
Abstract
Aims: This study sought to investigate the safety and efficacy of a biolimus-eluting stent with biodegradable polymer (BP-BES) (Nobori; Terumo Corp.) compared to an everolimus-eluting stent with durable polymer (DP-EES) (XIENCE V or Prime; Abbott Vascular, or PROMUS; Boston Scientific).
Methods and results: The all-comers NEXT and COMPARE II clinical trials randomly assigned 5,942 patients to BP-BES (N=3,412) or DP-EES (N=2,530). We conducted a patient level pooled analysis at three-year follow-up with specified study endpoints: definite stent thrombosis (ST), the combined safety endpoint cardiac death or target vessel myocardial infarction (TV-MI), and the efficacy endpoint target lesion revascularisation (TLR). At three-year follow-up, all endpoints, namely definite stent thrombosis (BP-BES 0.8% vs. 0.4%, p=0.20), death or TV-MI (BP-BES 7.8% vs. 6.7%, p=0.07), as well as TLR (BP-BES 6.4% vs. 6.4%, p=0.78) were similar between groups. Interestingly, unadjusted (BP-BES 5.6% vs. 4.5%, p=0.02) and adjusted (HR 1.36; 1.01-1.82, p=0.04) TV-MI rates were higher in the BP-BES group than in the DP-EES group.
Conclusions: In this large-scale patient level pooled analysis of the NEXT and COMPARE II randomised trials, the use of BP-BES compared with DP-EES resulted in similar outcomes, but with an observed higher rate of TV-MI in the BP-BES group.
Abbreviations
ACS: acute coronary syndrome
BP-BES: biodegradable polymer biolimus-eluting stent
CABG: coronary artery bypass grafting
CD: clinically driven
CI: confidence interval
COPD: chronic obstructive pulmonary disease
CVA: cerebrovascular accident
DAPT: dual antiplatelet therapy
DOCE: device-oriented composite endpoint
DP-EES: durable polymer everolimus-eluting stent
HR: hazard ratio
ITT: intention to treat
LAD: left anterior descending artery
LCX: left circumflex
LMT: left main trunk
MI: myocardial infarction
NSTEMI: non-ST-elevation myocardial infarction
P: p-value
PCI: percutaneous coronary intervention
POCE: patient-oriented composite endpoint
RCA: right coronary artery
RIND: reversible ischaemic neurological deficit
SD: standard deviation
STEMI: ST-elevation myocardial infarction
TIA: transient ischaemic attack
TLR: target lesion revascularisation
TVR: target vessel revascularisation
Introduction
Late adverse events such as very late stent thrombosis and restenosis after treatment with first-generation drug-eluting stents (DES) have been linked to vascular inflammatory response at the implantation site1-4. It has been hypothesised that the durable polymer responsible for drug elution plays a central role in promoting inflammation, thereby resulting in delayed re-endothelialisation and neoatherosclerosis5-9.
DES with biodegradable polymer have been developed to mitigate this dilemma by combining the best of both worlds, the early efficacy of DES and the late safety associated with BMS. Initial data from a randomised trial indicated a biodegradable polymer-based biolimus-eluting stent (BP-BES) to be non-inferior with lower rates of very late stent thrombosis compared to the first-generation sirolimus-eluting stent CYPHER Select® (Cordis, Johnson & Johnson, Warren, NJ, USA)10,11.
However, newer-generation DES using a durable polymer have also been designed to improve polymer biocompatibility. Currently, the cobalt-chromium or platinum-chromium everolimus-eluting stent with durable fluoropolymer (DP-EES) is regarded as the gold standard due to its safety and efficacy profile12. Subsequent randomised all-comers trials documented similar outcomes for BP-BES compared to DP-EES up to three years13-17. However, none of the randomised studies was individually powered to account for low frequency events such as stent thrombosis, death or myocardial infarction (MI). Additionally, potential benefits of BP-BES with regard to these low frequency events might emerge at later follow-up.
Therefore, we conducted the present pooled analysis of the long-term follow-up from the prospective multicentre NEXT and COMPARE II trials, which are the largest trials to date to compare BP-BES to second-generation DP-EES.
Methods
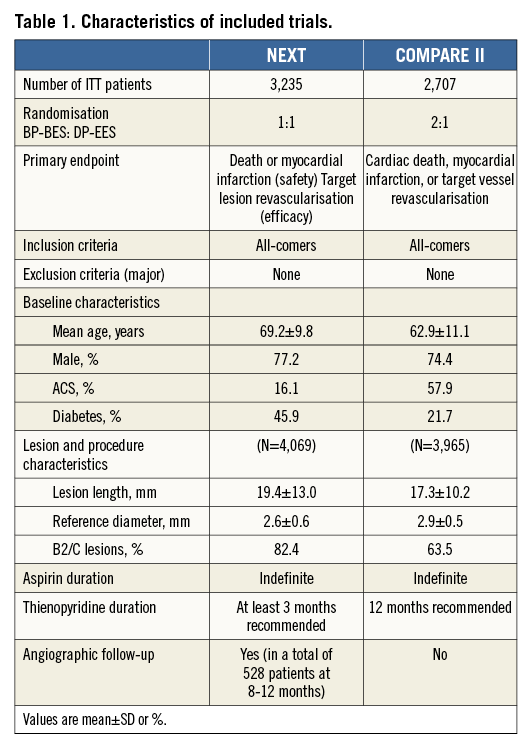
We performed a patient level pooled analysis of the NEXT (NOBORI Biolimus-Eluting Versus XIENCE/PROMUS Everolimus-eluting Stent Trial; NCT01303640) and COMPARE II (Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent; NCT 01233453) randomised, all-comers trials which are to date the largest studies comparing BES with biodegradable polymer (a 316L stainless steel stent with 120 µm strut thickness and with a biodegradable poly-lactic acid polymer eluting the drug Biolimus A9™ [Nobori®; Terumo Corp., Tokyo, Japan]) to EES with durable polymer (a cobalt- or platinum-chromium metallic stent with a strut thickness of 81 µm and with a durable fluoropolymer eluting the drug everolimus [XIENCE V® or XIENCE Prime®; Abbott Vascular, Santa Clara, CA, USA, or PROMUS™; Boston Scientific, Marlborough, MA, USA]). Primary results including detailed descriptions of study methodology of the individual trials have been published previously13,14. Both trials included an all-comers PCI population, applying the same endpoint definitions as well as similar pre-specified follow-up time points. Table 1 shows the main characteristics of the trials.
PROCEDURE
PCI was carried out according to standard techniques. Technical details such as predilation, use of adjunctive vessel and lesion modification via thrombectomy or rotational atherectomy and decision about stent post-dilatation were left to the discretion of the operator. All patients not being on dual antiplatelet therapy prior to the procedure received aspirin and an oral loading dose of clopidogrel 300-600 mg before the index procedure. During the procedure, unfractionated heparin (70-100 IU/kg) was given with additional boluses as needed to achieve the recommended activated clotting time of 250 sec or longer. Use of glycoprotein IIb/IIIa inhibitors was at the discretion of the operator in the COMPARE II trial, while glycoprotein IIb/IIIa inhibitors were not available in the NEXT trial. All patients were discharged with aspirin 100 mg once daily indefinitely as well as a thienopyridine (clopidogrel 75 mg, ticlopidine 200 mg or prasugrel 10 mg once daily), with the recommended minimal duration of dual antiplatelet therapy (DAPT) being three months in the NEXT and 12 months in the COMPARE II trial.
ENDPOINTS AND DEFINITIONS
The primary study outcome was defined by the endpoints which were specified before analysis implementation in conformity with the Academic Research Consortium, including (1) stent thrombosis (“definite” and “definite or probable”), (2) the combined safety endpoint of cardiac death or MI (not clearly attributable to a non-target vessel), and (3) target lesion revascularisation (TLR) as an efficacy measure.
Furthermore, the device-oriented composite endpoint (DOCE) consisting of cardiac death, MI (not clearly attributable to a non-target vessel), or TLR as well as the patient-oriented composite endpoint (POCE) defined as all-cause mortality, any MI, or any repeat revascularisation, and the respective individual endpoints were analysed.
All deaths were considered cardiac unless an unequivocal non-cardiac cause was established. MI and stent thrombosis were defined according to the Academic Research Consortium (ARC) definition18. TLR was defined as any repeat percutaneous intervention of the target lesion within the stent or within the 5 mm borders adjacent to the stent or bypass surgery of the target vessel performed for restenosis or other complication of the target lesion. Clinically driven revascularisation of any target lesion (or vessel) was defined by either (1) stenosis of 50% of the lesion (or vessel) diameter on the basis of quantitative coronary angiography in the presence of objective evidence of ischaemia from non-invasive or invasive testing or symptoms; or (2) stenosis of at least 70% of the diameter of the lesion (or vessel), irrespective of ischaemic signs or symptoms.
FOLLOW-UP AND DATA MANAGEMENT
Data were captured in an electronic data form. Follow-up was performed via outpatient visit or phone at 12 months and yearly thereafter; additional follow-up was conducted at one and six months in the COMPARE II trial. Reportable clinical events were adjudicated by an independent committee blinded to treatment allocation.
STATISTICAL ANALYSIS
Categorical variables are presented as number and percentage, and were compared with the chi-square or Fisher’s exact test. Continuous variables were expressed as mean±SD or medians with interquartile ranges. Normality was assessed by the visual inspection of QQ-plots and by the Shapiro-Wilk test. Continuous variables were compared using the Student’s t-test or Wilcoxon rank-sum test based on their distribution. Both pooled studies were designed as randomised, non-inferiority all-comers trials. Because of the similarity between the two trials with regard to study design and endpoint definitions as well as follow-up times, we performed a simple data pooling. All analysis was performed according to the intention-to-treat principle. Cox proportional hazards regression stratified by trial was used for survival analysis. Non-violation of the proportional hazards assumption was assessed on the basis of Schoenfeld’s residuals. To assess heterogeneity across trials, we calculated Q-statistics. A two-sided alpha level of <0.05 was considered statistically significant. All Kaplan-Meier failure functions are provided in a non-stratified, non-adjusted manner. Statistical analyses were performed using Stata/MP 13 (StataCorp LP, College Station, TX, USA).
Results
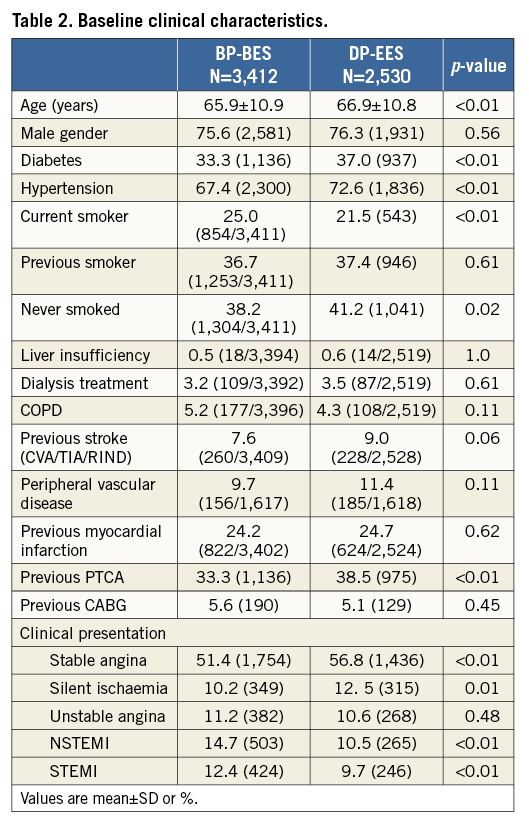
A total of 5,942 patients undergoing PCI were randomised to BP-BES (3,412 patients with 4,697 treated lesions) vs. DP-EES (2,530 patients with 3,397 treated lesions). Patient contribution from each trial as well as the key trial characteristics are shown in Table 1. Due to differences in baseline characteristics between the two trials coupled with the 2:1 (BP-BES:DP-EES) randomisation in the COMPARE II trial, significant baseline imbalances between treatment groups were observed after data pooling (Table 2). Briefly, patients treated with DP-EES were older and more often had diabetes and hypertension. On the other hand, patients treated with BP-BES more often presented with NSTEMI or STEMI.
Three-year clinical event rates are detailed in Table 3 and Figure 1. Rates of definite stent thrombosis (BP-BES 0.8% vs. 0.4%, HR 1.71, and 95% CI: 0.76-3.88, p=0.20) as well as the combined safety endpoint of death or MI (BP-BES 7.8% vs. 6.7%, and HR 1.22, 95% CI: 0.99-1.54, p=0.07) were numerically higher in the BP-BES compared to the DP-EES group without reaching a statistically significant difference. The efficacy endpoint TLR as well as device and patient-oriented composite endpoints were similar between groups (Table 3).
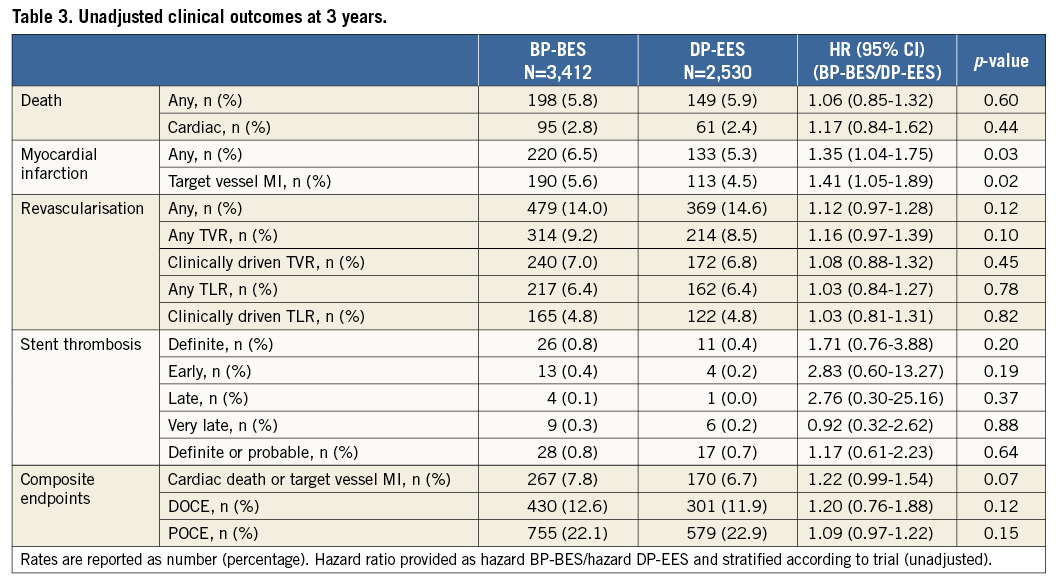
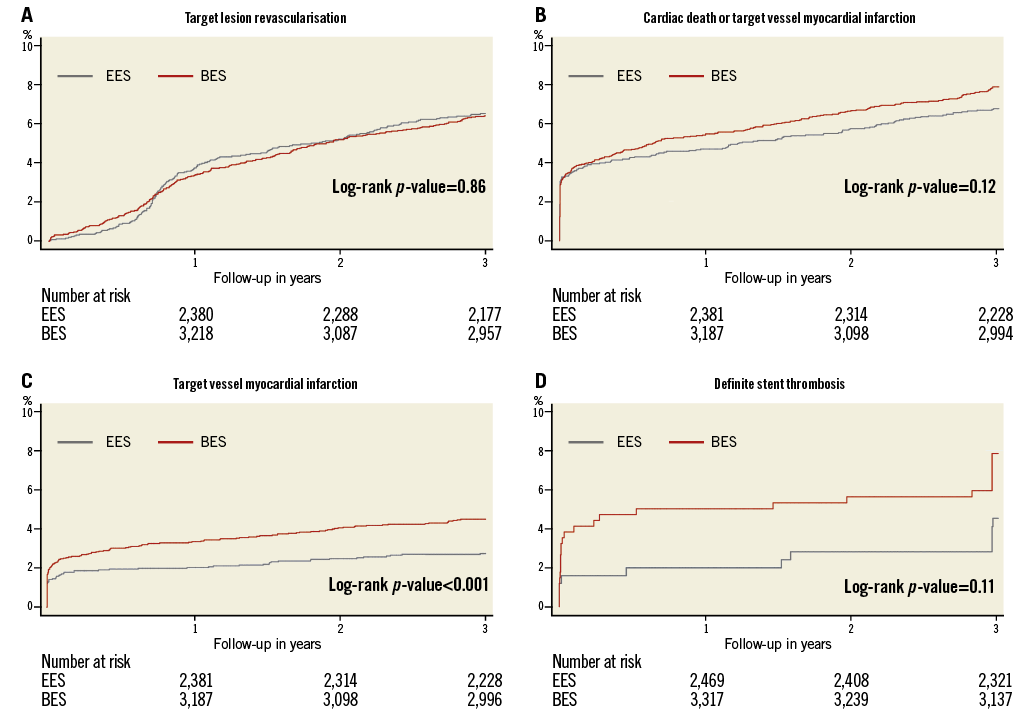
Figure 1. Kaplan-Meier cumulative event curves. A) Target lesion revascularisation. B) Cardiac death or target vessel myocardial infarction. C) Target vessel myocardial infarction. D) Definite stent thrombosis.
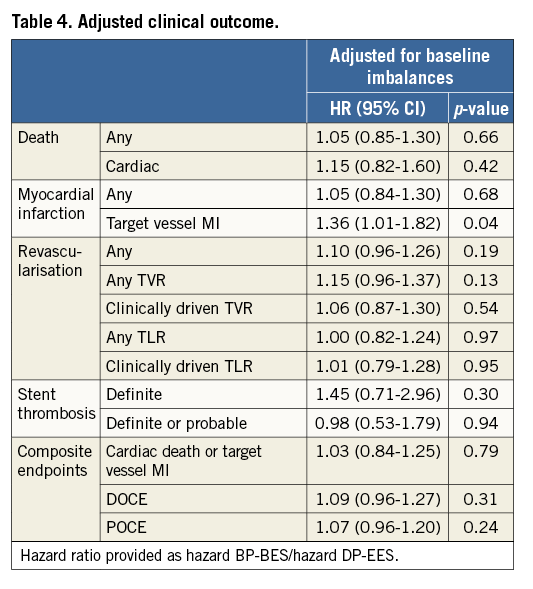
Interestingly, a significant increase in the occurrence of MI in the BP-BES group was observed (BP-BES 6.5% vs. DP-EES 5.3%, HR 1.35, CI: 1.04-1.75, p=0.03), which was driven by higher rates of target vessel MI (BP-BES 5.6% vs. DP-EES 4.5%, HR 1.41, CI: 1.05-1.89, p=0.02). The observed difference in target vessel MI is attenuated after adjustment for baseline imbalances but remains statistically significant (Table 4). There were no significant differences in treatment effects within any of the specified subgroups for the three main study outcomes or the DOCE, nor was any significant subgroup-treatment interaction found (data not shown).
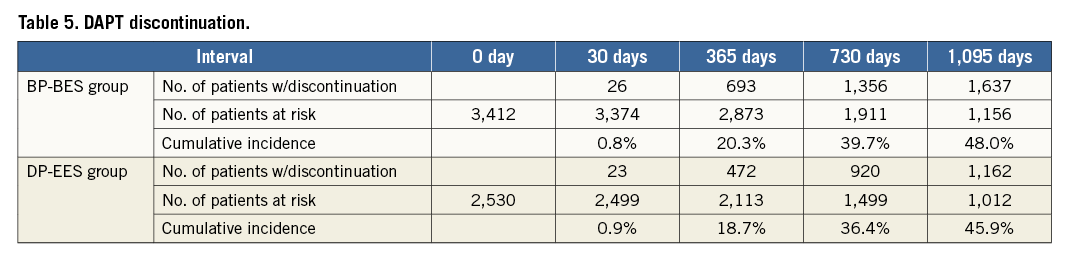
No significant difference was observed regarding DAPT discontinuation between groups over the period of three years (Figure 2, Table 5). About 20% of patients had interrupted DAPT before 12 months and about 2.5% before six months without any occurrence of stent thrombosis (data not shown).
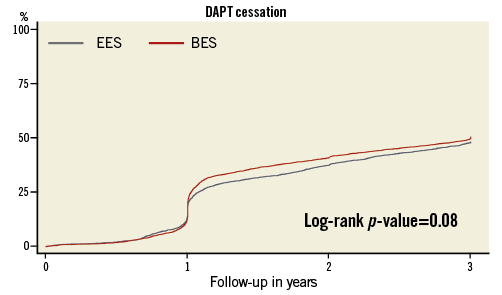
Figure 2. Kaplan-Meier cumulative incidence of discontinuation of dual antiplatelet therapy.
Discussion
The principal finding of this large-scale pooled analysis is that the rates of specified safety and efficacy endpoints were similar between groups, apart from target vessel myocardial infarction which occurred more often in the BP-BES group.
Network meta-analyses have indicated an excess risk of biodegradable polymer drug-eluting stents with regard to MI or stent thrombosis when compared to DP-EES, though these analyses were restricted due to limited follow-up duration19-21. Of note, while the meta-analyses by Kang et al and Navarese et al included BP-BES trials using the BioMatrix™ device (Biosensors Europe SA, Morges, Switzerland) which is similar to the Nobori stent, the meta-analysis of Bangalore et al also included trials using a sirolimus-eluting stent with biodegradable polymer (Yukon® Choice PC; Translumina GmbH, Hechingen, Germany).
However, the largest randomised all-comers trials, NEXT and COMPARE II, individually reported similar safety and efficacy results for the Nobori BP-BES compared to the second-generation DP-DES, namely DP-EES, at one and three years13,14,16,17. However, none of the individual trials was adequately powered for low frequency events such as stent thrombosis, death and MI. The present pooled analysis aimed to take these constraints into account, something which appears essential in the light of the attenuated power of the individual randomised studies due to lower than expected event rates of the primary endpoints used for sample-size calculation (almost 50% in the COMPARE II trial, about 30% in the NEXT trial).
Interestingly, while the present pooled analysis confirms results from the individual studies regarding similar efficacy outcomes, it indicates a significantly higher frequency of target vessel MI in the BP-BES group. This cannot be explained entirely through the numerically higher incidence of definite stent thrombosis in the BP-BES group. Thus, the pathophysiological mechanism triggering higher rates of target vessel MI remains partly unexplained. Drug and polymer properties, the stent platform design, material and, in particular, the strut thickness show significant differences between the tested devices which might lead to varying vascular healing responses. Indeed, strut thickness per se has been linked to higher rates of restenosis22,23.
Nevertheless, the benefit of biodegradable polymer-based stents is expected beyond one year. The LEADERS trial indicated lower very late stent thrombosis rates in patients treated with biodegradable polymer DES compared to the first-generation CYPHER DES10. However, the present analysis did not show benefits of the Nobori BP-BES at any of the analysed endpoints (including stent thrombosis) when compared to DP-EES. On the contrary, definite stent thrombosis occurred numerically more often in patients treated with BP-BES. Of note, the BP-BES cumulative definite stent thrombosis rate at three years was lower than the previously reported 1.4% in the SORT-OUT V trial17. Interestingly, we observed a very low definite stent thrombosis rate at three years in the DP-EES arm of 0.4%. Indeed, DP-EES possess an excellent safety profile when compared to other durable polymer DES and even BMS24. Finally, in both treatment arms no event of stent thrombosis was observed in patients who discontinued DAPT during the first 12 months after the index procedure.
By implication, the present results regarding the Nobori BP-BES cannot be generalised to other stent systems using biodegradable polymers, since the performance of these devices depends on interaction between the drug and its elution characteristics, the biodegradable polymer properties and the stent platform. Further data are warranted to indicate whether biodegradable polymer technologies with newer thin-strut stent platforms have added advantages in safety and efficacy when compared to current-generation thin-strut DES with durable polymer.
Limitations
The study is limited by its post hoc nature; thus, the results should be considered hypothesis-generating. Furthermore, this analysis combined data from studies which enrolled patients from different ethnicities. Moreover, although each trial documented well balanced baseline characteristics, we observed significant baseline imbalances after pooling, which were accounted for via trial affiliation analysis and confirmed by multivariable adjusted hazard ratios which were very similar to the unadjusted hazard ratios.
Conclusion
In this large-scale patient level pooled analysis from the NEXT and COMPARE II randomised trials, the use of Nobori BP-BES compared with DP-EES showed similar outcomes regarding the study specified endpoints but indicated a higher rate of target vessel-related myocardial infarction with BP-BES at three years.
| Impact on daily practice The present pooled analysis of the NEXT and COMPARE II trials shows that both the biodegradable polymer-coated biolimus-eluting stent (BP-BES) and the durable polymer-coated everolimus-eluting stents (DP-EES) have an excellent safety and efficacy profile for the specified endpoints, underlining the improved performance achieved with current-generation devices when compared to earlier-generation drug-eluting stents. Interestingly, the biodegradable polymer-coated BES does not indicate any benefit, in particular towards reduction of very late adverse events, and suggests higher rates of target vessel myocardial infarction, thus challenging the concept of biodegradable polymer coating in the tested setting. |
Acknowledgements
We would like to thank the following centres and principal investigators for COMPARE II patient inclusion: Department of Cardiology, Maasstad Hospital, Rotterdam, The Netherlands; Department of Cardiology, Medisch Centrum Leeuwarden, The Netherlands (S.H. Hofma, MD); Department of Cardiology, Hôpital Cantonal de Fribourg, Fribourg, Switzerland (M. Togni, MD, J-J. Goy, MD); Department of Cardiology, Complexo Hospitalario Juan Canalejo, Coruña, Spain (N. Vázquez, MD); Department of Cardiology, Hospital Virgen de la Arrixaca, Murcia, Spain (M. Valdés, MD); Department of Cardiology, Onassis Cardiac Surgery Centre, Athens, Greece (V. Voudris, MD, G. Karavolias, MD); Department of Cardiology, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands (T. Slagboom, MD); Department of Cardiology, Kantonsspital Aarau, Aarau, Switzerland (A. Vuilliomenet, MD); Department of Cardiology, Hospital del Mar, Barcelona, Spain (A. Serra, MD); Department of Cardiology, Hospital de Santiago de Compostela, Santiago de Compostela, Spain (R. Trillo Nouche, MD); and Department of Cardiology, Amphia Ziekenhuis, Breda, The Netherlands (P. den Heijer, MD). We would also like to thank Erik Spaepen and Tessa Rademaker-Havinga for their contribution to the statistical analysis and Claudia van Vliet for project management in the COMPARE II trial.
Funding
COMPARE II was supported by a grant from Terumo Europe (Leuven, Belgium) and the Research Foundation of the Cardiology Department, Maasstad Hospital (Rotterdam, The Netherlands). The sponsor had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review or approval of the manuscript.
Conflict of interest statement
G.J. Vlachojannis has received, in the last three years, lecture and consultation fees from Abbott Vascular. P.C. Smits has received, in the last three years, lecture fees from Abbott Vascular and institutional grants from Abbott Vascular, Terumo, and St. Jude. The other authors have no conflicts of interest to declare.

