KEYWORDS
Abstract
Aims: This study reports the 12-month clinical outcomes of the LEADERS clinical trial which compared a biolimus eluting stent with a biodegradable polymer (BES) to a sirolimus eluting stent with a durable polymer (SES).
Methods and results: The multicentre LEADERS trial employed an all-comers approach to recruit 1,707 patients who were randomised to treatment with either BES (n=857) or SES (n=850) in a non-inferiority design. The primary clinical endpoint of this study was a composite of cardiac death, myocardial infarction and clinical-indicated target vessel revascularisation. Follow-up was obtained in 97.6% of patients. At 12 months, BES remained non-inferior compared to SES for the primary endpoint (BES 10.6% vs. SES 12.0%, HR:0.88, 95% CI:0.66-1.17, p=0.37). Rates of cardiac death (2.1% vs. 2.7%, HR:0.77, 95% CI:0.42-1.44, p=0.42), MI (5.8% vs. 4.6%, HR:1.27, 95% CI:0.84-1.94, p=0.26) and clinically-indicated target vessel revascularisation (5.8% vs. 7.1%, HR:0.82, 95%CI:0.56-1.19, p=0.29) were similar for BES and SES. Similarly, there was no difference in the incidence of definite stent thrombosis at 12 months.
Conclusions: These findings support the safety and efficacy of the BES stent with a biodegradable polymer at 12-month clinical follow-up, and suggest it is a suitable alternative to the SES stent with a durable polymer.
Introduction
Drug eluting stents (DES) revolutionised the field of percutaneous coronary intervention (PCI) after their introduction in 2002, by significantly reducing rates of restenosis compared to bare metal stents (BMS)1. In recent times there have been concerns that when compared to BMS, DES are associated with an increased risk of very late (>1 year) stent thrombosis (ST)2-4. The cause of this is likely to be multi-factorial, however delayed re-endothelialisation plays an important role, and this in turn may be the result of a hypersensitivity reaction induced by the presence of a permanent polymer5,6 .
The Biomatrix™ Flex biolimus eluting stent (BES) (Biosensors, Morges, Switzerland) elutes biolimus from a polylactic acid (PLA) biodegradable polymer applied to the stent’s abluminal surface. The polymer is fully metabolised to water and carbon dioxide within six to nine months. Biolimus is a highly lipophilic sirolimus analogue7 which inhibits the mammalian target of rapamycin, and inhibits smooth muscle cell proliferation by causing the arrest of the cell cycle at G0 with similar potency to sirolimus. Grube et al were the first to demonstrate the feasibility of a BES with a biodegradable polymer, by reporting a significantly reduced late loss and neointimal volume with BES compared with a BMS. More recently, these findings have been confirmed by other studies of biolimus eluting stents which have enrolled more diverse patient populations8-12.
The BioMatrix™ Flex has previously been shown to be non-inferior to the Cypher® sirolimus eluting stent (SES) (Cordis, Warren, NJ, USA) in terms of major adverse cardiovascular events (MACE) at nine months follow-up (9% vs. 11%, p for non-inferiority=0·003, p for superiority=0·39) in the randomised LEADERS (Limus Eluted from A Durable versus ERodable Stent coating) clinical trial10. The current report presents the outcomes of the LEADERS trial at
12-month clinical follow-up, which represents a pre-specified secondary endpoint of the study.
Methods
Study population
The methods of the LEADERS trial have been published previously10. In brief the study applied an all-comers approach recruiting 1,707 patients with chronic stable coronary artery disease or acute coronary syndromes (ACS) including ST-elevation myocardial infarction (STEMI), who were eligible for enrolment if they had at ≥1 lesion with diameter stenosis (DS) ≥50% and a reference vessel diameter (RVD) 2.25-3.5 mm. The principle exclusion criteria were: known allergy to acetylsalicylic acid, clopidogrel, heparin, stainless steel, sirolimus, biolimus or contrast material that cannot be pre-medicated, planned surgery within six months of PCI unless the dual anti-platelet therapy (DAPT) could be maintained throughout the peri-operative period, pregnancy, participation in another trial before reaching the primary endpoint and lastly inability to give informed consent. The study complied with the Declaration of Helsinki and was approved by all institutional ethics committees. All patients provided written, informed consent for participation in the trial.
Randomisation and procedures
Patients were randomly allocated on a 1:1 basis to treatment with either a BES or SES, and to active angiographic follow-up at nine months or clinical follow-up only on a 1:3 basis with a factorial design.
BES were available in diameters of 2.25-3.5 mm and in lengths of 8-28 mm, whilst SES were available in diameters of 2.25-3.5 mm and in lengths of 8-33 mm. Balloon angioplasty and stent implantation were performed according to standard technique, and direct stenting was allowed. The aim was to obtain full lesion coverage with one or several stents. No mixture of DES was permitted within a given patient, unless the operator was unable to insert the study stent, in which case crossover to another device of the operator’s choice was possible. Procedural anticoagulation was achieved with unfractionated heparin 5,000 IU or 70-100 IU/kg, whilst the use of glycoprotein IIb/IIIa inhibitors was left to the operator’s discretion. Pre-procedure all patients enrolled into the study received ≥75 mg of acetylsalicylic acid, and at least 300 mg of clopidogrel. All patients were discharged on ≥75 mg of acetylsalicylic acid indefinitely, and clopidogrel 75 mg for a minimum of 12 months following the index procedure. In the case of inter-current revascularisation procedures needing stent implantation, treating cardiologists were encouraged to use study stents.
Follow-up
Adverse events were assessed in hospital, and clinical follow-up was performed at 1, 6, 9, and 12 months. Additional clinical follow-up is planned at yearly intervals to five years. One in four patients was asked to return for angiographic follow-up at nine months.
Study endpoints
The clinical primary endpoint of this study was MACE, defined as the composite of cardiac death, myocardial infarction (MI), and clinically-indicated target vessel revascularisation (TVR) at 12 months. Secondary endpoints were death from any cause, cardiac death, MI, any target lesion revascularisation (TLR) (both clinically and non-clinically indicated); any TVR, and ST.
A blinded independent clinical events committee adjudicated all endpoints, and independent study monitors verified all case reports from data on-site. The operators were by necessity aware of the assigned study stent during PCI and angiographic follow-up, but patients and staff involved in follow-up assessment were blinded to the allocated stent type. Angiography films were centrally assessed at one angiographic core laboratory (Cardialysis, Rotterdam, Netherlands) with assessors unaware of the allocated stent.
Definitions
Definitions of all endpoints are provided in full elsewhere10. MI was defined using the electrocardiographic criteria of the Minnesota code, or by a measured level of creatinine kinase (CK) two times the upper limit of normal (ULN), with either a positive concentration of CK-myoglobin fraction, or troponin I or T. Revascularisation was regarded as clinically indicated if on quantitative coronary angiography (QCA) the lumen DS of the treated lesion was ≥50% in the presence of ischaemic signs or symptoms, or ≥70% in the absence of ischaemia. TVR was defined as any repeat PCI or surgical bypass of any segment within the entire major coronary vessel proximal and distal to a target lesion, including upstream and downstream branches and the target lesion itself. TLR was defined as a repeat revascularisation due to a stenosis within the stent or within a 5 mm border proximal or distal to the stent. ST was defined according to the Academic Research Consortium definitions13.
Statistics
This trial was powered for non-inferiority on the primary clinical endpoint at nine months. The rationale behind this and for the sample size is reported elsewhere10. In this paper continuous variables are expressed as mean ±standard deviation; and categorical data is presented as frequency (percentages). Patient demographic data was compared using the Student t-test, whilst χ2 was used for categorical data. Survival curves were constructed for time-to-event variables using Kaplan-Meier estimates, and compared by the log-rank test. The Mantel-Cox model was used for the rate ratios of clinical outcome. All analyses were performed using SAS 8.02 by a dedicated statistician. All p-values and confidence intervals were two-sided; p<0.05 was considered statistically significant.
Results
Follow-up
Figure 1 shows the clinical follow-up of patients from enrolment to 12 months, on an intention to treat basis. Overall clinical follow-up was available in 1,666 patients (97.6%) made up of 837 of the original 857 BES patients, (97.7%) and 829 of the original 850 SES patients (97.5%). The reasons for incomplete follow up are shown in Figure 1.
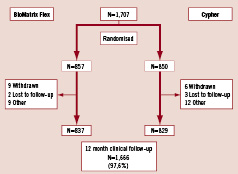
Figure 1. Flow chart of clinical follow-up of patient population.
Patient population and lesion characteristics
The baseline demographic, clinical and angiographic characteristics, have been published previously10 and are summarised in Table 1.
Procedural results and outcomes of nine month clinical and angiographic follow-up have all been presented elsewhere10. There was no significant difference in procedural characteristics between both groups. Similarly clinical outcomes at nine months in terms of death, cardiac death, MI, clinically-indicated TLR and TVR, any TLR and TVR were also comparable for both stents. Overall the primary endpoint at nine months met the pre-specified criteria for non-inferiority (BES 9% vs. SES 11%, p for non-inferiority=0.003, p for superiority=0.39).
Nine-month angiographic follow-up results were available in 335 patients (168 BES, and 167 SES), representing 78.5% of those allocated to angiographic follow-up. BES was non-inferior to SES for the angiographic outcome in-stent percentage stenosis (20.9% vs. 23.3%, p for non-inferiority=0.001). There were no significant differences in superiority testing in other angiographic parameters such as in-stent and in-segment percentage stenosis, late loss, and binary restenosis.
Symptom control and medication at 12 months
Symptomatically at 12 months 14.5% of patients (13.2% BES, 15.7% SES) still experienced angina pectoris, or had clinical evidence of on-going silent ischaemia. In the majority of these patients the angina was stable, and at Canadian Cardiovascular Society (CCS) angina class 1. Over 80% of patients were on a beta-blocker, a statin and either an angiotensin converting enzyme inhibitor or angiotension-II blocker.
Clinical outcomes at 12 months
The hierarchical and non-hierarchical events at one year clinical follow-up are shown in Table 2. At 12-month follow-up the use of BES was associated with similar rates of death and cardiac-death. Whilst patients treated with BES experienced a numerically greater number of MIs at 12-month follow-up (5.8% vs. 4.6%, p=0.26), which was largely driven by the 0.9% higher rate of periprocedural MI recorded with BES, they also experienced numerically lower rates of TLR and TVR. The absolute differences between event rates observed with BES and SES were similar at nine and 12 months as can be appreciated in the Kaplan Meier survival curves (Figure 2).
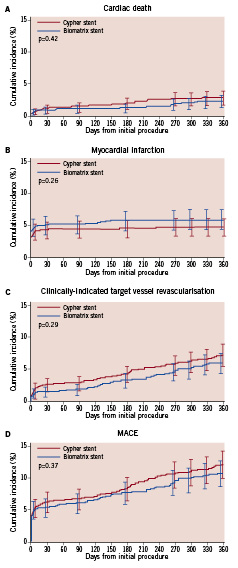
Figure 2. Kaplan Meier event curves at 12 months for (A) cardiac death, (B) myocardial infarction, (C) clinically-indicated target vessel revascularisation and (D) major adverse cardiovascular events.
The rates of ST as per ARC definitions are listed in Table 3. There was no significant difference in definite, probable or possible, early or late ST between both groups. Table 4 gives a detailed description of each late ST, and indicates that eight of the nine (88.9%) events occurred in patients still taking DAPT. Furthermore, the only ST event to occur after nine months, when the BES polymer is expected to have completely biodegraded, occurred in a patient treated with BES. This patient however was also the only patient who prematurely discontinued DAPT in view of an impending elective operation.
Discussion
This randomised prospective study has confirmed the comparable clinical outcomes at one year follow-up, in an ‘all-comers’ population, of the BES with a biodegradable polymer when compared to the sirolimus eluting stent with a durable polymer.
Early trials of new DES recruited patients with simple, de novo lesions, and although important, their results are not applicable to the 60-70% of today’s PCI patients who receive DES for ‘off-label’ indications.14 Compared to ‘on-label’ use, the use of DES for ‘off-label’ indications is associated with poorer outcomes and a higher risk of ST14,15. The current study had an ‘all-comers’ design, such that over half of the patients enrolled had an acute coronary syndrome (unstable angina, Non-ST elevation MI, ST-elevation MI), and over three-quarters of patients had stenting for an ‘off-label’ indication. Therefore, it comes as no surprise that the overall event rates reported in this study are somewhat higher than those observed in earlier trials of new DESs16. Notwithstanding this, these results can be regarded as being more applicable to routine clinical practice.
The formal comparison of outcomes in patients treated for ‘on-label’ versus ‘off-label’ indications is not yet available. However post hoc sub-group analysis of diabetic patients indicates significantly reduced in-stent restenosis with the use of BES compared to SES at nine months angiographic follow-up (21.79%±19.42 vs. 33.57%±25.42, p=0.01), whilst at 12-month clinical follow-up, insulin treated diabetics treated with BES had a significantly reduced rate of mortality (p<0.01)17. Overall no significant difference in clinical outcomes were observed between patients with ACS treated with either stent, however, in patients with STEMI, the use of BES was associated with a significantly lower rate of 12 month cardiac death (p=0.04) and MACE (p=0.02) compared with SES. This was largely driven by reduction in sub-acute ST and TVR within the first 30 days18. Other available analyses indicates similar performance between both stents in the management of patients with bifurcation lesions19, lesions in vessels less than 2.75 mm in diameter20, and lesions longer than 20 mm21.
The higher rate of MI noted with BES was largely driven by periprocedural events, as opposed to spontaneous MIs. Post hoc analysis has demonstrated over half of these periprocedural events occurred in patients who had at least one bifurcation lesion, with procedural factors, i.e., more frequent pre-dilatation suggested as a possible cause19. Furthermore these periprocedural MIs did not have any effect on cardiac death. At 30 days, 42 patients experienced an MI, and only three patients experienced a cardiac death. Currently the significance of a periprocedural MI has not been clearly established; recent studies demonstrate that rises in cardiac enzymes post-PCI are common and may predict short-term prognosis22, but do not influence long-term prognosis22, especially if the procedure is successful23.
ST is one of the most prominent concerns with the widespread use of DES in daily clinically practice1-4. The occurrence of ST remains largely unpredictable and no specific causative factor has been identified. One area where concern has been focused is the potential of durable, or permanent polymers to precipitate very late ST. This may be the result of inducing a hypersensitivity reaction5,6, however recent histopathological studies have also shown that durable polymers can also cause localised vascular inflammation, hyper-eosinophilia, thrombogenic reactions, and apoptosis of smooth muscle cells, all of which may precipitate ST24-26. Specifically the non-erodible polymers poly (ethylene co-vinyl acetate) and poly (n-butyl methacrylate) found on the first generation Cypher® SES have been shown to induce hypersensitivity reactions in animal models and humans27,28. One recent advance in polymer technology has been the development of PLA biodegradable polymers, as found on the BioMatrix BES stent used in this study. This polymer is located only on the abluminal surface of the stent, which not only allows for better targeted drug release, but also reduces systemic exposure to both the polymer and biolimus. Furthermore, the polymer is co-released with biolimus, and biodegrades to carbon dioxide and water, such that only a stainless steel stent, which is free of any primer polymer, remains after
6-9 months of stent deployment. In theory this should potentially reduce the risk of precipitating late and very late ST.
The similar rates of ST observed in the current study however should not be considered to indicate the lack of benefit of a biodegradable polymer. This is primarily because the current study is underpowered to detect differences in ST. In reality a considerably larger study population, followed long term will be required to enable definitive conclusions to be drawn about whether a biodegradable polymer will impact significantly on rates of late/very late ST.
The limited long-term data that is available on metallic stents with biodegradable polymers show promising results, and suggests, as indicated in this study, the absence of significant repeat revascularisations or clinical events following the complete biodegradation of the polymer. The Excel stent (JW Medical Systems, China) is a stainless steel stent, coated with sirolimus and a PLA biodegradable polymer, which completes degradation in 6-9 months. A registry of over 2,000 patients has recently shown at 18-month follow-up, a rate of MACE of 3.1%, and despite 80.5% of patients discontinuing clopidogrel at six months, a rate of ST of 0.87%29. The longest follow-up data is available from the Nobori phase I trial which randomised the Nobori™ (Terumo, Japan) BES with a PLA biodegradable polymer
to the TAXUS® Express2 (Boston Scientific, Natick, MA, USA) paclitaxel eluting stent in 120 patients. After biodegradation of the polymer at 6-9 months there were no ST events, TVR or target vessel failures (cardiac death, MI-target vessel related, clinically driven TVR) in those treated with the Nobori stent, however the population enrolled was considerably less complex than the current study30.
This limited data set indicates the importance of the long term follow-up results of the present study, which should help in establishing whether biodegradable polymers will be vital components of future DES.
Limitations
One limitation with the results of the current study are their reproducibility when considering that the PCI procedures were performed by experienced operators, in high volume centres throughout Europe.
Conclusions
The present report demonstrates the safety and efficacy during 12-month clinical follow-up of a biolimus eluting stent with a biodegradable polymer, and indicates that this stent is a suitable alternative to a sirolimus eluting stent with a durable polymer, in patients with simple and complex coronary artery disease.

