definitions7. TLR was defined as revascularisation for a stenosis within the stent or within the 5 mm borders adjacent to the stent. We regarded revascularisation of the target lesion and vessel as clinically indicated if stenosis of any target lesion or vessel was at least 50% of the diameter of the vessel on the basis of quantitative coronary angiography in the presence of objective evidence of ischaemia from non-invasive or invasive testing or symptoms. We also regarded revascularisation as clinically indicated if stenosis was at least 70% of the diameter of the vessel irrespective of ischaemic signs or symptoms. Bleeding events were recorded using the Bleeding Academic Research Consortium (BARC) definition. Net adverse clinical and cerebral events (NACCE) was defined as a composite of all-cause death, MI, stroke, or major bleeding (BARC type ≥3 bleeding).
Patients were followed up by telephone or hospital visit at 1, 6, 9, and 12 months and thereafter annually for five years, as predefined in the original protocol.
STATISTICAL ANALYSIS
Sample size calculation has been reported previously5. A total of 2,790 subjects would need to be enrolled. Non-inferiority would be achieved if the upper limit of the one-sided 95% confidence interval of the difference was less than the margin.
Following the conduct of the study, based on the observed TLF rate of 6.3% and actual number per group, there was 80% power to exclude a 0.028 non-inferiority margin (margin/event ratio 44.5%, the same as originally planned).
Categorical variables are reported as counts and percentages, and between-group differences were assessed with chi-square or Fisher’s exact tests. Continuous variables are presented as means±SD and were compared with the use of a two-sample t-test. The Kaplan-Meier method was used to calculate time to clinical endpoints, and the log-rank test was used to compare between-group differences. An exploratory Cox regression analysis was used to identify demographic and clinical factors predictive of the endpoints. Unless otherwise specified, a two-sided p-value of less than 0.05 was considered to indicate statistical significance. Landmark analyses for primary and secondary endpoints were performed from randomisation to one year and from one year to five years. Statistical analysis was performed using SAS software version 9.1.3 (SAS Institute, Cary, NC, USA).
Results
A total of 2,737 Chinese patients were randomised to treatment with the BP-SES (n=1,829) or DP-SES (n=908) (Figure 1). Baseline information between groups is shown in Supplementary Table 1 and Supplementary Table 2. Overall, 167 patients (9.1%) allocated to the BP-SES group and 85 (9.4%) allocated to the DP-SES group were lost to follow-up before reaching five years, without any between-group differences. Baseline patient characteristics have been shown previously5. The two groups of patients were generally well balanced in terms of their baseline clinical and lesion characteristics5.
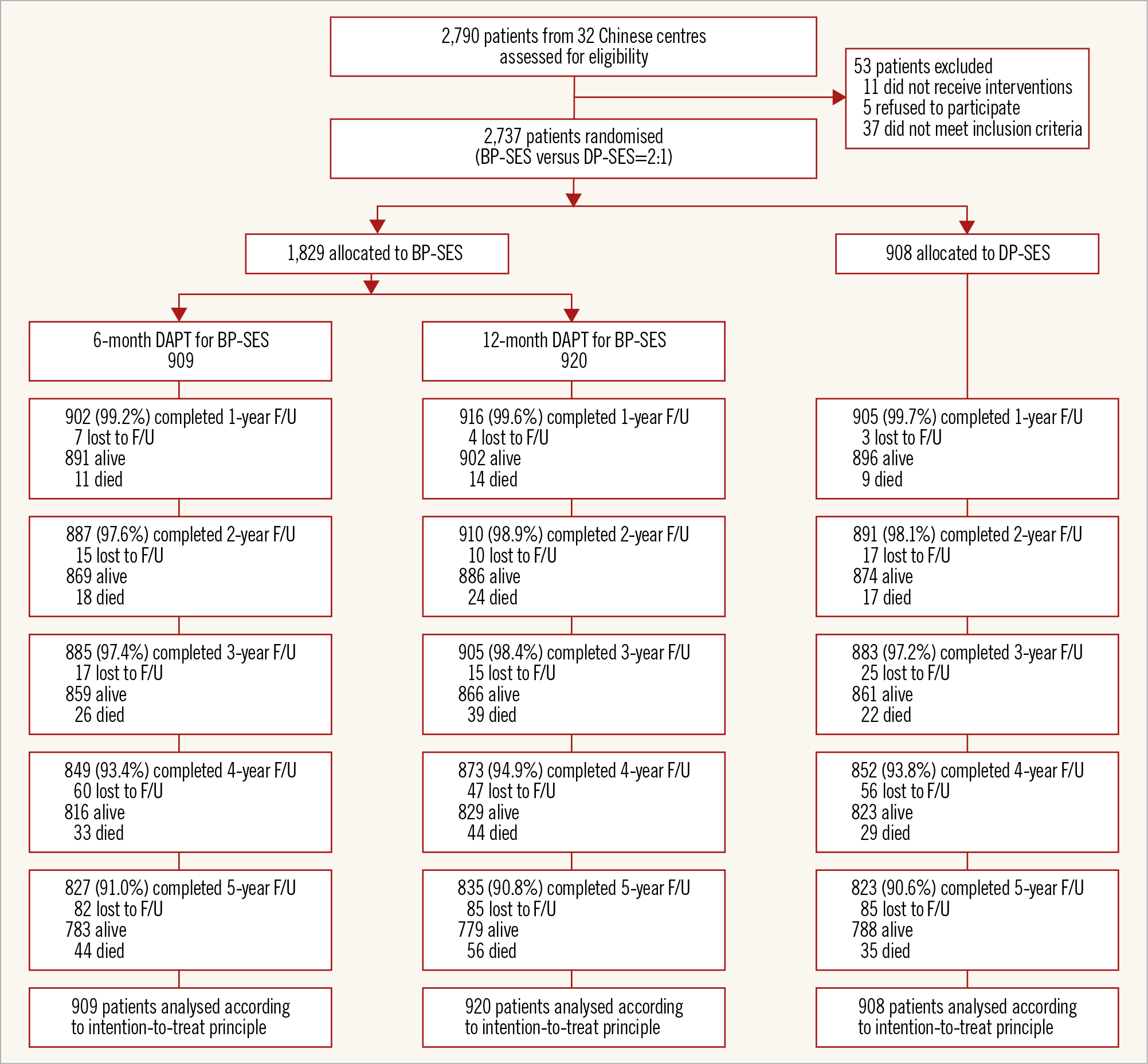
Figure 1. Patient flow. BP-SES: biodegradable polymer sirolimus-eluting stent(s); DAPT: dual antiplatelet therapy; DP-SES: durable polymer sirolimus-eluting stent(s)
At five years, the incidence of TLF as the primary endpoint was similar between the BP-SES and the DP-SES groups (11.4% vs 11.1%, p=0.89) (Table 1, Figure 2A). The secondary endpoints for the individual components of TLF, including cardiac death, TV-MI, and clinically indicated TLR, were comparable between the two arms (Figure 2B-Figure 2D). Similarly, there was no difference between the two groups in the PoCE (19.6% vs 18.8%, p=0.68) (Table 1, Figure 2E), or definite/probable ST (1.2% vs 1.4%, p=0.80) (Table 1, Figure 2F).
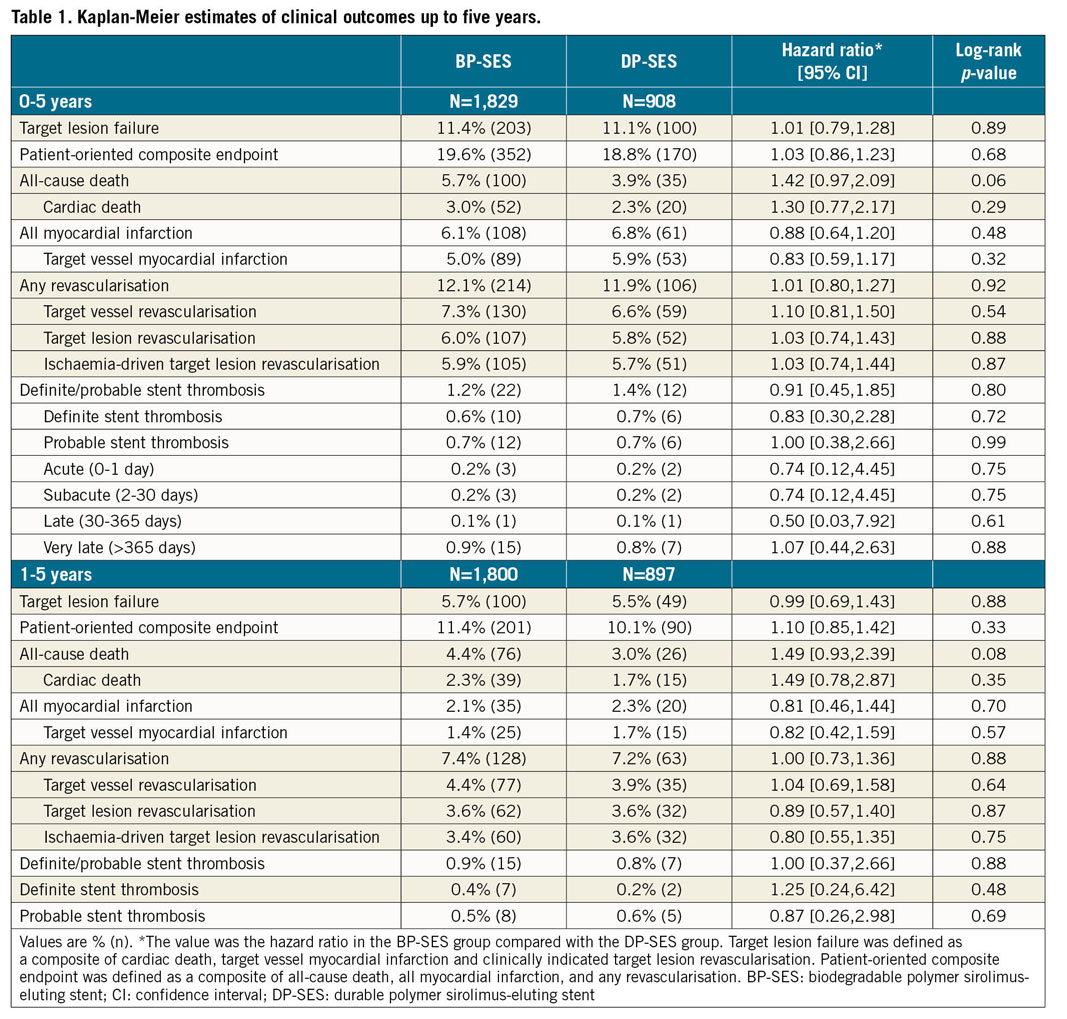
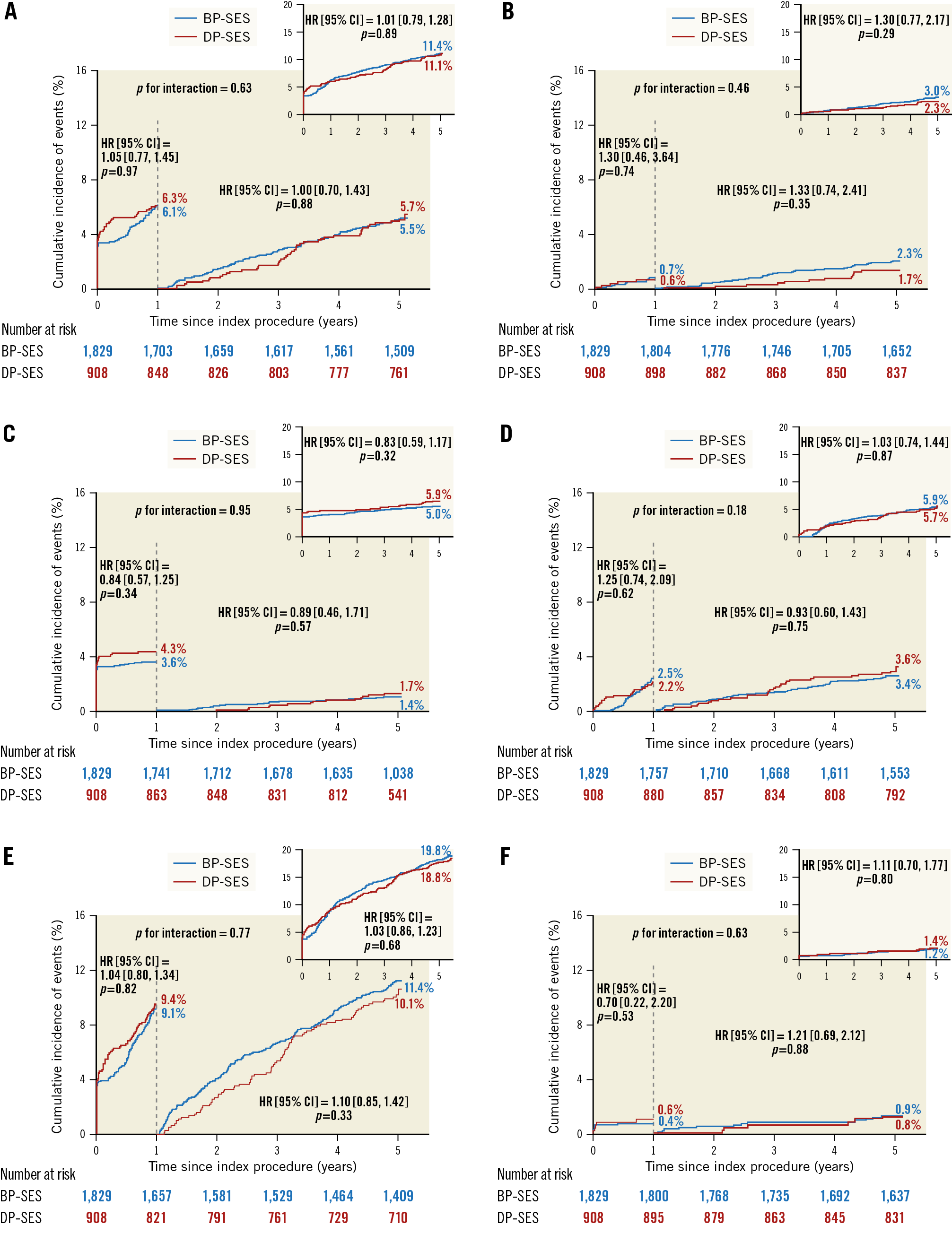
Figure 2. Kaplan-Meier estimates for TLF, PoCE and Def/Prob ST in the overall population up to five years. Cumulative incidence of target lesion failure (A), cardiac death (B), target vessel MI (C), ID-TLR (D), PoCE (E), and Def/Prob ST (F). BP-SES: biodegradable polymer sirolimus-eluting stent(s); Def/Prob ST: definite/probable stent thrombosis; DP-SES: durable polymer sirolimus-eluting stent(s); ID-TLR: ischaemia-driven target lesion revascularisation; MI: myocardial infarction; PoCE: patient-oriented composite endpoint
In a landmark analysis of the primary endpoint and its components, with the cut-off set at one year, we found no difference in the rate of late events between one and five years (Figure 2, Table 1). The very late ST rates were low, with no significant between-group differences (BP-SES vs DP-SES, 0.9% vs 0.8%, p=0.88) (Table 1, Figure 2F). Most patients discontinued DAPT after one year; cumulative event rates were similar between the six-month and 12-month DAPT groups after BP-SES implantation (Table 2, Supplementary Figure 1).
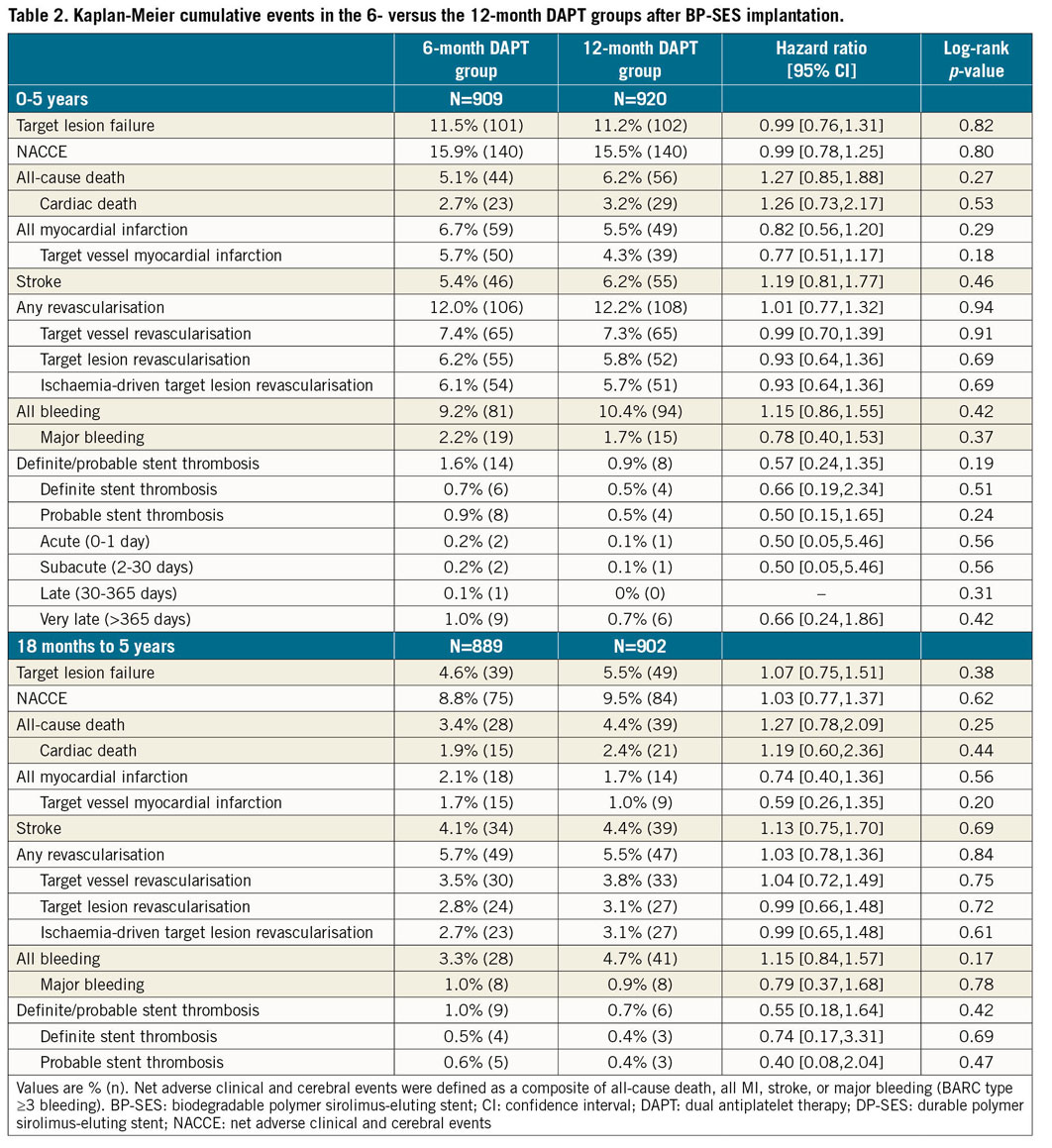
In the subgroup analysis, the incidence of TLF was not significantly different between the BP-SES and DP-SES groups for any pre-specified subgroups, except that patients with long lesions tended to benefit more from DP-SES implantation over five years (Figure 3). There was also no statistically significant heterogeneity between duration of DAPT and the occurrence of NACCE among the subgroups (Supplementary Figure 2).
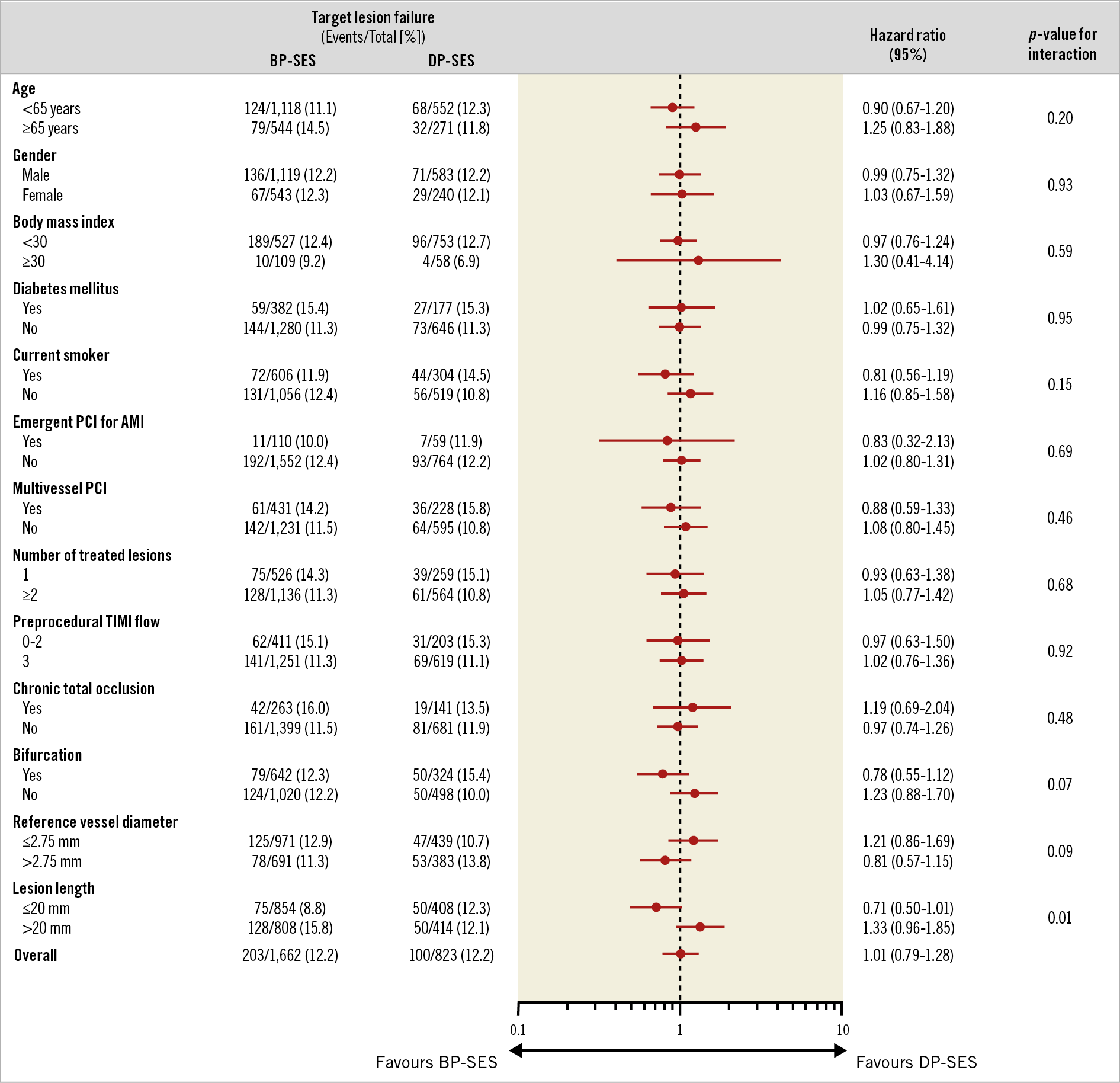
Figure 3. Subgroup analysis of the five-year TLF. Complex lesions were defined by presence of at least one of the following lesion characteristics: unprotected left main coronary artery, bifurcation, ostial lesion, total occlusion, severely tortuous or angulated lesion, and moderate to heavy calcification. AMI: acute myocardial infarction; BP-SES: biodegradable polymer sirolimus-eluting stent(s); CI: confidence interval; DP-SES: durable polymer sirolimus-eluting stent(s); PCI: percutaneous coronary intervention
Discussion
The current article is the first report on long-term follow-up data up to five years, comparing treatment with BP-coated versus DP-coated cobalt-chromium (CoCr) sirolimus-eluting stents. The major findings of this report were: (1) similar long-term safety and efficacy outcomes between BP-SES and DP-SES; (2) similar rates of TLF and its components between BP-SES and DP-SES by landmark analysis after one year; and (3) similar rates of cumulative events between the six and 12 months of DAPT groups after BP-SES implantation.
Whether there is benefit of the BP stent over the permanent polymer stent has been a topic of discussion in interventional cardiology. To the best of our knowledge, I-LOVE-IT 2 was the first study that directly compared biodegradable and durable polymer stents, which were quite similar except for slight differences in strut thickness, stent profile, coating thickness, and available stent lengths5 (Supplementary Table 3). Our long-term follow-up results have shown that BP-SES were similar to DP-SES in terms of clinical outcomes at five-year follow-up, including ST, which might firstly be explained by both devices being thin-strut CoCr DES. Previous studies had shown a similar safety profile in terms of MI or ST beyond one year for thin-strut BP-SES and DP-DES8,9; improvements in strut platform and strut thickness of new-generation DP-DES may have resulted in extended safety and efficacy outcomes that are comparable to those of BP-DES. In the multicentre, randomised EVOLVE II trial (EVOLVE II Clinical Trial to Assess the SYNERGY Stent System for the Treatment of Atherosclerotic Lesion[s]), BP-DES with thin struts demonstrated comparable outcomes to DP-DES, with low rates of ST and adverse events up to five years of follow-up10. A recent study showed that optical coherence tomography (OCT) performed at a median follow-up of seven months displayed decreased neointimal growth and a higher risk for uncovered struts after implantation of thick-strut BP-DES in comparison to new-generation DP-DES11; the subgroup analysis found that thick-strut but not thin-strut BP-DES had less neointimal hyperplasia (NIH) thickness as compared with new-generation DP-DES. Those findings confirmed that thin-strut stents allow accelerated endothelial recovery, faster integration into the vessel wall and complete re-endothelialisation in comparison with thick-strut platforms. Secondly, lesions in patients in the study were of lower complexity (mean baseline SYNTAX score 11.7), and the sample size was calculated based on primary endpoints at one year. The rates of clinical events in the study were lower than those in other similar studies12,13; a larger study with a greater proportion of patients with higher lesion complexity is warranted to elucidate finally whether BP-DES are superior to DP-DES or not.
One of the hypotheses of the study was that the combination of short-term DAPT and a BP-DES could reduce the incidence of both thrombotic events and bleeding complications. The previously reported 18-month follow-up results showed that the incidence of TLF and NACCE was similar between the six-month and 12-month DAPT groups14. The present five-year follow-up results again showed similar results for cumulative events between the six- and 12-month DAPT groups after BP-SES implantation, which was consistent with a recent study15. Some concerns arise when the present data are interpreted in daily practice. Firstly, the incidence of the primary endpoint was lower than expected, suggesting that patients in the study might represent a low-risk cohort. The mean total lesion length in both groups was around 21 mm, and average stent diameters were over 3 mm in size. Therefore, whether six-month DAPT after BP-DES implantation is feasible in high-risk patients with more complex lesions needs to be studied. Secondly, new-generation antiplatelet drugs, such as ticagrelor and prasugrel, were not used in our trial. Discontinuation of aspirin and continuation of new-generation antiplatelet drugs may be another option after BP-DES implantation, as suggested by the GLOBAL LEADERS study16. Finally, biodegradable polymer-based DES have wide-ranging differences in degradation products and kinetics, which may affect DAPT duration significantly. Therefore, the results of the study cannot be extrapolated to other biodegradable polymer DES.
Limitations
In addition to the aforementioned points, several important study limitations need to be taken into consideration. Firstly, although this study focused on comparisons between BP-DES and DP-DES, there remains limited evidence supporting the TIVOLI as a prototype BP-DES when compared with US Food and Drug Administration-approved new-generation thin-strut BP-DES. Secondly, a double-blinded design was not used for the comparison between the two DAPT strategies within the BP-DES group; early discontinuation of DAPT (0.3%) and crossing over to prolonged DAPT (6.5%) occurred in some of the patients. Third, 58% of patients presented with unstable angina. In recent years, because high-sensitivity cardiac troponin assays have improved the detection of acute MI, the number of patients labelled as having non-ST-elevation myocardial infarction (NSTEMI) instead of unstable angina is increasing. Fourth, all patients, including those with acute coronary syndrome (ACS), were treated with clopidogrel, not with more potent antiplatelet agents. Fifth, differences in the available lengths of the two stents may have had effects on clinical outcomes. Finally, all patients enrolled in the present study were Chinese, so generalisability of the study findings needs to be proved in future studies.
Conclusions
I-LOVE-IT 2, the largest randomised trial of a cardiovascular device in China, has shown similar five-year safety and efficacy for BP-SES and DP-SES, and also for patients who received six or 12 months of DAPT after BP-SES implantation. The present long-term analysis adds valuable new data to the evidence pool of randomised comparisons between BP-DES and DP-DES, and shorter versus standard duration of DAPT.
|
Impact on daily practice BP-SES and DP-SES have similar long-term safety and efficacy outcomes, similar rates of TLF and its components between BP-SES and DP-SES by landmark analysis after one year, and similar rates of cumulative events between six- and 12-month DAPT groups after BP-SES implantation. |
Funding
The study was sponsored by Essen Technology (Beijing, China) and was also supported by the National Key Technology R&D Program in the 12th Five-Year Plan of China (2011BAI11B07) and the Key Project of the National 12th Five-Year Research Program of China (2012ZX093016 - 002).
Conflict of interest statement
The authors/study collaborators have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

