Abstract
Aims: Arterial plaque rupture and thrombus characterise ST-elevation myocardial infarction (STEMI) and may aggravate delayed arterial healing following durable polymer drug-eluting stent (DP-DES) implantation. Biodegradable polymer (BP) may improve biocompatibility. We compared long-term outcomes in STEMI patients receiving BP-DES vs. durable polymer sirolimus-eluting stents (DP-SES).
Methods and results: We pooled individual patient-level data from three randomised clinical trials (ISAR-TEST-3, ISAR-TEST-4 and LEADERS) comparing outcomes from BP-DES with DP-SES at four years. The primary endpoint (MACE) comprised cardiac death, MI, or target lesion revascularisation (TLR). Secondary endpoints were TLR, cardiac death or MI, and definite or probable stent thrombosis. Of 497 patients with STEMI, 291 received BP-DES and 206 DP-SES. At four years, MACE was significantly reduced following treatment with BP-DES (hazard ratio [HR] 0.59, 95% CI: 0.39-0.90; p=0.01) driven by reduced TLR (HR 0.54, 95% CI: 0.30-0.98; p=0.04). Trends towards reduction were seen for cardiac death or MI (HR 0.63, 95% CI: 0.37-1.05; p=0.07) and definite or probable stent thrombosis (3.6% vs. 7.1%; HR 0.49, 95% CI: 0.22-1.11; p=0.09).
Conclusions: In STEMI, BP-DES demonstrated superior clinical outcomes to DP-SES at four years. Trends towards reduced cardiac death or myocardial infarction and reduced stent thrombosis require corroboration in specifically powered trials.
Introduction
Biodegradable polymer-based drug-eluting stents (BP-DES) provide controlled drug release followed by degradation of the polymer coating, eventually rendering the stent surface similar to that of a bare metal stent (BMS). This design has been hypothesised to reduce the incidence of late adverse events, which have been linked to durable polymer coatings in some patients1,2. Studies of BP-DES have demonstrated safety and long-term efficacy versus durable polymer DES in a broadly inclusive patient population, with a reduction in very late definite stent thrombosis seen up to four years2.
Patients with ST-segment elevation myocardial infarction (STEMI) have poorer outcomes after percutaneous coronary intervention (PCI) compared to those with stable coronary artery disease, with higher rates of stent thrombosis and increased risk of reinfarction persisting at long-term follow-up3,4. Although PCI with DES improves efficacy outcomes in patients with STEMI, maintenance of superior safety outcomes in the long term has not yet been conclusively demonstrated in comparison to BMS in STEMI, even for newer-generation DES5-8. Late adverse events have been linked to an inflammatory reaction and delayed arterial healing – at least in part due to durable polymer coatings – and the extent of this problem may be more pronounced in the setting of STEMI.
Consequently, the definition of the optimal DES device in STEMI remains unresolved and the search for ideal DES therapy for this condition continues. In this pooled analysis we aimed to evaluate long-term performance of BP-DES in comparison to durable polymer DES in a high-risk subset of patients with STEMI.
Methods
PATIENT POPULATION AND DEVICE DESCRIPTION
We performed a patient-level pooled analysis of the three largest multicentre, randomised clinical trials comparing biodegradable polymer DES with a leading first-generation durable polymer sirolimus-eluting stent (SES) for coronary revascularisation (the ISAR-TEST-3 trial [ClinicalTrials.gov identifier: NCT00350454]9, the ISAR-TEST-4 trial [ClinicalTrials.gov identifier: NCT00598676]10 and the LEADERS trial [ClinicalTrials.gov identifier: NCT00389220]11) and analysed outcomes in the subset of patients with STEMI. From an overall population of 4,062 patients in the original trials, a total of 497 patients with STEMI were included in the present analysis: 291 of these were randomly allocated to treatment with biodegradable polymer DES (either Yukon PC Choice, backbone produced by Translumina, Hechingen, Germany, or BioMatrix Flex; Biosensors Inc., Newport Beach, CA, USA), and 206 were allocated to treatment with durable polymer SES (Cypher Select; Cordis, Miami Lakes, FL, USA). The Yukon PC Choice stent comprised a pre-mounted, sandblasted microporous 316L stainless steel stent coated with sirolimus and a polylactate polymer which fully degraded within nine weeks; the BioMatrix Flex stent is a stainless steel stent with an abluminal coating of biolimus and a polylactate polymer degrading fully within six months. Detailed descriptions of the design of the three trials are reported in the primary publications9-11. A summary of the principal trial characteristics was reported previously12. Patients were followed up clinically up to four years after enrolment by the investigating sites.
PROCEDURAL AND DISCHARGE MEDICATION
In all three trials, an oral loading dose of 300-600 mg clopidogrel was administered before or at the time of the PCI procedure. During the procedure, all patients received unfractionated heparin or bivalirudin. The use of glycoprotein IIb/IIIa antagonists was left at the discretion of the operators. On discharge, all patients were prescribed aspirin at least 75 mg daily indefinitely and clopidogrel 75 mg daily for at least 12 months.
ENDPOINTS AND DEFINITIONS
The pre-specified primary endpoint was the occurrence of major adverse cardiac events (MACE), defined as the composite of cardiac death, myocardial infarction (MI), and target lesion revascularisation (TLR). Secondary endpoints were TLR (efficacy endpoint), the composite of MI or cardiac death and definite or probable stent thrombosis (safety endpoint). Cardiac death was defined as death due to immediate cardiac causes or complications related to the procedure, as well as any death in which a cardiac cause could not be excluded. Definitions of MI were consistent across the pooled trials; however, in the ISAR-TEST-4 trial (and in patients included from ISAR-TEST-3) target-vessel MI was adjudicated, whereas in the LEADERS trial any MI was included. TLR was defined as any clinically indicated repeat revascularisation (percutaneous or surgical) of the target lesion. The definition of clinically indicated revascularisation was consistent across the included trials. Stent thrombosis was defined according to the Academic Research Consortium criteria in all studies13.
TRIAL QUALITY ASSESSMENT
All trials were assessed for bias using components recommended by the Cochrane Collaboration, including: sequence generation of the allocation; allocation concealment; blinding of participants, personnel, and outcome assessors; selective outcome reporting; and other sources of bias14. Trials with high or unclear risk for bias for any one of the first three components were considered as having high risk of bias. Otherwise, they were considered as having low risk of bias.
STATISTICAL ANALYSIS
Categorical data are presented as counts and proportions (%). Continuous data are presented as mean (±SD) or median [25th-75th percentiles]. Individual patient data were pooled and analysed according to intention to treat. Survival analysis was performed using the Mantel-Cox method stratified by the trial. Fulfilment of the proportional hazards assumption was checked before each analysis. Trials in which the event of interest was not observed in either treatment group were omitted from the analysis of that event. In the event that only one of the treatment groups from a trial had no event of interest, then the estimated treatment effect estimate and its standard error were calculated after adding 0.5 to each cell of the 2×2 table for that trial15. Cochrane tests were used to assess heterogeneity across trials. Consistency between trials was measured by calculating the I2 statistic - with values of 25, 50, and 75% indicating low, moderate, and high inconsistency, respectively16. Results were considered statistically significant at two-sided p<0.05. Statistical analysis was performed using the Stata software package, version 9.2 (StataCorp, College Station, TX, USA). Survival curves are presented as simple, non-stratified Kaplan-Meier curves and were constructed with the use of S-Plus software version 4.5 (Insightful Corporation, Seattle, WA, USA).
Results
From a total of 4,062 enrolled patients in the original trials, 2,358 received BP-DES and 1,704 DP-SES. In total, 497 patients with STEMI were included in the present analysis, of whom 291 received BP-DES and 206 received durable polymer SES. A summary of included trials is shown in Table 1.
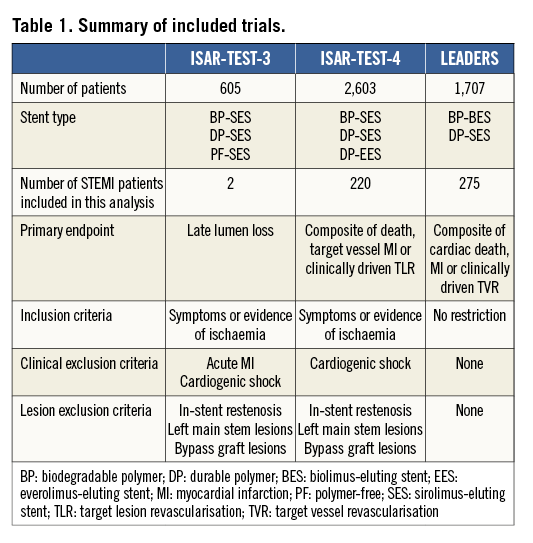
Patient characteristics were broadly similar; however, those receiving biodegradable polymer DES had a lower prevalence of hypercholesterolaemia, a greater prevalence of multivessel disease at baseline and a lower percentage diameter stenosis immediately post intervention (Table 2). Clinical outcomes up to four years as well as landmark analyses are summarised in Table 3. There was no heterogeneity across trials and no interaction between treatment effect of BP-DES and trial origin for any of the endpoints analysed. At four years, the incidence of MACE was significantly reduced with BP-DES versus durable polymer SES (14.2% vs. 23%; HR 0.59, 95% CI: 0.39-0.90; p=0.01) (Figure 1). This was driven by a reduction in target lesion revascularisation (7.4% vs. 13.1%; HR 0.54, 95% CI: 0.30-0.98; p=0.04) (Figure 2) and a trend for reduction in cardiac death or MI (9.5% vs. 15.0%; HR 0.63, 95% CI: 0.37-1.05; p=0.07) (Figure 3). Stent thrombosis was numerically lower in those receiving BP-DES; however, this did not reach statistical significance for definite stent thrombosis (2.5% vs. 5.5%; HR 0.44, 95% CI: 0.17-1.14; p=0.08) or definite or probable stent thrombosis (3.6% vs. 7.1%; HR 0.49, 95% CI: 0.22-1.11; p=0.09) (Figure 4).
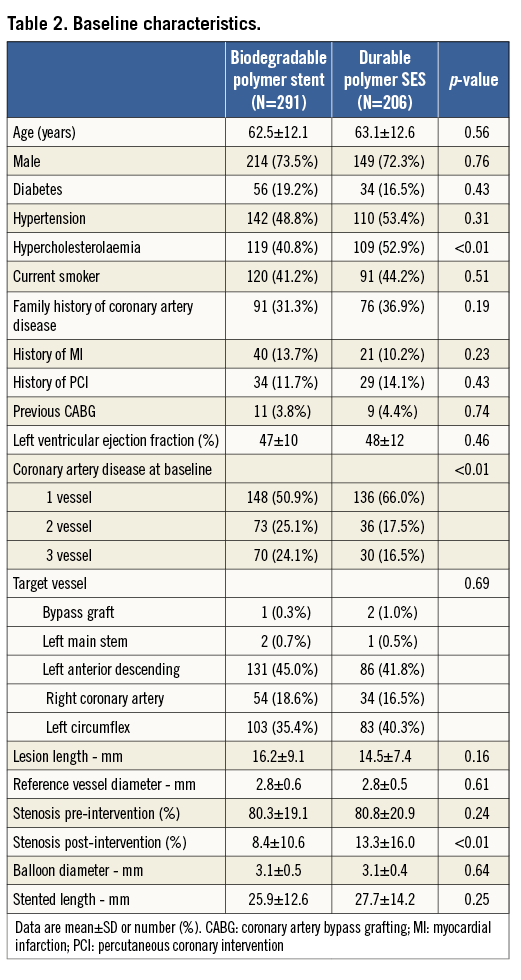
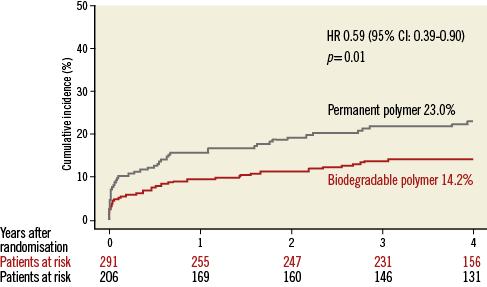
Figure 1. Kaplan-Meier curves for cardiac death, myocardial infarction or target lesion revascularisation for the pooled population in each of the treatment groups. CI: confidence interval; HR: hazard ratio
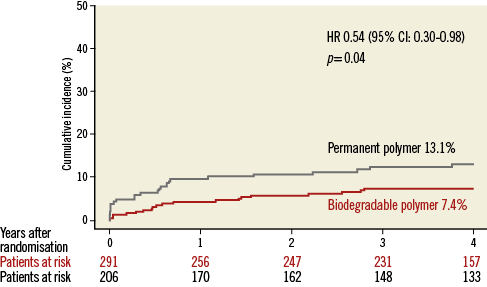
Figure 2. Kaplan-Meier curves for target lesion revascularisation for the pooled population in each of the treatment groups. CI: confidence interval; HR: hazard ratio
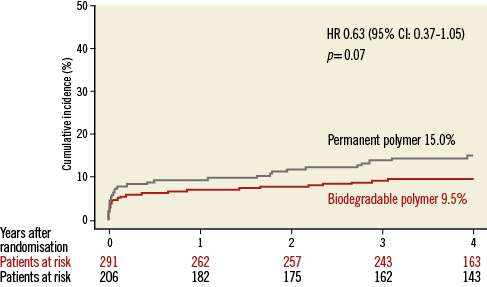
Figure 3. Kaplan-Meier curves for cardiac death or myocardial infarction for the pooled population in each of the treatment groups. CI: confidence interval; HR: hazard ratio
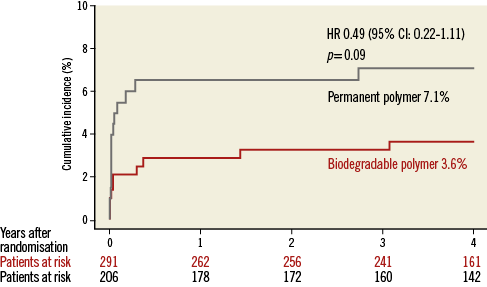
Figure 4. Kaplan-Meier curves for definite or probable stent thrombosis for the pooled population in each of the treatment groups. CI: confidence interval; HR: hazard ratio
Discussion
In the present study, we analysed individual patient data from three randomised trials comparing biodegradable polymer DES with the leading first-generation durable polymer SES in 497 patients with STEMI up to four years. The main finding was that treatment with biodegradable polymer DES significantly improved clinical outcomes. All components of MACE favoured the BP-DES: TLR demonstrated a significant reduction and cardiac death or MI showed a trend towards reduction. Although stent thrombosis was numerically lower following BP-DES, the reduction seen in this group did not achieve statistical significance.
DES achieve greater antirestenotic efficacy in comparison with bare metal stents at the cost of delayed healing of the treated arterial segment17-19. Pathological studies have shown evidence of a persistent inflammatory response in the arterial wall, and durable polymer coatings have been heavily implicated in the aetiology of this response in some patients18,19. Delayed arterial healing following DES is a pathophysiological process characterised by persistent fibrin deposition, inflammatory cell infiltration, ongoing platelet activation and delayed endothelial regrowth and has been associated with adverse vessel remodelling, stent thrombosis and ongoing late restenosis17-19.
Numerous reports have highlighted STEMI as a risk factor for late reinfarction and late stent thrombosis following primary intervention3,4,20. This is thought to be due to a combination of factors including adverse lesion morphology and high thrombus burden, persistent inflammation and increased platelet reactivity, as well as chronic processes associated with delayed healing and vessel remodelling21. Additionally, intravascular imaging following DES implantation in STEMI has shown that at follow-up a higher proportion of struts remains uncovered compared to BMS22-24 and that incomplete DES apposition25,26 is observed more frequently, a finding that appears to indicate adverse vessel remodelling and confer a higher risk of subsequent stent thrombosis.
Given this background, BP-DES may represent an attractive solution for patients with STEMI, as complete polymer biodegradation after drug elution is expected to result in a vessel wall-stent interaction resembling that seen after bare metal stenting. Intravascular imaging studies have shown encouraging data regarding vessel healing following biodegradable polymer DES implantation in man27,28. Moreover, in a broadly inclusive patient cohort, BP-DES demonstrated a reduction in clinically indicated target lesion revascularisation and very late definite stent thrombosis up to four years in comparison to durable polymer SES12, an effect potentially magnified in patients with STEMI.
Our study represents the first analysis of long-term outcomes in patients with STEMI treated with BP-DES and the first specific comparison of BP-DES and durable polymer DES in STEMI. There are several significant findings. First, although biodegradable polymer DES have been hypothesised to offer improved outcomes mainly in the long term, differences in stent thrombosis are evident early, and analysis shows a significant difference in MACE and TLR already evident at one year and persisting up to four years. Although the reduction in MACE of 42% seen at one year in comparison to durable polymer SES was similar to the margin demonstrated by a biodegradable polymer biolimus-eluting stent in comparison to a BMS seen in the recent COMFORTABLE AMI trial (4.3% vs. 8.7%; p<0.01)29, this does not help to explain the onset of the difference or to define the role of biodegradable polymer in this regard. Although polymer may fully biodegrade in as little as nine weeks, it is difficult to attribute all of the observed effects to biodegradable polymer in isolation, and other factors may play a role.
Second, rates of stent thrombosis were numerically lower in those treated with biodegradable polymer DES, although this narrowly failed to reach statistical significance at one or four years, a finding similar to that demonstrated in the STEMI subgroup of the COMFORTABLE AMI trial at one year29.
Third, the reduction seen regarding MACE in patients with STEMI treated with BP-DES was driven by a reduction in TLR and a trend for reduced death or MI. In actual fact, the absolute magnitude of the treatment effects was greater than that seen in the overall study cohort12. This suggests that new-generation BP-DES with enhanced biocompatibility may have particular benefit in the high-risk subgroup of patients who present with STEMI and who undergo primary percutaneous coronary intervention.
STUDY LIMITATIONS
The present study has several limitations. First, although the three source studies were all randomised, they were not specifically designed for STEMI patients and did not perform stratified randomisation according to the presence of STEMI. As a post hoc analysis, the results should be considered hypothesis-generating.
Second, the present study comprises a large-scale comparison of biodegradable polymer versus durable polymer DES in patients with STEMI; however, the sample size remains inadequate to determine statistical significance of differences in incidence of rare clinical outcomes between the two treatment groups.
Third, even though inclusion criteria were broad across all three included trials, there remained slight differences in the characteristics of patients enrolled in the individual trials. Despite this, our analysis showed no evidence of any heterogeneity across the trials. Fourth, the comparator SES was the leading stent of its generation at the time when the studies were conducted. Thus, the comparative efficacy results cannot be extrapolated to current DES platforms using other limus family drugs and different polymers.
Finally, the study not only compared biodegradable and durable polymers but also three separate stent platforms with differences which may in part explain the observed outcomes. Although the lack of interaction between BP-DES treatment effect and trial origin suggests clinical homogeneity and both biodegradable polymer stents contain polylactic acid monomer coatings and elute limus-agent drugs, differences in polymer degradation, drug efficacy and stent backbone mean that significant differences in their relative performance may be expected.
Conclusion
In the current pooled analysis of patients with STEMI undergoing PCI, biodegradable polymer DES compared to durable polymer SES demonstrated improved overall clinical outcome, reduced need for revascularisation as well as trends towards lower incidence of cardiac death or MI and reduced stent thrombosis at long-term follow-up. These findings support the use of biodegradable polymer DES in patients with STEMI but should be confirmed in dedicated randomised controlled trials against leading new-generation comparators.
| Impact on daily practice The choice of stent implanted in patients undergoing PCI for STEMI affects arterial healing and influences clinical outcomes. Biodegradable polymer DES provide, when used in this high-risk setting, high clinical efficacy and good patient safety which remain comparable to durable polymer DES out to the long term. Dedicated randomised controlled trials with long-term follow-up trials are required to investigate the observed trend towards a reduction in stent thrombosis and cardiac death or MI with biodegradable polymer DES in STEMI. |
Guest Editor
This paper was guest edited by Morton J. Kern, MD, FSCAI, FAHA, FACC, Long Beach Veterans Administration Hospital, Long Beach, CA, USA, and University of California, Irvine, CA, USA.
Funding
The ISAR-TEST-3 and ISAR-TEST-4 trials were funded in part by the Bavarian Research Foundation (BFS-ISAR Aktenzeichen AZ: 504/02 and BFS-DES Aktenzeichen AZ: 668/05). The LEADERS trial was funded by Biosensors Europe SA, Switzerland. Funding for the current analysis was provided in part by a grant of the Swiss National Science Foundation to S. Windecker (number 33CM30-140336) and in part by the DZHK (German Centre for Cardiovascular Research) and BMBF (German Ministry of Education and Research) as part of the Munich Heart Alliance. G. Stefanini is the recipient of a research fellowship (SPUM) funded by the Swiss National Science Foundation.
Conflict of interest statement
B. Meier has received educational and research support to the institution from Abbott, Cordis, Boston Scientific, and Medtronic. A. Kastrati holds a patent related to the stent coating used on the biodegradable polymer DES studied in the ISAR-TEST-3 and ISAR-TEST-4 trials, and reports having received lecture fees from Abbott, Biotronik, Cordis and Medtronic. S. Windecker has received research contracts to the institution from Abbott, Biosensors, Biotronik, Boston Scientific, Cordis, Medtronic, and St. Jude. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

