
Abstract
Aims: The aim of this study was to establish the long-term safety and efficacy of a sirolimus-eluting stent with bioresorbable polymer (BP-SES; Ultimaster) by comparison with an everolimus-eluting stent with permanent polymer (PP-EES; XIENCE).
Methods and results: CENTURY II (Clinical Evaluation of New Terumo Drug-Eluting Coronary Stent System in the Treatment of Patients with Coronary Artery Disease) is a large-scale, prospective, multicentre, randomised single-blind, controlled, non-inferiority trial conducted at 58 study sites globally, including Europe, Japan and Korea, powered to prove non-inferiority for freedom from target lesion failure (TLF: cardiac death, target vessel-related myocardial infarction [MI] and target lesion revascularisation) at nine months. Patients requiring a percutaneous coronary intervention (PCI) were randomised (1:1) to BP-SES (n=551) or PP-EES (n=550). Freedom from TLF at five years was 90.0% in the BP-SES and 91.1% in the PP-EES group (p=0.54). The patient-oriented composite endpoint (all death, any MI, any revascularisation) was 24.1 and 25.6% (p=0.57) with BP-SES and PP-EES, respectively. The very late stent thrombosis rate from one to five years was especially low at 0.2% in both arms.
Conclusions: This randomised clinical trial showed that the BP-SES stent was non-inferior to the benchmark PP-EES stent for TLF. Safety and efficacy measures were comparable up to five-year follow-up after PCI.
Abbreviations
ACS: acute coronary syndrome
BARC: Bleeding Academic Research Consortium
BMS: bare metal stent
BP-SES: bioresorbable polymer sirolimus-eluting stent
CAD: coronary artery disease
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
MI: myocardial infarction
(N)STEMI: (non-)ST-segment elevation myocardial infarction
PCI: percutaneous coronary intervention
PP-EES: permanent polymer everolimus-eluting stent
ST: stent thrombosis
TLF: target lesion failure
TLR: target lesion revascularisation
TVF: target vessel failure
TVR: target vessel revascularisation
(VL)ST: (very late) stent thrombosis
Introduction
Percutaneous coronary intervention (PCI) with drug-eluting stents (DES) significantly reduced neointimal proliferation and restenosis risk, when compared to bare metal stents (BMS)1. Concerns were raised when, after DES treatment, reports of a higher risk of (very) late stent thrombosis (VLST) appeared in a few studies2,3. Delayed vessel healing and stent endothelialisation, linked to a lack of biocompatibility of stent polymers used in first-generation DES, were identified as the main culprits of increased ST4.
Since then, major advances have taken place in stent design, including changes in platform structure, use of antiproliferative agents and polymer composition. The Ultimaster® stent (Terumo Corp., Tokyo, Japan) belongs to this new generation of DES systems. It comprises a thin-strut cobalt-chromium platform, with gradient, abluminal, bioresorbable coating (a polymeric matrix carrier with incorporated sirolimus drug)5.
The CENTURY II trial (Clinical Evaluation of New Terumo Drug-Eluting Coronary Stent System in the Treatment of Patients with Coronary Artery Disease study) was designed to assess, in a general population of coronary artery disease (CAD) patients referred for PCI, whether Ultimaster (BP-SES) achieves similar (non-inferiority study design) clinical outcomes to the gold standard, everolimus-eluting XIENCE stent (Abbott Vascular, Santa Clara, CA, USA), which has a circumferential (biocompatible) permanent polymer (PP-EES) coating. The trial reached the primary endpoint of freedom from target lesion failure (TLF: a composite of cardiac death, target vessel-related myocardial infarction [MI] and target lesion revascularisation) at nine months. In the per-protocol analysis, 95.6% of patients treated with BP-SES and 95.1% patients treated with PP-EES were free from TLF at nine months, with an absolute risk difference of 0.55% in favour of the BP-SES group (pnon-inferiority<0.0001)6.
The present study reports long-term clinical safety and efficacy data and compares five-year clinical outcomes in both treatment arms.
Methods
Detailed methods can be found in the Supplementary Appendix 1.
STUDY DESIGN
The CENTURY II trial is a prospective multicentre randomised, single-blind, controlled, non-inferiority two-arm clinical trial, comparing BP-SES with PP-EES (study registration number: UMIN000006940). Details of the study design have been reported elsewhere6.
STUDY POPULATION AND RANDOMISATION
A total of 1,119 eligible CAD patients, scheduled for PCI, were enrolled at 58 centres in Europe (42 sites), Japan (15 sites) and Korea (one site) (see list in Supplementary Appendix 2.). Patients were randomly assigned in a 1:1 proportion to undergo PCI with either BP-SES or PP-EES. Randomisation was stratified for cohort JR (Japanese requirements: the subset of patients matching requirements for DES in Japan) and balanced for diabetes mellitus, acute coronary syndrome (ACS) and multivessel disease. Detailed inclusion and exclusion criteria have been reported previously6. The study complied with the Declaration of Helsinki and was approved by the institutional review board at each participating centre and the competent authority of each participating country. All patients provided written informed consent before undergoing any study-specific procedure.
STUDY DEVICES
The bioresorbable polymer-containing sirolimus-eluting Ultimaster stent (BP-SES) uses a cobalt-chromium metal platform with thin struts (80 µm) with bioresorbable PDLLA-PCL (poly D,L-lactide-co-caprolactone) polymer. The permanent polymer everolimus-eluting stent XIENCE (PP-EES) is a second-generation DES, based on a cobalt-chromium alloy platform with a strut thickness of 81 µm.
ENDPOINTS AND DEFINITIONS
Clinical outcomes at one-year and five-year follow-up include: (i) freedom from TLF, a device-oriented composite endpoint (cardiac death, MI not clearly attributable to a non-target vessel, and clinically driven target lesion revascularisation [TLR]); (ii) rate of target vessel failure (TVF), defined as a composite of cardiac death and MI not clearly attributable to a non-target vessel, and clinically driven target vessel revascularisation (TVR); (iii) patient-oriented composite endpoint composed of all deaths, all MI and all coronary revascularisations; (iv) rates of TLR, TVR, ST, cardiac death, MI; (v) composite of cardiac death and MI; (vi) rate of bleeding and vascular complications according to Bleeding Academic Research Consortium (BARC) definitions7. The endpoints are defined as per Academic Research Consortium recommendations8.
STATISTICAL ANALYSIS
Differences between randomisation arms were assessed by the Student’s t-test, analysis of variance, or non-parametric test (i.e., Mann-Whitney), as appropriate. The Kaplan-Meier method was used to estimate event rates for time-to-event outcomes, and data were compared with the log-rank test. A landmark Kaplan-Meier analysis was performed at one year. To explore whether TLF with BP-SES vs. PP-EES was consistent across subgroups, logistic regression analysis was performed. All analyses were carried out using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
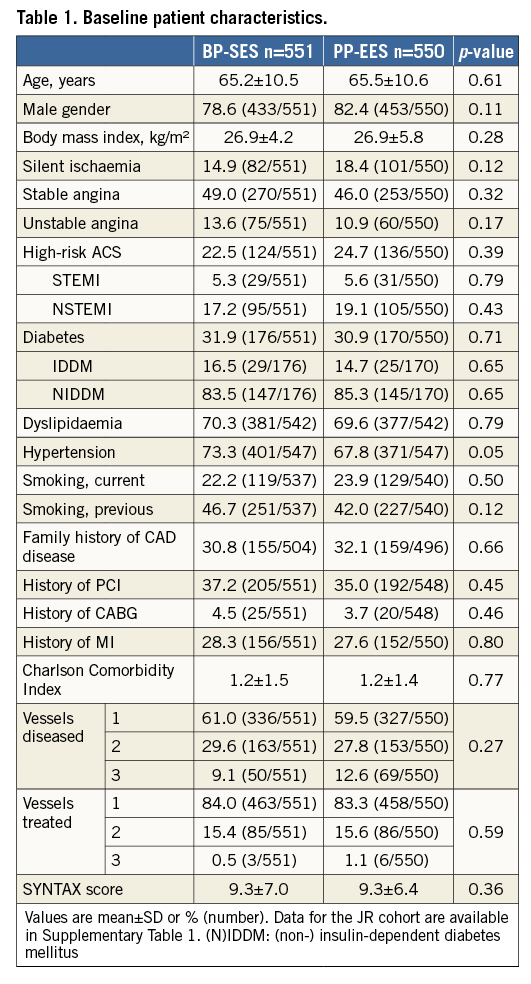
BASELINE PATIENT AND PROCEDURAL CHARACTERISTICS
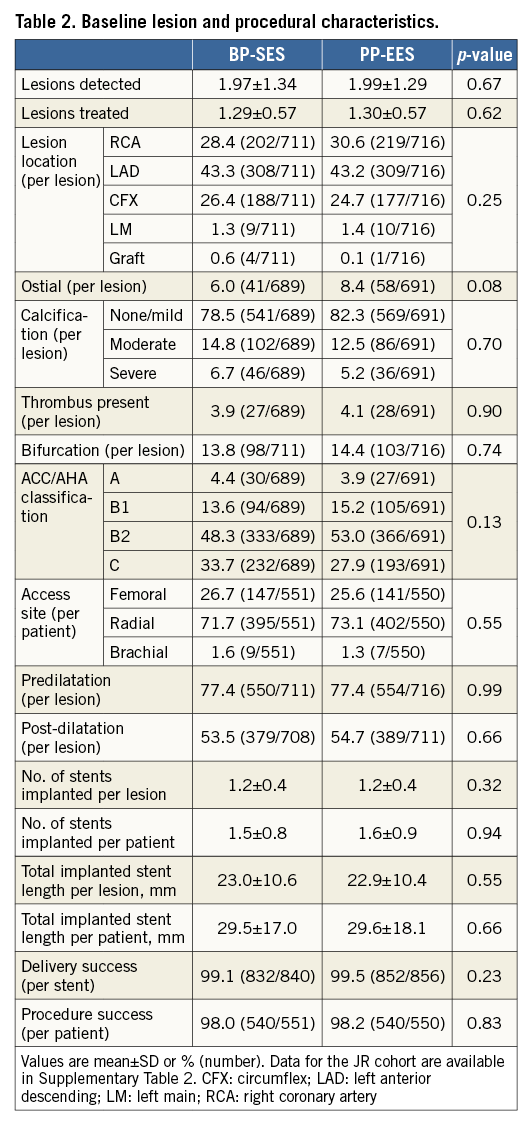
Out of 1,119 patients (intention-to-treat), 1,101 patients (551 in the BP-SES and 550 in the PP-EES arm) were included in the per-protocol analysis (Figure 1). The main baseline characteristics were similar in both arms (Table 1). The frequency of cardiovascular risk factors was similar in both groups (Table 1), apart from a slightly higher frequency of arterial hypertension in the BP-SES arm. A total of 1,427 lesions were treated (711 in BP-SES and 716 in PP-EES). Type B2/C lesions were frequently present and most lesions were treated through the radial access (Table 2). The per-protocol analysis in the cohort with Japanese requirements (cohort JR) included 362 patients treated with BP-SES and 353 patients treated with PP-EES (Supplementary Table 1, Supplementary Table 2).
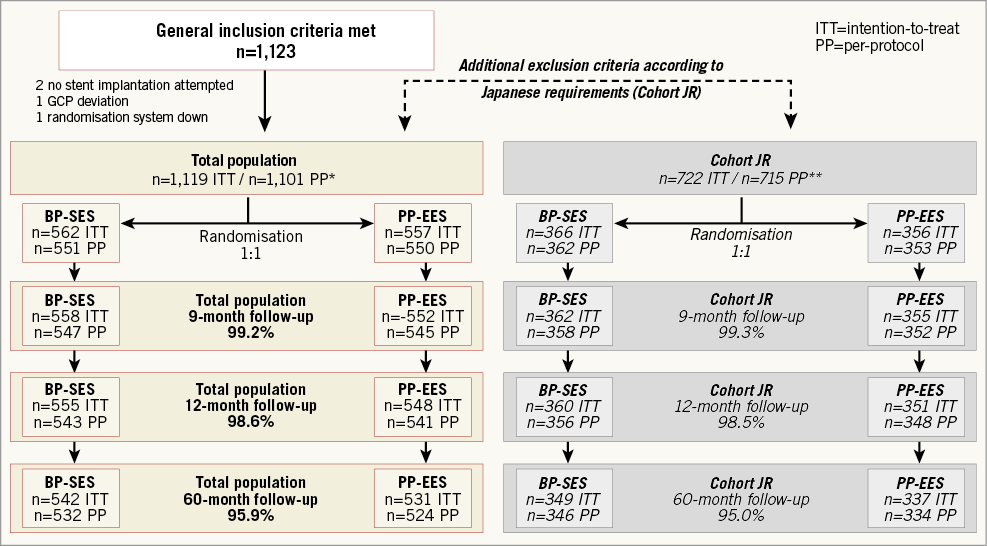
Figure 1. Study flow chart. *1,101 patients analysed per protocol for total population: 22 major protocol deviations. **715 patients analysed per protocol for cohort JR: nine major protocol deviations. Japanese requirement (JR): patients who met criteria matching approved indication for drug-eluting stents in Japan. BP-SES: bioresorbable polymer sirolimus-eluting stent; PP-EES: permanent polymer everolimus-eluting stent
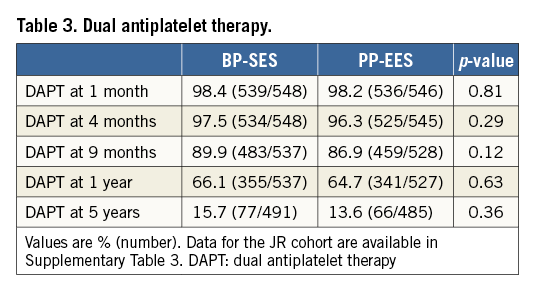
DUAL ANTIPLATELET THERAPY
Table 3 gives the number of patients in each treatment arm using DAPT at 1, 4, 9 months, 1- and 5-year follow-up. The use of DAPT in the cohort JR is shown in Supplementary Table 3.
LONG-TERM CLINICAL OUTCOMES
One-year clinical outcomes (Table 4) showed no significant difference between BP-SES and PP-EES in all-cause death, any MI, revascularisations or any of the composite endpoints. Rates of ST, bleeding and vascular complications were similar between the two arms. These findings were observed in the total population and in the cohort JR (Supplementary Table 4).
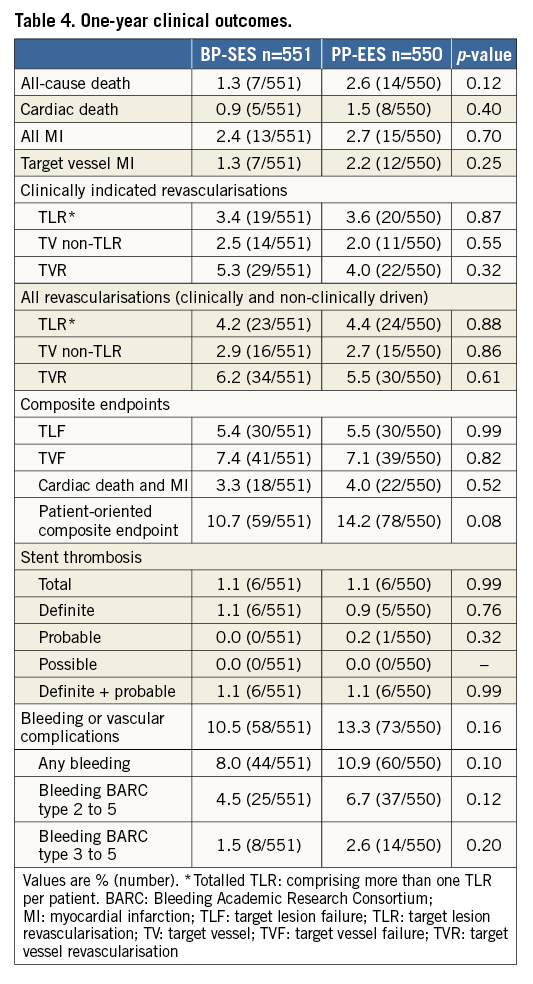
Figure 2 shows the TLF-free rates at five-year follow-up. No differences were found between the two treatment arms (90.0% for BP-SES versus 91.1% for PP-EES; p=0.54 in the total population). At five-year follow-up, all-cause death, any MI or revascularisations did not differ (Table 5) in the total population or in the cohort JR (Supplementary Table 5). At five years, the composite safety endpoint of cardiac death and MI was 5.8% for BP-SES and 7.1% for PP-EES (p=0.39), and the patient-oriented composite endpoint was 24.1% for BP-SES and 25.6% for PP-EES (p=0.57) (Table 5, Figure 3). Rates of ST were also similar in both arms (Table 5). Bleeding and vascular complications at five-year follow-up were reported in 19.2% of patients in the BP-SES arm and 19.1% of patients in the PP-EES arm (p=0.95).
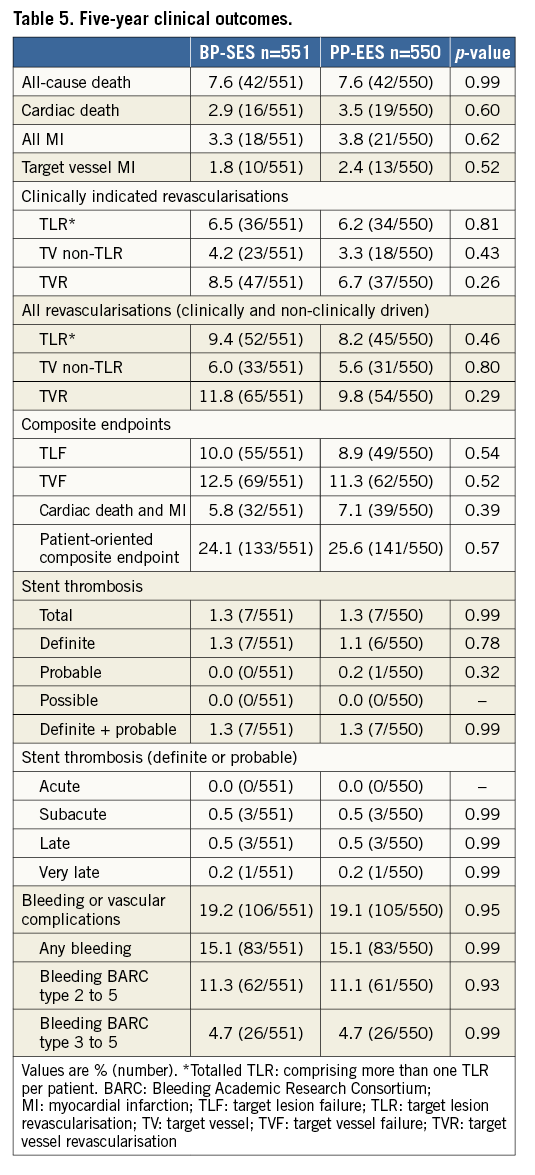
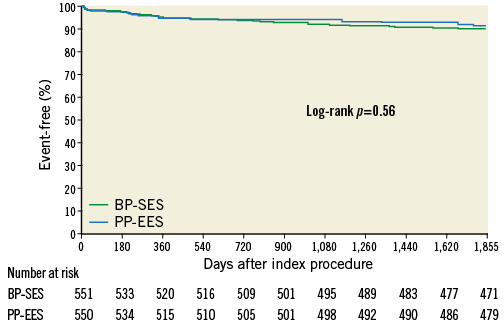
Figure 2. Kaplan-Meier curve for target lesion failure-free event rate (%) at five-year follow-up. BP-SES: bioresorbable polymer sirolimus-eluting stent; PP-EES: permanent polymer everolimus-eluting stent
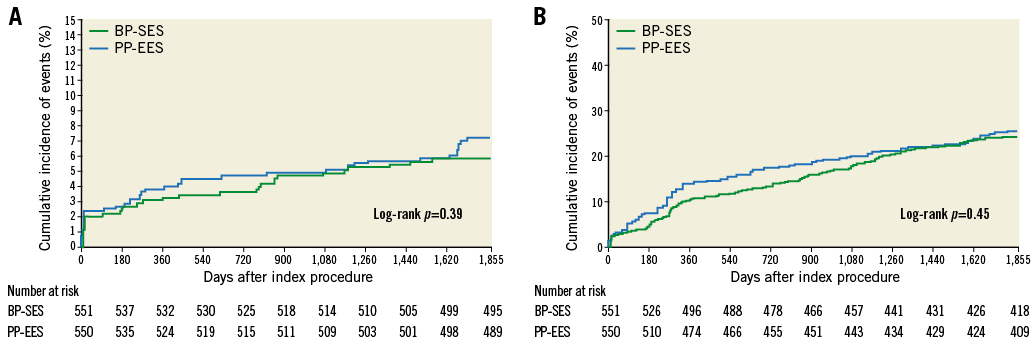
Figure 3. Kaplan-Meier curves for five-year safety endpoints. Composite of cardiac death and MI (A) and patient-oriented composite endpoint (POCE) of any death, any MI and any coronary revascularisation (B). BP-SES: bioresorbable polymer sirolimus-eluting stent; PP-EES: permanent polymer everolimus-eluting stent
Results of a landmark analysis between one and five years are reported in Supplementary Table 6 and Supplementary Figure 1.
SUBGROUP ANALYSIS
Figure 4 shows the relative risk of five-year TLF in high-risk subgroups including diabetes, patients with long lesions (>25 mm), bifurcation, high-risk ACS and multivessel disease. No significant differences were observed between treatment arms.
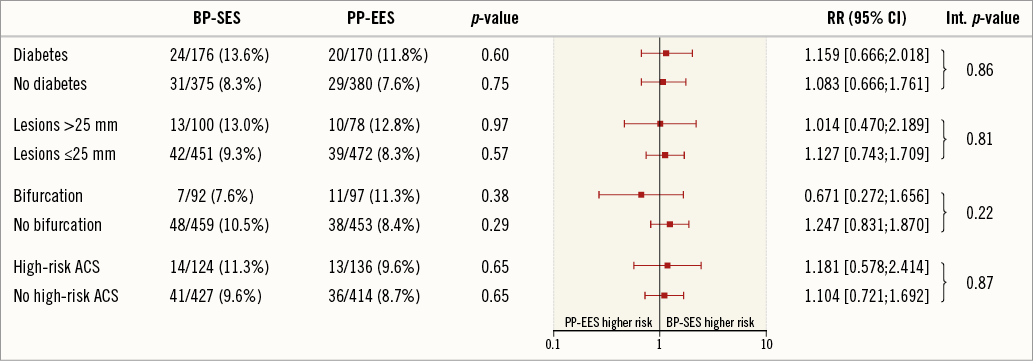
Figure 4. Subgroup analysis: relative risk with 95% confidence interval (CI) of target lesion failure (TLF) at five years. Int. p-value: p-value for interaction
Discussion
We report the longest available clinical data concerning safety and efficacy following bioresorbable polymer sirolimus-eluting stent implantation, showing comparable clinical outcomes to the current benchmark device, a durable polymer everolimus-eluting stent, up to five years of follow-up. Rates of VLST are remarkably low, showing excellent safety of both investigated devices.
OVERALL DEVICE PERFORMANCE
The principal findings of this analysis are that device-related events (TLF) were low and comparable with both stents up to five years of follow-up (10.0% [BP-SES] vs. 8.9% [PP-EES]; p=0.54). The low incidence of device-related events with bioresorbable polymer is in line with prior analyses of this subclass of devices. In the ISAR-TEST 4 trial, TLF at five years was not significantly different between BP-DES and PP-EES (20.5% vs. 19.5%, respectively, hazard ratio [HR] 1.04, 95% CI: 0.84-1.29; p=0.71)9. In the COMPARE II trial (Abluminal Biodegradable Polymer Biolimus-Eluting Stent Versus Durable Polymer Everolimus-Eluting Stent), TLF rates at five years for BP-biolimus-eluting stents and PP-EES were comparable (respectively, 13.4% vs. 11.5%; p=0.17)10, and similar to this report. Five-year results from the NEXT trial confirm these findings, showing comparable TLF rates of 13.9% vs. 13.6% (p=0.84) for BP-BES vs. PP-EES11.
Since then, the XIENCE stent has built up a favourable efficacy and safety profile during short-, medium- and long-term follow-up and is considered to be the benchmark device for evaluation of emerging new DES9. Hence, the observed clinical performance of the Ultimaster stent resembles that of XIENCE at all analysed time points and places it alongside the best-in-class DES.
VERY LATE STENT THROMBOSIS
One of the most noteworthy findings was the very low rate of VLST with both stents (0.2% vs. 0.2%, respectively; p=0.99). Following landmark publications such as the LEADERS trial (Limus Eluted From A Durable Versus ERodable Stent Coating) and network meta-analysis, concerns were raised about the long-term safety of first-generation DES. Increased VLST rates were intuitively attributed to persistent inflammatory stimulus arising from the mere presence of durable polymer, long after complete drug elution12. LEADERS was the first randomised trial to show long-term benefit of BP-DES over first-generation PP-DES. At five years, the BP-DES stent was statistically non-inferior to the PP-DES stent for the primary composite endpoint of cardiac death, MI, and clinically indicated TVR, with observed rates of 22.3% and 26.1%, respectively. One of the most notable findings of the study was the increased safety of the BP-DES, due to a significant reduction in VLST, with event cases continuing to accumulate from one (2.2%) to five years (4.2%) in the PP-DES group only, while almost plateauing in BP-DES patients during the same period (2.0% to 2.6%). Similar findings came from a pooled analysis of 4,062 patients from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS study evaluating four-year clinical outcomes. The risk of ST was lower with BP-DES than with PP-DES controls, predominantly due to a significantly lower risk of VLST (HR 0.22, 95% CI: 0.08-0.61; p=0.004)13.
It was hypothesised that bioresorbable polymer leaving an inert bare metallic platform following optimal drug release would prevent VLST. Results from NOBORI-1 showed some superiority to first-generation PP-DES with rates of definite and probable ST of 0.0% vs. 3.2% with bioresorbable and durable polymer stents, respectively (p=0.01)14. However, as Kang et al concluded in their meta-analysis, further all-round refinements in device design, reduced strut thickness and optimised elution process with reduced drug concentration and shortened elution periods may also have contributed to the observed findings15. Initial reports of randomised studies between BP-DES and new-generation PP-DES did not reproduce observed differences in early studies with respect to short- and medium-term safety outcomes16-18. However, the expected advantage of BP-DES was to be detected during longer observation periods, which are needed to elucidate the true value of biodegradable technology in contemporary PCI practice. Long-term reports of COMPARE II, ISAR-TEST 4 and EVOLVE failed to show any significant advantage in long-term safety of BP-DES compared to new-generation, biocompatible PP-DES9,10,19. Rates of ST were low and comparable in treatment groups, with just a few events occurring after 12 months across all studied populations. Further to this, the recent meta-analysis that included 16 RCTs, comprising 19,886 patients, addressed the effect of BP-DES design characteristics such as strut thickness, polymer degradation time and metallic platform material. None of the above proved to contribute to improved safety and efficacy compared to new PP-DES20. It is possible that active bioresorption during polymer elimination causes inflammation, making late benefit barely noticeable21. Very thin durable polymer DES designs also minimise metallic stimulus for thrombogenicity22 and increase the biocompatibility of new polymers, causing little or no inflammation23. Finally, given the current very low rates of VLST, none of the above-mentioned trials was powered to detect such minute differences in late safety outcomes.
Study limitations
This study has a number of limitations. First, the study was powered for non-inferiority of BP-SES over PP-DES, in terms of TLF at nine months. This long-term report mainly provides hypothesis-generating insights. Second, the observed findings may not be applicable to all subgroups of this all-comer population. The overall SYNTAX score of 9 is low, mainly due to the additional exclusion criteria required in Japanese patients. In the overall population, patients with high-risk ACS, bifurcation lesions, left main and ostial lesions were included, adding evidence to the generalisability of our findings to an everyday population of CAD patients.
Conclusions
Biodegradable polymer and permanent polymer DES showed comparable clinical outcomes at five years. The observed rates of VLST were remarkably low, confirming the excellent safety of both investigated devices.
| Impact on daily practice This report presents the longest available clinical data regarding efficacy and safety following bioresorbable polymer sirolimus-eluting stent implantation, showing that comparable clinical outcomes to the current benchmark device, a durable polymer everolimus-eluting stent, are maintained up to five years. Notably, observed rates of very late stent thrombosis are remarkably low, supporting the safe use of this thin-strut stent in routine clinical PCI practice. |
Funding
CENTURY II was funded by Terumo Corporation, Tokyo, Japan.
Conflict of interest statement
B. Merkely reports personal fees from Abbott, Biotronik, Boston Scientific and Medtronic during the conduct of the study. Except for the institutional research grant received in the framework of the CENTURY II study, the other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Methods.
Supplementary Appendix 2. CENTURY II investigators.
Supplementary Figure 1. Kaplan-Meier curve for landmark analysis of target lesion failure-free event rate (%) up to one-year follow-up and between one-year and five-year follow-up.
Supplementary Table 1. Baseline patient characteristics of cohort JR.
Supplementary Table 2. Baseline lesion and procedural characteristics of cohort JR.
Supplementary Table 3. Dual antiplatelet therapy of cohort JR.
Supplementary Table 4. One-year clinical outcomes of cohort JR.
Supplementary Table 5. Five-year clinical outcomes of cohort JR.
Supplementary Table 6. Landmark analysis between 1 year and 5 years.
To read the full content of this article, please download the PDF.

