Abstract
Aims: Second-generation everolimus-eluting stents (EES) are safer and more efficient than first-generation paclitaxel-eluting stents (PES). Third-generation biolimus-eluting stents (BES) have been found to be non-inferior to PES. To date, there is no available comparative study between EES and BES. We aimed to investigate the safety and efficacy of BES with biodegradable polymer compared to EES with durable polymer at a follow-up of two years in an unselected population of consecutively enrolled patients.
Methods and results: A group of 814 consecutive patients undergoing percutaneous coronary intervention (PCI) was enrolled between 2007 and 2010, of which 527 were treated with EES and 287 with BES implantation. Clinical outcome was compared in 200 pairs using propensity score matching. The primary endpoint was a composite of death, myocardial infarction (MI) and target vessel revascularisation (TVR) at two-year follow-up. Median follow-up was 22 months. The primary outcome occurred in 11.5% of EES and 10.5% of BES patients (HR 1.11, 95% CI: 0.61-2.00, p=0.74). At two years, there was no significant difference with regard to death (HR 0.49, 95% CI: 0.18-1.34, p=0.17), cardiac death (HR 0.14, 95% CI: 0.02-1.14, p=0.66) or MI (HR 6.10, 95% CI: 0.73-50.9, p=0.10). Stent thrombosis (ST) incidence was evenly distributed between EES (n=2) and BES (n=2) (p-value=1.0).
Conclusions: This first clinical study failed to demonstrate any significant difference regarding safety or efficacy between these two types and generations of drug-eluting stents (DES).
Abbreviations
BES: biolimus-eluting stent
DES: drug-eluting stent
EES: everolimus-eluting stent
MACE: major adverse cardiac events
MI: myocardial infarction
PCI: percutaneous coronary intervention
TLR: target lesion revascularisation
TVR: target vessel revascularisation
Introduction
Drug-eluting stents (DES) lower neointimal hyperplasia and subsequent revascularisation in comparison to bare metal stents (BMS). However, first-generation DES are associated with a low but significant risk of late occurring clinical events such as stent thrombosis or restenosis. These delayed complications are linked to certain DES components responsible for chronic inflammatory reactions. Therefore, it is considered that changes in key components of first-generation DES might diminish local inflammatory reactions and thus decrease the risk of late events.
Everolimus-eluting stents (EES) – a second-generation DES – are characterised by thinner stent struts and a lower amount of drug released through a durable polymer when compared to paclitaxel-eluting stents (PES). Two large randomised studies (SPIRIT IV and COMPARE) have proved EES superiority to PES, and one propensity score matched registry (LESSON-1) has shown a trend towards a lower risk of the patient-oriented safety and efficacy endpoint of death, MI and TVR as compared to sirolimus-eluting stents (SES) during follow-up to three years1-3.These three trials showed a significant reduction in definite and probable stent thrombosis during long-term follow-up according to the definitions of the Academic Research Consortium (ARC)1,4. Other recently published trials, such as ISAR-TEST-4 or EXCELLENT, have proved the non-inferiority of EES in terms of safety and efficacy when compared with SES at three years and nine months, respectively5,6. EES is currently the most used DES in the USA and in Europe7.
Similarily, biolimus-eluting stents (BES) – a third-generation DES – are characterised by a bioabsorbable abluminal polymer coating. It is reported that the polylactic polymer is completely converted to lactic acid by six months and, via the Krebs cycle, to carbon dioxide and water by six to nine months. BES induce less neointimal proliferation than first-generation PES and were non-inferior for clinical endpoints at nine months in the randomised controlled NOBORI trial8. Moreover, in the four-year follow-up LEADERS trial, BES were demonstrated to be non-inferior to first-generation SES and associated with a reduced risk of cardiac events associated with very late ST when compared to SES9-12.
To date, no study has directly assessed the safety and efficacy of EES versus BES in real life practice. We performed a propensity-matched analysis to compare the outcomes of consecutively enrolled patients who were treated with implantation of EES and BES over two years, trying to demonstrate that no difference in mortality, myocardial infarction or TVR existed between these types of stent.
Methods
STUDY POPULATION AND DATA COLLECTION
A total of 527 consecutive patients were treated with EES (XIENCE V; Abbott Vascular, Santa Clara, CA, USA, or Promus®; Boston Scientific, Natick, MA, USA) between January 2007 and November 2010, whereas 287 consecutive patients underwent treatment with BES (Nobori®; Terumo Corporation, Tokyo, Japan, or BiomatrixTM; Biosensors International, Singapore) between March 2008 and November 2010 at our institution in one single cathlab. All patients having received at least one other stent (crossover with study or non-study stent), patients included in the COMPARE-2 (NCT01233453) trial and those with a clinical follow-up of less than 12 months were excluded in order to decrease events non-attributable to the studied devices. Stent selection in the individual patient was left entirely to the operator’s discretion and therefore depended solely upon his routine. No indications favouring the implantation of EES rather than BES or vice versa were present. A prospective clinical follow-up was completed in the first 300 patients treated with EES and in the first 200 patients treated with BES (Figure 1). The study complied with the Declaration of Helsinki and was approved by the local ethics committee at Fribourg University & Hospital, Switzerland (003-REP-CER-FR). Written and informed consent for prospective follow-up was obtained from every patient.
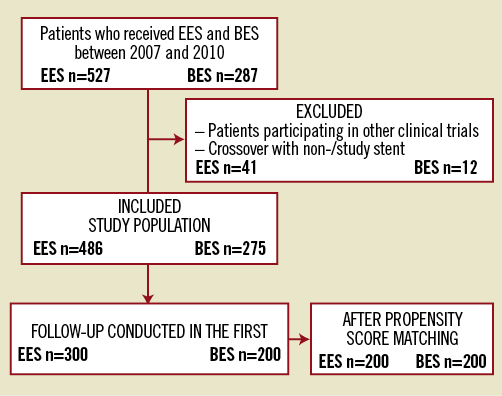
Figure 1. Flowchart illustrating patient exclusion and inclusion. BES: biolimus-eluting stent; EES: everolimus-eluting stent
Patients were clinically followed for at least two years. Information regarding clinical status was collected at clinic visits or by telephone interview. No blinding was present during the collection of outcome data. When the patient was not accessible, data were retrieved from the referring physician or hospital electronic database. The institutional angiographic and imaging core laboratories at Fribourg University reviewed the cases. Events were adjudicated by the local events adjudication committee. Event adjudication was blinded for stent type. Every event was reviewed in detail using the medical record; if uncertainties were present, the case was reviewed with the responsible physician at the time the event occurred.
Procedures
The treatment guidelines, including periprocedural and post-procedural medication regimens, were carried out according to current practice guidelines and did not change between the inclusion of the first and last patients of both EES and BES cohorts. All patients received a 600 mg clopidogrel loading dose during the procedure and were prescribed lifelong aspirin once daily as well as clopidogrel for 12 months. The use of glycoprotein IIb/IIIa antagonists was at the discretion of the operator. Creatinine kinase (CK), CK-MB, and troponin I were routinely assessed at baseline and four to six hours post-PCI as was a 12-lead electrocardiogram (ECG). Biomarkers were sampled every six to eight hours in patients with signs of ischaemia until identification of peak levels. IVUS-guided stenting was not routinely performed at our institution.
Definitions
The primary endpoint was the composite of death, MI, and target vessel revascularisation (TVR) up to a maximum two-year follow-up. Hypercholesterolaemia was defined according to the Adult Treatment Panel III13. Heart failure was classified as being either present or absent according to the clinical definition of the New York Heart Association14. Acute coronary syndromes (ACS) were defined according to the consensus paper from the ESC–ACC–AHA–WHF Joint Force15. Cardiogenic shock was defined as sustained hypotension (systolic blood pressure [BP] <90 mmHg lasting >30 minutes) accompanied by signs of tissue hypoperfusion in the setting of clinically adequate or elevated left ventricular filling pressures16. The definition of cardiac death included any death due to immediate cardiac cause, procedure-related deaths, and death of unknown cause. The diagnosis of Q-wave MI required ischaemic signs or symptoms and new pathological Q-waves in ≥2 contiguous ECG leads. In the absence of Q-waves, the diagnosis of MI was based on an elevation of CK to higher than twice the upper reference limit and elevation of CK-MB or troponin to higher than the upper reference limit. Periprocedural MI was defined as troponin or CK-MB elevation of at least three times URL during intervention or in the subsequent 48 hours after PCI, and was included in the total number of MI.
TVR was defined as repeat revascularisation of any segment within the entire major coronary vessel proximal and distal to a target lesion. Target lesion revascularisation (TLR) was defined as revascularisation for a stenosis within the stent or the 5 mm borders adjacent to the stent. ST was defined according to Academic Research Consortium (ARC) definitions4. MACE were equally defined according to the ARC definitions and were therefore the composite of cardiac death, non-fatal MI and TVR. Of note, staged procedures performed >3 months after the index procedure were considered as revascularisation.
Statistical analysis
This was a propensity score (PS) matched analysis. Analyses were performed using SPSS software 18.0 (SPSS Inc., Chicago, IL, USA).
A power analysis revealed that, assuming a small effect size and an alpha of 0.05, a two-sample comparison of proportions with 200 patients in each group would yield a power of 34%. We compared baseline characteristics between patients treated with EES and BES using a chi-square test for categorical variables and an unpaired t-test for continuous variables with a normal distribution and non-parametric tests such as the Wilcoxon rank sum test for continuous variables with a non-Gaussian distribution. We then used PS matching to account for differences in baseline characteristics. A PS for receiving EES was estimated using a probit model including age, gender, and pre-treatment variables associated with stent selection in the multivariable model at p<0.10 as independent variables (hypercholesterolaemia, heart failure and a history of previous PCI). The matching balance of covariates was then assessed using standard chi-square algorithms. The multivariable model was computed using the forward stepwise selection. In the matching procedure we used the caliper matching approach that randomly selected a patient treated with EES with one treated with BES from the pool of patients within a caliper of ±0.05 on the propensity score. We used Cox proportional hazard models that accounted for the 1:1 matching, including all variables that significantly differed between the two groups in order to calculate hazard ratios (HR) comparing the two stents, and in that way tried to adjust for confounders of the relation between stent choice and clinical outcomes. All p-values and 95% confidence intervals (CIs) are two-sided.
Results
BASELINE PATIENT CHARACTERISTICS
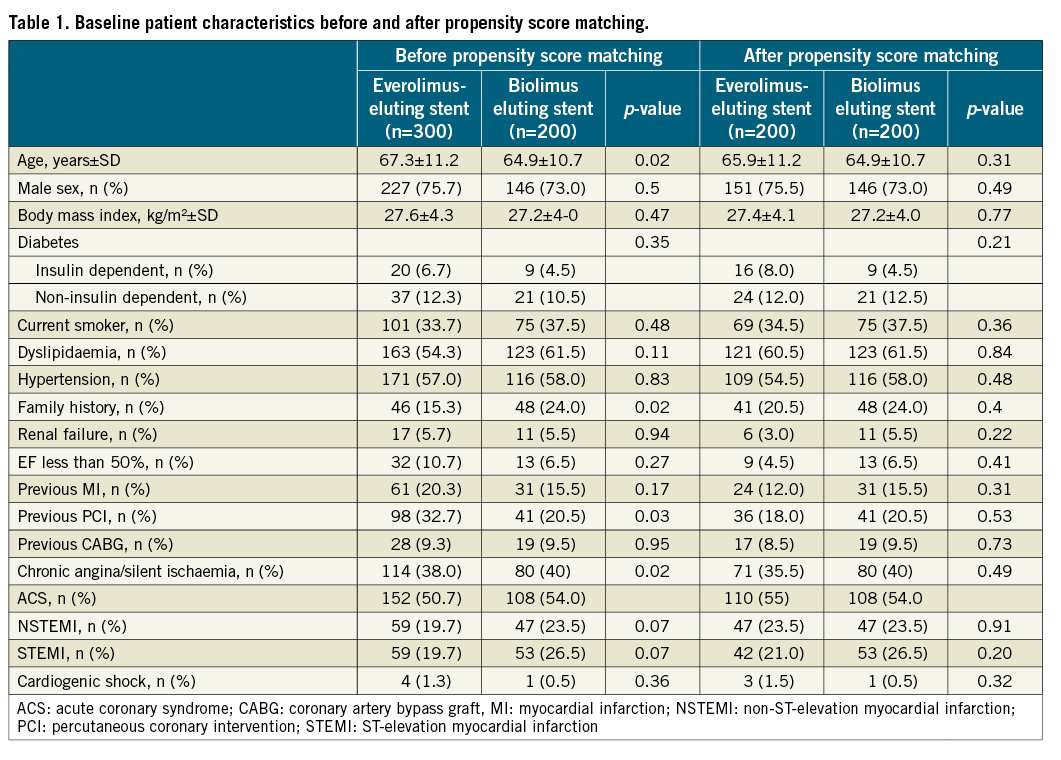
Baseline patient characteristics before and after PS are summarised in Table 1. Of 200 patients who received a BES, the Biomatrix stent was implanted in 148 (74%) and the Nobori stent was implanted in 52 (26%) patients. Before PS matching, EES patients were older (67.3±11.2 vs. 64.9±10.7 years, p=0.02), presented less family history of coronary artery disease (CAD) (15.3% vs. 24.0%, p=0.02), were more prone to heart failure (11.7% vs. 3.0%, p=0.001) and more frequently had a history of previous PCI (32.7% vs. 20.5%, p=0.03) than BES. No statistically significant differences remained after PS matching.
BASELINE LESION AND PROCEDURAL CHARACTERISTICS
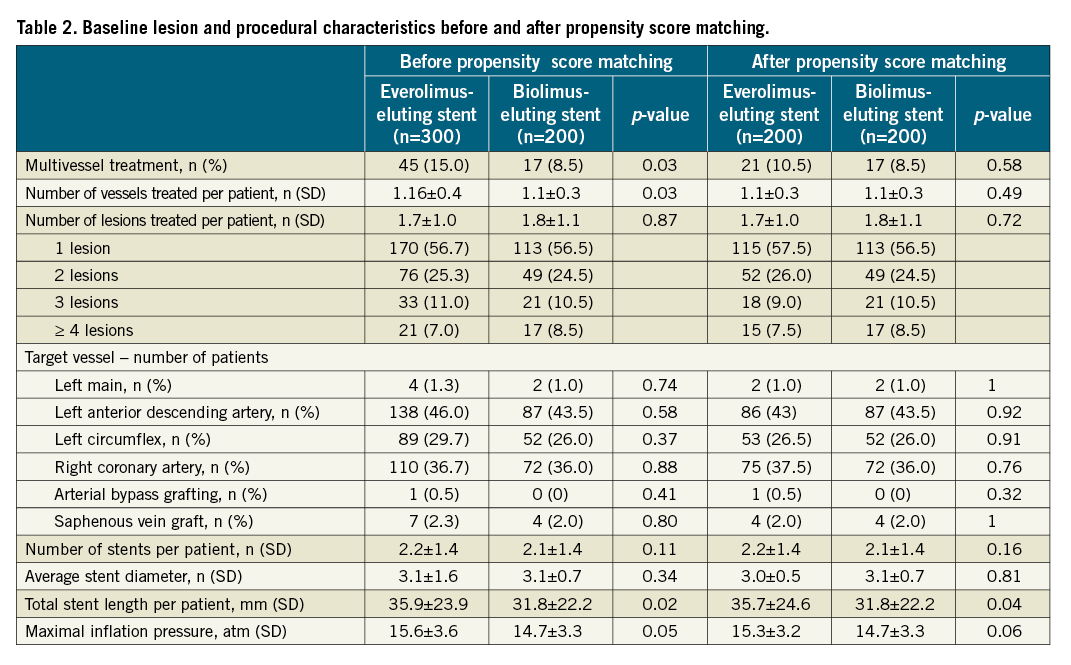
Baseline lesion and procedural characteristics are summarised in Table 2. The two groups were well balanced according to the number of lesions and vessels treated, rate of multivessel treatment, target vessel distribution, number of stents implanted and average stent diameter. However, a small but significant difference was seen in total stent length (EES: 35.7±24.6 mm vs. BES: 31.8±22.2 mm, p=0.04) and a trend towards higher implantation pressure in the EES group (EES: 15.3±3.2 atm vs. BES: 14.7±3.3 atm, p=0.06).
CLINICAL FOLLOW-UP
The median follow-up duration was 21.3 months [IQR 13.8-27.1] before and 22.0 months [IQR 14.0-30.9] after PS matching. Table 3 presents clinical outcomes up to two years. The primary outcome occurred in 11.5% of EES-treated and 10.5% of BES-treated patients up to two years (HR 1.11; 95% CI: 0.61-2.00; p=0.74). Figure 2 and Figure 3 depict Kaplan-Meier survival-free curves for death, MACE and the primary outcome up to two-year follow-up before and after propensity score matching. Rates of all-cause and cardiac mortality were similar, whereas myocardial infarction (3.0% vs. 0.5%; HR 6.10, 95% CI: 0.73-50.9; p=0.10) was slightly less frequent with BES. The incidence rate for the primary endpoint was 5.2% per year in the EES group and 6.6% per year in the BES group. Regarding MACE, the incidence rates were 4.1% and 5.4% per year in the EES and BES groups, respectively. Angiographically documented and therefore definite ST occurred in two patients (1%) within the EES group (one subacute and one late ST) and in one BES patient (subacute ST). The stent thromboses accounted for two of the three cardiac deaths in the first month in the BES group. The other patient died due to cardiac tamponade. Rates of overall probable and definite ST were similar with EES and BES (definite and probable ST - EES 2 (1.0%) versus BES 2 (1.0%); p=1.0).
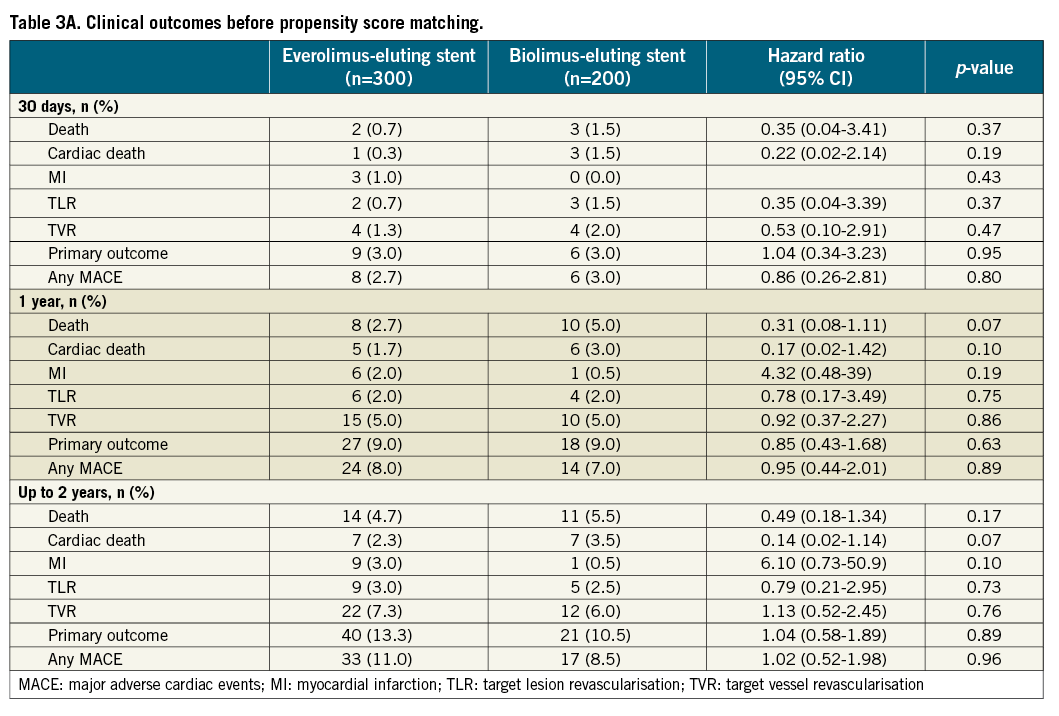
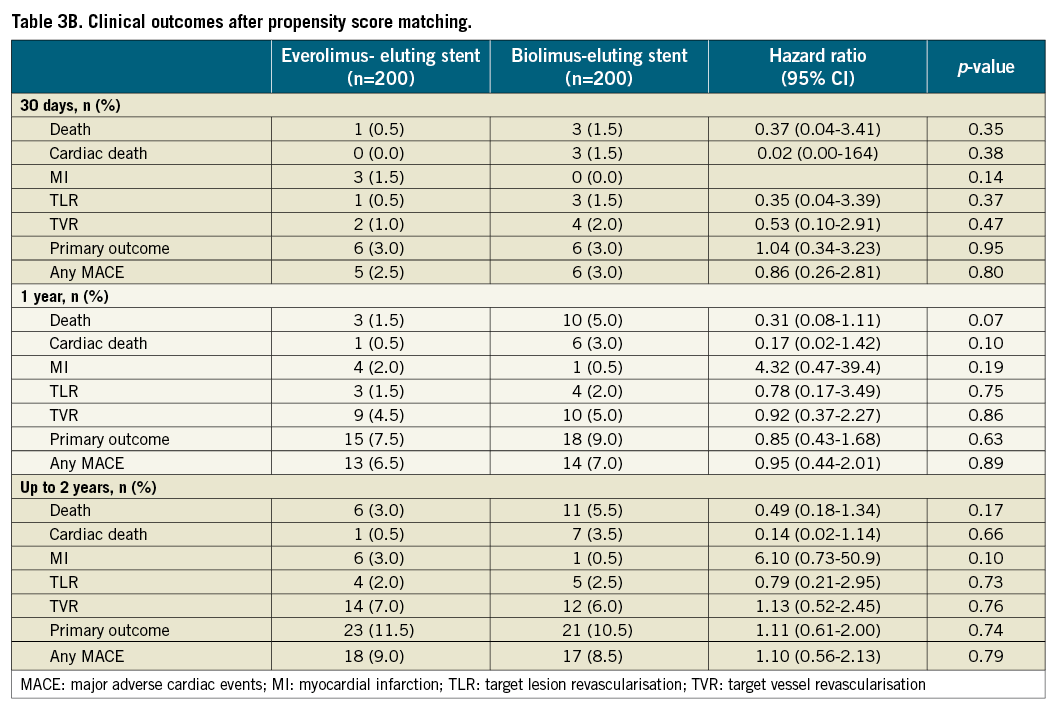
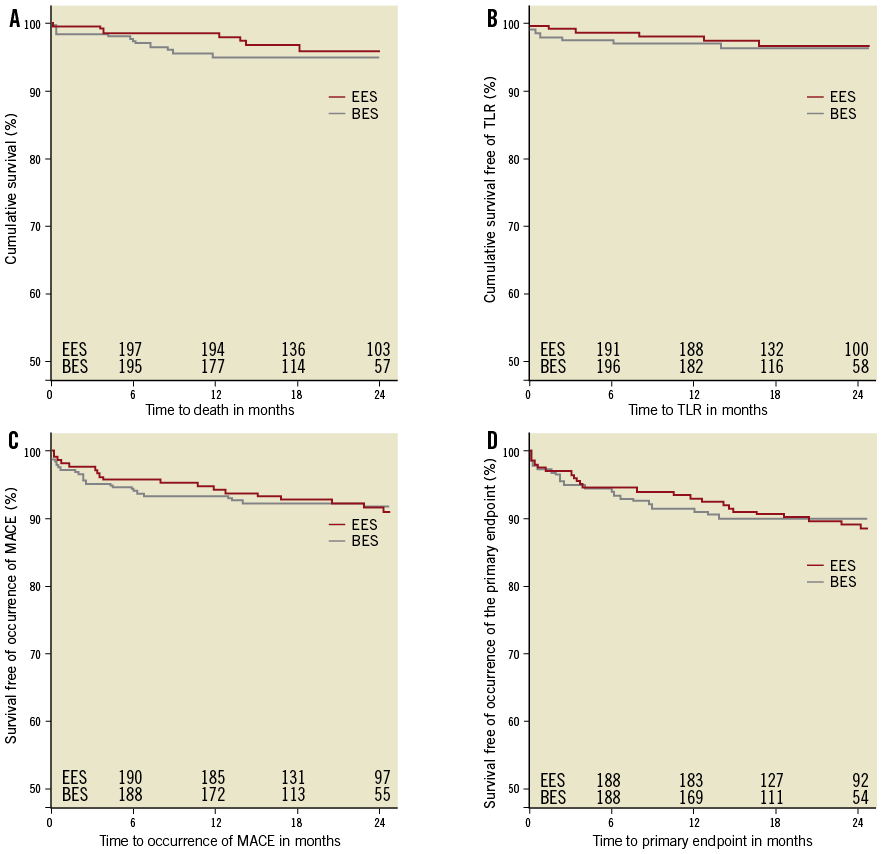
Figure 2. Kaplan-Meier curves for survival (A), survival free of TLR (B), MACE (C) and the primary endpoint (D) according to stent type implanted after propensity score matching. BES: biolimus-eluting stent; EES: everolimus-eluting stent; MACE: major adverse cardiac events
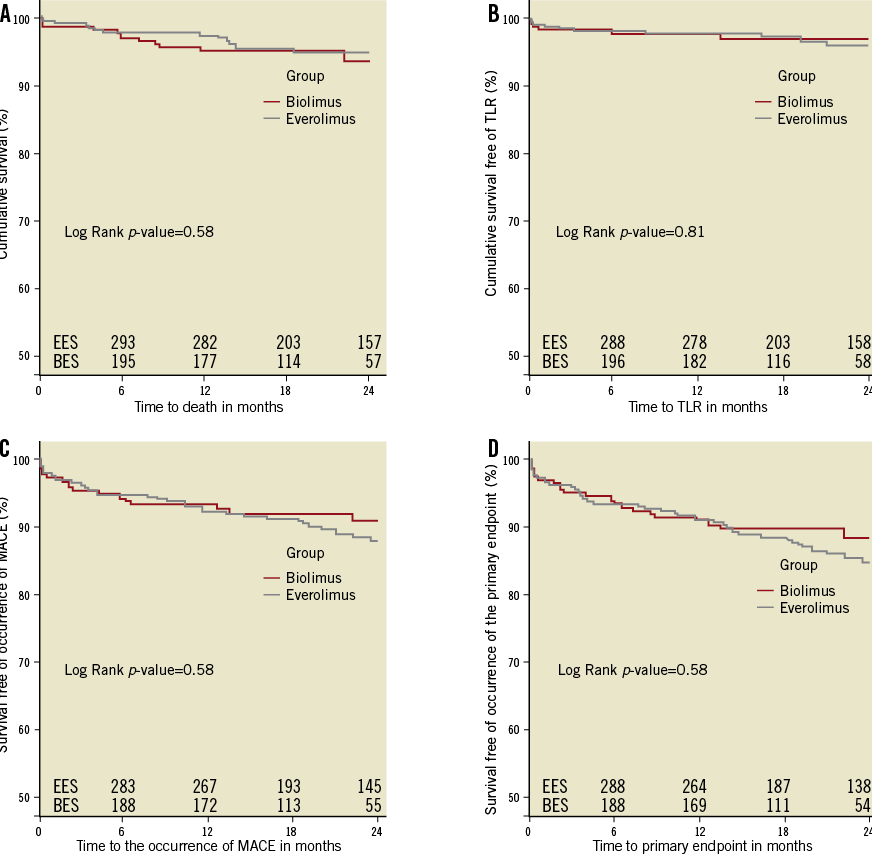
Figure 3. Kaplan-Meier curves for survival (A), survival free of TLR (B), MACE (C) and the primary endpoint (D) according to stent type implanted before propensity score matching. BES: biolimus-eluting stent; EES: everolimus-eluting stent, MACE: major adverse cardiac events
Discussion
This first EES versus BES comparative study has the following principal finding: BES with biodegradable polymer is similar to EES with durable polymer in the composite primary endpoint of death, myocardial infarction, and TVR up to two years in an unselected patient population. No differences regarding efficacy – such as TLR – or safety outcomes – such as ST – could be seen between the new-generation biolimus-releasing stent from a biodegradable polymer and an everolimus-releasing stent from a durable polymer as used in routine clinical practice.
Considering that durable polymers are incriminated in the pathogenesis of late fatalities (restenosis or thrombosis), BES with bioresorbable polymer offers a theoretical advantage over EES. Our study, however, failed to demonstrate this benefit on a mean 22 months follow-up. Several putative factors could interplay and be responsible for this lack of drastically improved effectiveness. Firstly, this is in line with previous findings from the LEADERS study. If LEADERS could demonstrate a marginal advantage of BES over the first-generation SES during extended follow-up, the advantage remained statistically insignificant at two-year follow-up. This has recently been confirmed in a pooled individual analysis of patient data from ISAR-TEST-3, -4, and LEADERS17. Secondly, EES is associated with lower drug content and a more biocompatible polymer than first-generation stents18. As such, the number of very late events has been significantly decreased in comparison with other DES19. This is nicely demonstrated in a pooled data analysis of the SPIRIT and COMPARE trials in 6,789 patients with a two-year follow-up, where significant reductions of acute MI (2.9% in EES versus 5.5% in PES), TLR (4.1% vs. 6.6%) and ST (definite/probable: 0.7% versus 2.3%) were demonstrated20. The same holds true in the EES versus SES LESSON-1 trial at two years. Moreover, Baber and colleagues demonstrated the low incidence of ST in a meta-analysis of 13 randomised controlled trials21. Finally, BES is no panacea since the lactic acidification of the media from the polymer could impact on vascular healing in the stent vicinity and promote an inflammatory reaction22.
It will therefore be difficult to see any significant differences between BES and EES. Large-scale randomised controlled trials, such as the COMPARE-2 (n=2,400 patients) or BASKET-PROVE 2 (n=2,400 patients) should present results that could further help to demonstrate the relative safety of BES compared to EES and should have enough statistical power to evaluate individual endpoints and MACE predictors.
Limitations
There are some threats to the internal validity of our study. First and foremost, we have to note the lack of randomisation. A non-randomised population suffers from a selection bias that even a propensity score cannot account for, as some relevant subject-related variables have not been distributed by chance alone. Furthermore, we have to note the experimenter bias which exists in the present study in the form of stent selection by the operator who was not blinded, and the equally non-blinded collection of outcome data.
The threat to external validity is the lack of power, resulting from the small sample size, and therefore the lack of statistical significance with regard to type II errors. Furthermore, external validity is compromised by selection, and generalisations must be drawn with caution from a population that is subject to such selection.
Conclusion
This first clinical study failed to demonstrate any significant differences regarding safety or efficacy between these two types and generations of drug-eluting stents. This is reassuring but might also reflect too small a sample size. Therefore, large randomised trials are needed to corroborate the findings of the present study.
Funding
FSC Fonds Scientifique Cardiovasculaire, Fribourg, Switzerland.
Conflict of interest statement
The authors have no conflicts of interest to declare.

