
Abstract
Aims: The aim of the study was to evaluate the two-year clinical outcome of all-comer trial participants who were treated with two very different thin-strut biodegradable polymer versus thin-strut durable polymer drug-eluting stents (DES). Prolonged clinical outcome after discontinuation of dual antiplatelet therapy is of particular interest, given the highly dissimilar polymer types, amount, distribution, and degradation speed of both biodegradable polymer DES.
Methods and results: The BIO-RESORT trial (NCT01674803) randomly assigned 3,514 patients to treatment with biodegradable polymer SYNERGY everolimus-eluting stents (EES) or Orsiro sirolimus-eluting stents (SES), or durable polymer Resolute Integrity zotarolimus-eluting stents (ZES). At two-year follow-up (available in 98.8%), the rate of the primary composite endpoint target vessel failure (TVF) was 8.3% in ZES versus 6.8% in EES (p=0.19) and 6.6% in SES (p=0.12). Landmark analyses at one year revealed differences between SES and ZES in the rates of target lesion revascularisation and target lesion failure (0.6% vs. 1.5%, p=0.04, and 1.1% vs. 2.4%, p=0.02, respectively) as well as other composite secondary endpoints that reached statistical significance.
Conclusions: At two-year follow-up, there was no significant between-DES difference in the rates of the primary endpoint. Landmark analyses provided a signal that the use of SES versus ZES might reduce the risk of repeat revascularisation after one-year follow-up.
Abbreviations
DAPT: dual antiplatelet therapy
EES: everolimus-eluting stent
MACE: major adverse cardiac events
MI: myocardial infarction
PCI: percutaneous coronary intervention
POCE: patient-oriented composite endpoint
SES: sirolimus-eluting stent
TLR: target lesion revascularisation
TLF: target lesion failure
TVF: target vessel failure
ZES: zotarolimus-eluting stent
Introduction
The lifelong presence of durable polymer-coated drug-eluting stents in coronary arteries has been associated with chronic inflammation, delayed arterial healing, and neoatherosclerosis, which may result in late adverse clinical events1. New-generation biodegradable polymer DES were designed to overcome these limitations by providing the antiproliferative benefits of durable polymer DES combined with the long-term safety of bare metal stents due to the absence of polymer residues2. The early-generation biodegradable polymer DES had thick struts and, in a large all-comers trial, showed similar efficacy and somewhat better long-term safety as compared to early-generation thick-strut durable polymer DES2,3. Novel biodegradable polymer DES have uncoated struts that are up to half as thick.
These very thin-strut biodegradable polymer DES have flexible designs and thin, refined coatings4 to accelerate re-endothelialisation and reduce the risk of ischaemic coronary events. The rapid resorption of the abluminal polymer in SYNERGY™ everolimus-eluting stents (EES) (Boston Scientific, Marlborough, MA, USA) results in a bare metal platform within four months, while the encompassing polymer coating of the Orsiro sirolimus-eluting stent (SES) (Biotronik, Bülach, Switzerland) is slowly degraded within approximately 18 months5. The one-year follow-up of the randomised BIO-RESORT trial demonstrated non-inferiority of both biodegradable polymer DES versus Resolute Integrity® zotarolimus-eluting stents (ZES) (Medtronic, Santa Rosa, CA, USA) in 3,514 all-comer patients, but there was no short-term advantage6. Nevertheless, biodegradable polymer DES might still improve midterm or long-term outcomes.
Clinical outcome after discontinuation of dual antiplatelet therapy (DAPT) is of particular interest, given the highly dissimilar polymer types, amount, distribution, and degradation speed of the two biodegradable polymer DES tested. In the present study, DAPT was prescribed for 12 months in most patients and then stringently discontinued. Notably, BIO-RESORT is 1) the first randomised trial to assess both the biodegradable polymer SYNERGY and Orsiro stents, and 2) the first randomised trial to test SYNERGY EES in all-comers6. In the present study, we assessed the two-year clinical outcome of the BIO-RESORT all-comers who were treated with EES and SES versus ZES and followed a stringent DAPT discontinuation policy after one year.
Methods
STUDY DESIGN AND PATIENTS
The investigator-initiated, multicentre randomised BIO-RESORT trial (TWENTE III) enrolled all-comers requiring percutaneous coronary intervention (PCI) with DES at four sites in the Netherlands (Thoraxcentrum Twente, Medisch Spectrum Twente, Enschede; Rijnstate Hospital, Arnhem; Haga Hospital, The Hague; Albert Schweitzer Hospital, Dordrecht)5,6. In this three-arm clinical trial (ClinicalTrials.gov Identifier: NCT01674803), patients were randomly (1:1:1) assigned to either a platinum-chromium EES (SYNERGY), a cobalt-chromium SES (Orsiro), or a new-generation thin-strut ZES (Resolute Integrity). All coronary syndromes, de novo and restenotic lesions, and coronary or bypass lesions were permitted. There was no limit for lesion length, reference vessel size, and number of lesions or vessels to be treated. The study design has been described previously5,6.
The trial complied with the CONSORT 2010 Statement and the Declaration of Helsinki and was approved by the Medical Ethics Committee Twente and the institutional review boards of all participating centres. All patients provided written informed consent.
PROCEDURES AND FOLLOW-UP
Treatment was performed according to current medical guidelines and the physician’s judgement6. Lesion predilation, direct stenting and stent post-dilation were left to the operator’s discretion. The SYNERGY stent elutes everolimus within three months from a 4 μm biodegradable PLGA (poly[lactic-co-glycolic acid]) coating that is located only on the abluminal side of 74 μm/79 μm/81 μm platinum-chromium struts (for stent sizes ≤2.5 mm/3.0-3.5 mm/4.0 mm, respectively) and resorbed within four months5,7. The sirolimus-eluting Orsiro stent has 60 μm or 80 μm (for stents ≤3.0 mm or >3.0 mm) cobalt-chromium struts that are circumferentially covered by an asymmetrical hybrid coating that is thicker on the abluminal side (7.4/3.5 μm)5. Its biodegradable PLLA (poly[L-lactide] acid) elutes the drug within three months, is fully resorbed within 18 months, and covers a thin passive coating of amorphous silicon carbide5. The zotarolimus-eluting Resolute Integrity stent has thin 91 μm cobalt-chromium struts, covered by a 6 μm zotarolimus-eluting blend of three durable polymers.
Clinical follow-up was obtained at visits to outpatient clinics or, if not feasible, by telephone follow-up or medical questionnaire. A clinical research organisation (Cardiovascular Research and Education, Enschede, the Netherlands) coordinated trial and data management.
Clinical endpoints were pre-specified, using definitions of the Academic Research Consortium5,8. The primary composite endpoint of target vessel failure (TVF) assessed device efficacy and patient safety and consisted of cardiac death, target vessel-related myocardial infarction (MI), or clinically indicated target vessel revascularisation. Secondary endpoints included: target lesion revascularisation (TLR), target lesion failure (TLF, a composite of cardiac death, any MI not clearly attributable to a non-target vessel, or clinically driven TLR), major adverse cardiac events (MACE, a composite of all-cause death, any MI, or emergent coronary bypass surgery, or repeat clinically indicated TLR), the most global patient-oriented composite endpoint (POCE, a composite of all-cause death, any MI, or any repeat coronary revascularisation), and definite-or-probable stent thrombosis.
Data monitoring, processing of clinical outcome data, and independent clinical event adjudication were performed by an independent clinical research organisation (Diagram, Zwolle, the Netherlands).
STATISTICAL ANALYSIS
Categorical variables were assessed with the chi-square or Fisher’s exact test, as appropriate, while continuous variables were assessed with the Student’s t-test. The time to primary endpoint and components thereof was assessed according to Kaplan-Meier methods; the log-rank test was applied for between-group comparisons. Hazard ratios were computed using Cox proportional hazards regression analysis. We performed landmark analyses of the primary endpoint and all secondary endpoints by using the one-year landmark. P-values <0.05 were considered significant. P-values and confidence intervals were two-sided. Statistical analyses were performed with SPSS, Version 22.0 (IBM Corp., Armonk, NY, USA).
Results
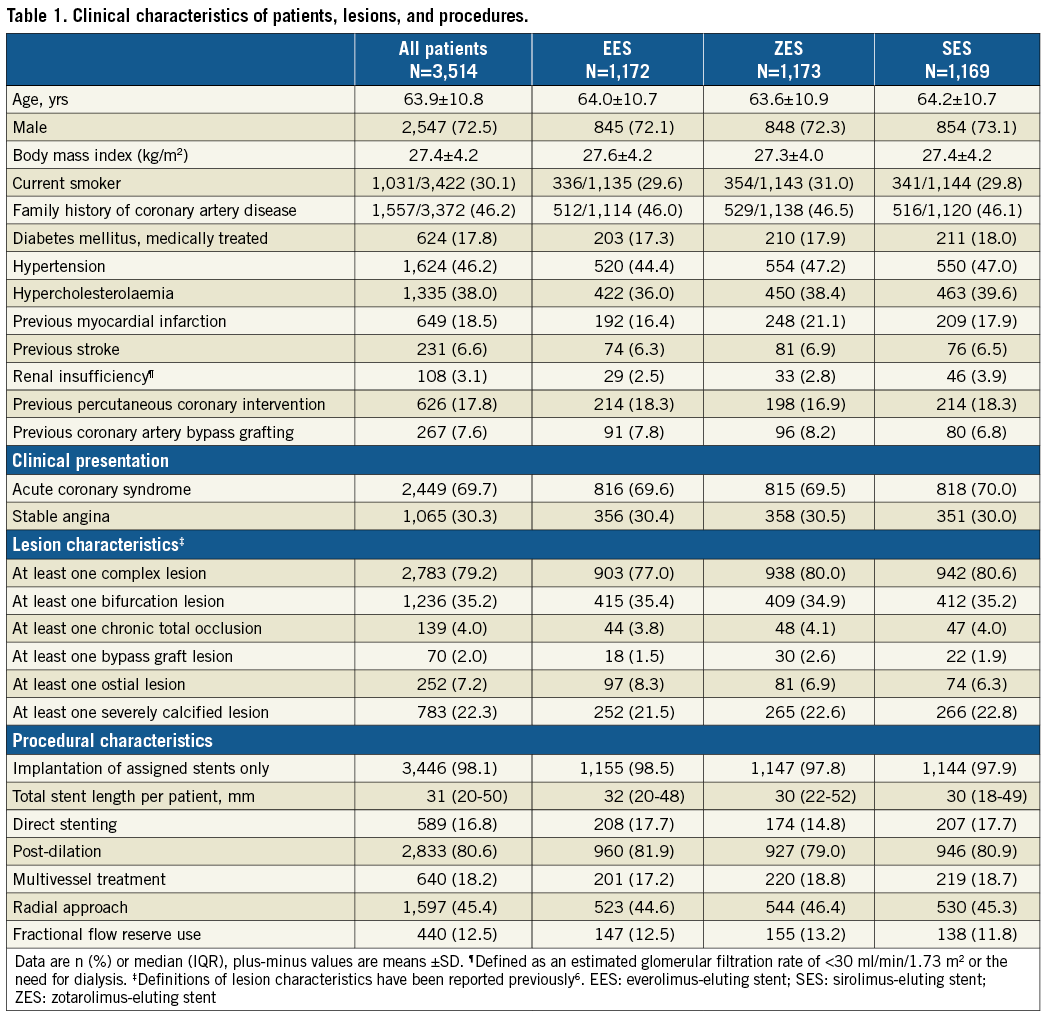
From December 2012 to August 2015, a total of 3,514 patients were randomised and assessed at four clinical sites in the Netherlands, of whom 3,472 (98.8%) completed two-year follow-up or had died. Eleven (0.3%) patients were lost to follow-up, and 31 (0.9%) withdrew their consent (censored at the moment of dropout).
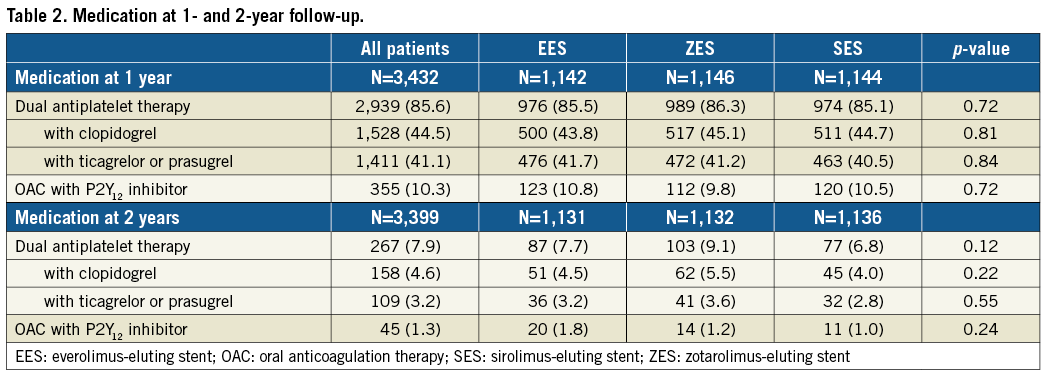
Table 1 presents the baseline characteristics of the trial participants, stratified for assigned treatment arms. Most patients presented with acute coronary syndromes (69.7%) and 79.2% of patients had ≥1 complex target lesion. At two years, DAPT rates were low (Table 2). Between stent arms, there were no statistically significant differences in the use of antiplatelet agents and oral anticoagulation therapy.
The two-year clinical outcome data are presented in Table 3. At two-year follow-up, the primary endpoint TVF occurred in 79/1,172 (6.8%) patients assigned to EES, 76/1,169 (6.6%) patients assigned to SES, and 96/1,173 (8.3%) patients assigned to ZES. These differences were statistically non-significant for both EES and SES versus ZES (p=0.19, and p=0.12, respectively). The event rates of TVF and its individual components are displayed in Figure 1. The event rates for the primary endpoint were consistent across subgroups, except for patients who were treated for bypass grafts (Supplementary Table 1A, Supplementary Table 1B). Definite stent thrombosis was an infrequent event that occurred in 7 (0.6%), 5 (0.4%) and 6 (0.5%) patients, respectively. Definite-or-probable stent thrombosis rates were similar among treatment groups (11 [1.0%], 7 [0.6%], and 9 [0.8%], respectively; p=0.65 and p=0.62) (Table 3, Figure 2).
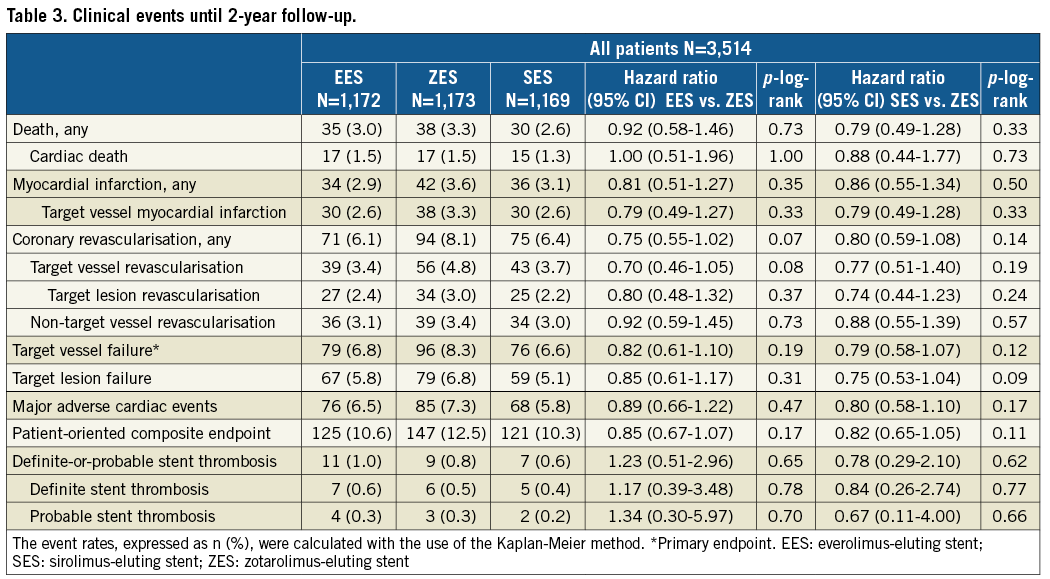
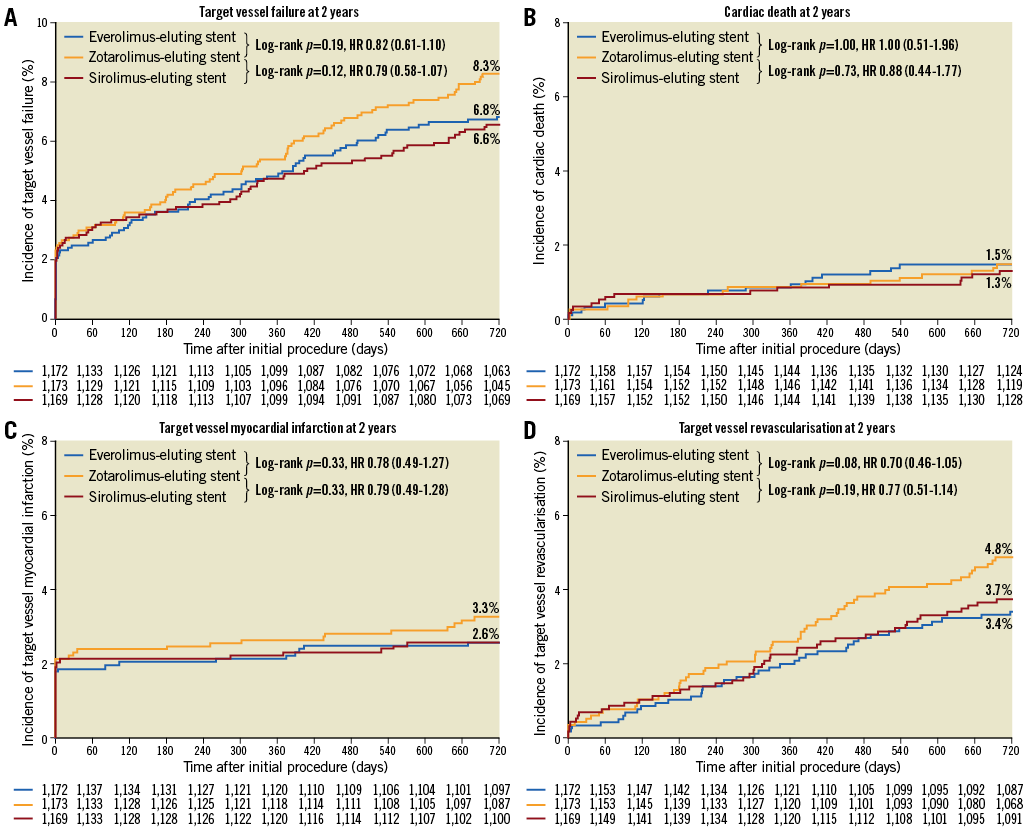
Figure 1. Kaplan-Meier cumulative event curves for the primary endpoint target vessel failure and its individual components at two-year follow-up. The primary endpoint target vessel failure (A), a composite of cardiac death (B), target vessel-related myocardial infarction (C), or clinically indicated target vessel revascularisation (D).
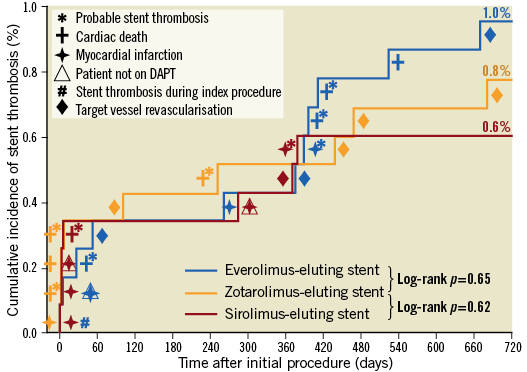
Figure 2. Incidence of definite or probable stent thrombosis at two-year follow-up. Symbols indicate the adverse events associated with stent thrombosis.
In landmark analyses between one- and two-year follow-up, patients assigned to SES had, compared to patients assigned to ZES, significantly lower rates of TLR (0.6% vs. 1.5%, p=0.04) and TLF (1.1% vs. 2.4%, p=0.02) (Table 4, Figure 3). In addition, the rates of the composite endpoints MACE and POCE were significantly lower in SES versus ZES (0.8% vs. 2.2% and 3.2% vs. 5.3%, respectively, both p=0.01), while the TVF rate was 1.9% versus 3.0% (p=0.10). TLR as well as non-cardiovascular death contributed to the differences in MACE and POCE. A detailed description of the TLR cases is presented in Supplementary Table 2. Landmark analyses between one and two years for TVF and secondary endpoints showed no statistically significant differences for EES versus ZES (Table 4).
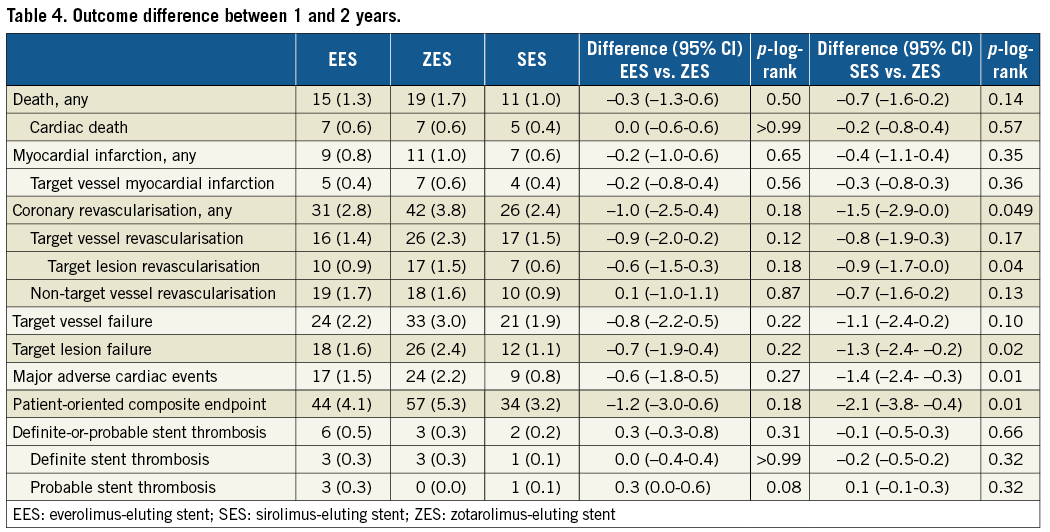
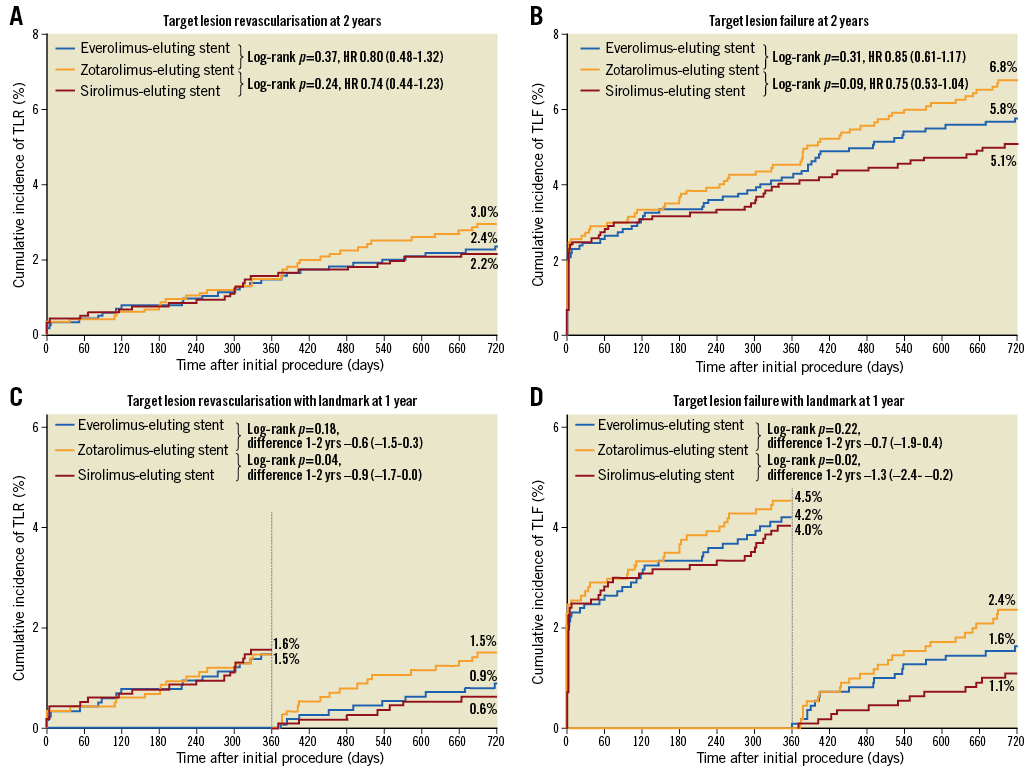
Figure 3. Kaplan-Meier cumulative event curves and landmark analyses for target lesion revascularisation and target lesion failure. Target lesion failure is a composite of cardiac death, target lesion-related myocardial infarction, or clinically driven target lesion revascularisation.
Discussion
The present analysis shows that, at two-year follow-up, the rate of the primary endpoint TVF is similar in both very thin-strut biodegradable polymer DES and the reference durable polymer DES. This supports the concept in PCI all-comers that the combination of very thin struts with biodegradable polymers is associated with safety and efficacy which, during the first two years from implantation, are similar to DES with durable polymer coatings.
Landmark analyses revealed lower rates of TLR and several secondary composite endpoints (TLF, MACE, and POCE) in SES versus ZES during the second year of follow-up. This signal of a potentially lower long-term risk of target lesion recurrence following treatment with SES is of interest but should be interpreted cautiously. During the second year of follow-up, the sirolimus has already been eluted for >9 months, while the process of polymer resorption is just finishing. Clinical data suggest that this very slow polymer degradation might be advantageous, potentially by minimising vascular inflammation. In addition, the very high rate of stent post-dilation in >80% of patients could have resulted in a deeper embedding of the very thin stent struts, which might have contributed to the overall favourable event rates.
Potential benefits of the biodegradable polymer DES might be seen no earlier than after several years3,9,10. Clinical event rates after discontinuation of DAPT are of particular interest, given the highly dissimilar polymer types, amount, distribution, and degradation speed of SYNERGY and Orsiro tested in the BIO-RESORT trial. In our study, DAPT was prescribed for 12 months in most patients and then stringently discontinued, as is common practice in the Netherlands. At two-year follow-up, DAPT use was 8%, while in another large-scale randomised DES trial that studied SYNERGY EES the DAPT rate was 47% (Kereiakes DJ et al. Late clinical outcomes after bioresorbable or permanent polymer everolimus-eluting stents: 2-year results from the EVOLVE II randomized trial. Presented at ACC 2017, Washington DC, USA, 17 March 2017).
RANDOMISED DES STUDIES WITH SYNERGY AND ORSIRO
BIO-RESORT is the first randomised study to examine both SYNERGY and Orsiro biodegradable polymer DES, and the first randomised trial to assess SYNERGY EES in all-comers. This and other randomised trials have demonstrated the short-term safety and efficacy of both novel biodegradable polymer DES, which were found to be similar to established new-generation durable polymer DES6,11.
The large-scale EVOLVE II trial examined 1,684 patients with up to moderate clinical risk, treated with SYNERGY EES versus durable polymer platinum-chromium EES, and demonstrated non-inferiority of SYNERGY at one-year follow-up7. The two-year outcome showed similar safety and efficacy outcomes for both devices. Theoretically, the rapid polymer resorption in the SYNERGY may justify an abbreviated DAPT regime, which may be most advantageous in patients with an increased bleeding risk. In the randomised SYNERGY II Senior trial, elderly patients, treated with one or six months of DAPT after PCI, showed superior outcomes after PCI with SYNERGY EES versus bare metal stents12.
The biodegradable polymer Orsiro stent was previously tested against other DES in large randomised studies, which ascertained the safety and efficacy of Orsiro in greatly unrestricted patient populations13-15. The SORT OUT VII trial studied 2,525 PCI patients, randomly assigned to the sirolimus-eluting Orsiro versus thick-strut biolimus-eluting biodegradable polymer DES and showed similar two-year clinical outcomes16. In addition, the two-year clinical outcome of the randomised BIOSCIENCE trial in 2,119 patients was similar for patients treated with Orsiro versus durable polymer XIENCE EES17. In the BIOFLOW V randomised trial, Orsiro outperformed the durable polymer EES in a complex patient population, mainly based on a lower incidence of in-hospital MI, which did not translate into a difference in mortality18.
BIODEGRADABLE POLYMER DES
The struts of the novel biodegradable polymer stents are substantially thinner than those of the early biodegradable polymer stents2. These very thin struts may have the advantage of reducing the incidence of side branch occlusion and periprocedural MI. A recent meta-analysis confirmed low MI rates after PCI with these devices but observed no benefit in clinically driven TLR versus other contemporary DES15. The reduction in strut thickness needs to be balanced against a decrease in radial strength, which can be achieved by modifications in stent design or the use of metal alloys with a higher strength. In addition, a potential disadvantage of the thin stent struts is their inferior radiographic visibility that is more marked in cobalt-chromium devices than in platinum-chromium stents19; theoretically, a lower visibility increases the risk of geometrical miss and related adverse cardiovascular events. Our current analysis, obtained in a broad population of PCI all-comers, shows no signal of increased adverse event risk for both very-thin strut biodegradable polymer DES but excellent outcomes until two-year follow-up.
The type and pharmacokinetic release profile of the antiproliferative drug as well as the reproducibility of drug elution varies across different biodegradable polymer DES3,20-22. Variance in polymer formulation and properties, such as polymer chain length and hydrophobicity, determines the course and products of polymer degradation18. All of the above may have an effect on the biological response of the coronary vessel, the speed of endothelialisation, the required minimum DAPT duration, and finally clinical outcome. Therefore, the various types of biodegradable polymer DES should not be considered a homogeneous class of devices. As a matter of fact, it is of the utmost importance to assess all individual biodegradable polymer DES carefully with a longer-term follow-up and to report clinical findings of the specific devices with their highly unique features in stent design, metal backbone, polymer type, composition of polymer and drug, and profiles of drug elution and polymer resorption.
Limitations
The findings of the landmark analyses should be considered hypothesis-generating. In addition, this study is not powered for reliable assessment of secondary clinical endpoints and, in particular, adverse events with a low incidence such as stent thrombosis. Despite high follow-up rates, systematic assessment of biomarkers, electrocardiographs and independent monitoring, the clinical event rates of the present study were low6.
Conclusions
Two years after stenting, the biodegradable polymer EES and SES showed favourable clinical outcomes that were comparable to the reference durable polymer ZES in a broad all-comers population, including many patients with acute coronary syndromes. Long-term follow-up will be of interest, as landmark analyses provided a signal that the use of SES might reduce the risk of repeat revascularisation after the first year of follow-up.
| Impact on daily practice Novel DES with very thin-strut biodegradable polymers may have advantages over stents with thin-strut durable polymers. The current two-year outcome data of the randomised BIO-RESORT trial show no statistically significant difference in adverse event rates between each of two novel very thin-strut biodegradable polymer DES and a thin-strut durable polymer reference DES. However, we found a signal that the biodegradable polymer sirolimus-eluting stent might be associated with a lower repeat revascularisation risk beyond one-year follow-up. Further long-term follow-up assessment is warranted and certainly of interest. |
Funding
Biotronik, Boston Scientific, Medtronic.
Conflict of interest statement
C. von Birgelen indicates that the research department of Thoraxcentrum Twente has received institutional research grants from Biotronik, Boston Scientific, and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1A. Subgroup analyses for the 2-year rates of target vessel failure: EES versus ZES.
Supplementary Table 1B. Subgroup analyses for the 2-year rates of target vessel failure: SES versus ZES.
Supplementary Table 2. Circumstances and consequences of TLR between 1 and 2 years.
To read the full content of this article, please download the PDF.

