
Abstract
Aims: CENTURY II is a prospective, multicentre, randomised, single-blind trial comparing the bioresorbable polymer sirolimus-eluting Ultimaster® stent (BP-SES) with the permanent polymer everolimus-eluting XIENCE stent (PP-EES). Here we present a pre-specified analysis of safety and efficacy outcomes in a subgroup of patients with small vessel coronary artery disease.
Methods and results: CENTURY II included 525 patients with lesions of reference diameter ≤2.5 mm. Treatment was randomly assigned: 277 patients received BP-SES (399 lesions) and 248 patients received PP-EES (377 lesions). The primary outcome was target lesion failure (TLF), which is a composite of cardiac death, target vessel-related myocardial infarction (MI) and target lesion revascularisation (TLR). There was no significant difference between treatment groups in baseline or procedural data. Mean pre-procedural reference diameter was similar (BP-SES 2.30±0.40 mm, PP-EES 2.31±0.42 mm, p=0.59). Stented length was 24.0±11.7 mm for BP-SES and 23.5±11.5 mm for PP-EES (p=0.45). At 12 months, there was no significant difference between the BP-SES and PP-EES groups in TLF (6.9% vs. 7.7%; p=0.72), cardiac death (1.1% vs. 1.2%; p=0.90), target vessel MI (1.8% vs. 3.2%; p=0.30), TLR (4.0% vs. 5.7%; p=0.37), or definite or probable stent thrombosis (0.7% vs. 1.2%; p=0.57).
Conclusions: In the large-scale, randomised CENTURY II trial, use of BP-SES and PP-EES for the treatment of small vessel coronary artery disease resulted in similar outcomes at 12 months.
Introduction
Treating coronary artery disease in small vessels is associated with a higher restenosis rate and a higher risk of stent thrombosis after implantation of drug-eluting stents (DES) than treating larger vessels1-4. Small vessel coronary artery disease is frequently seen in coronary angiography and is often associated with diabetes mellitus or female gender3. In such a complex population, the chance to observe differences in safety or efficacy between modern DES is greater than in a population with stenosis of a large vessel. Recent data show that thinner struts reduce restenosis in small vessel coronary artery disease5-7. The risk of stent thrombosis8-10 and the need for target vessel revascularisation (TVR)8,9 was less with permanent polymer everolimus-eluting stents (PP-EES) than with other DES.
The randomised, multicentre, single-blind CENTURY II study compared PP-EES with the bioresorbable polymer sirolimus-eluting Ultimaster® stent (BP-SES) (Terumo Corporation, Tokyo, Japan). At nine months, BP-SES showed similar safety and efficacy to PP-EES in 1,123 patients11. Here we evaluate the clinical outcomes at 12 months in a subgroup of patients with small vessel coronary artery disease.
Methods
CENTURY II (Clinical Evaluation of New TerUmo dRug-eluting coronarY stent system in the treatment of patients with coronary artery disease) is a prospective, multicentre, randomised, single-blind trial comparing BP-SES with PP-EES (UMIN000006940). A total of 1,123 patients were included from 58 centres11. Of these, 525 patients (46.7%) suffered from small vessel disease with a reference diameter ≤2.5 mm by off-line quantitative coronary angiography (QCA). This is a pre-specified analysis of safety and efficacy outcomes in the subgroup of patients with small vessel coronary artery disease. Eligible patients were older than 18 years, had clinical evidence of ischaemic heart disease and/or a positive stress test. Exclusion criteria were: an allergy to sirolimus, everolimus, or dual antiplatelet treatment (DAPT); left ventricular ejection fraction <25%; bleeding diathesis or coagulopathy; cardiogenic shock; need for dialysis; or inability to provide written informed consent. The study complied with the Declaration of Helsinki and was approved by the institutional review board at each participating centre. All patients provided written informed consent.
Patients were randomised (1:1 ratio) to treatment with BP-SES or PP-EES. Randomisation was stratified for diabetes mellitus, ST-segment elevation myocardial infarction (STEMI) or non-ST-segment elevation myocardial infarction (NSTEMI), and presence of multivessel disease. Coronary interventions were performed according to standard hospital practice, including predilation, post-dilation and use of anticoagulation. DAPT was recommended for at least six months. Patients were scheduled for clinical follow-up at one, four, nine and 12 months (and yearly up to five years). A data monitoring committee reviewed all data. An independent clinical events committee reviewed and adjudicated all major adverse cardiac events. The study was managed by independent contract research organisations responsible for monitoring, data management and analysis. Data were collected and stored on an independent electronic data collection eClinical portal, Veracity (Merge Healthcare, Inc., an IBM Company, Morrisville, NC, USA). All data on case report forms were 100% verified on-site versus source documents.
The Ultimaster coronary stent system
The bioresorbable polymer sirolimus-eluting Ultimaster stent (BP-SES) uses a cobalt-chromium bare metal stent platform with thin struts (80 µm) and an open cell design. Within three to four months, the drug from the bioresorbable gradient coating is released and the bioresorbable abluminal polymer is metabolised. The Ultimaster DES was available in 2.5, 3.0, 3.5 and 4.0 mm diameters and in 12, 15, 18, 24 and 28 mm lengths.
The XIENCE stent
The permanent polymer (fluorinated copolymer) everolimus-eluting stent XIENCE® (PP-EES) (Abbott Vascular, Santa Clara, CA, USA) is based on a cobalt-chromium alloy stent with a strut thickness of 81 µm. To enable comparison, the sizes of XIENCE DES allowed in the CENTURY II study were diameters of 2.5, 3.0, 3.5 and 4.0 mm and lengths of 12, 15, 23 and 28 mm.
Quantitative coronary angiography
All pre-procedural, post-procedural and follow-up angiograms were analysed by an independent core laboratory (K.I.C. co Ltd, Kanagawa, Japan) using dedicated software (QAngio® XA, ver. 7.1; Medis, Leiden, The Netherlands). The following angiographic parameters were measured: minimum lumen diameter (MLD) before and after procedure, percent diameter stenosis (DS%), acute gain (defined as the change in MLD from baseline to the final procedural angiogram), and reference vessel diameter. Results were obtained for in-stent and in-segment regions.
Endpoints and definitions
The primary outcome was the rate of target lesion failure (TLF), a device-oriented composite endpoint (cardiac death, MI not clearly attributable to a non-target vessel, and clinically driven target lesion revascularisation [TLR]) at nine months post stent implantation. Secondary outcome measures were the rate of target vessel failure (TVF), defined as a composite of cardiac death, MI not clearly attributable to a non-target vessel, and clinically driven target vessel revascularisation (TVR), rate of TLR, stent thrombosis, and target vessel MI. The endpoints were defined according to the Academic Research Consortium12. Any death was considered cardiac unless clear non-cardiac causes could be determined. MI was defined either as the development of pathological Q-waves in at least two contiguous leads with or without elevated cardiac enzymes, or, in the absence of pathological Q-waves, as an elevation in creatinine kinase levels to greater than twice the upper limit of normal in the presence of an elevated level of creatine kinase MB (CK-MB) fraction or troponin. TLR was defined as repeat percutaneous intervention of the stented lesion including 5 mm proximal and distal from the edge of the stent, or bypass surgery of the target vessel that was performed for a clinical indication and was due to restenosis or occlusion of the target lesion. Device success was defined as achievement of a final diameter stenosis of <50% by QCA, and/or <30% by visual assessment, using the assigned device only.
Statistical analysis
The CENTURY II randomised study was powered for non-inferiority of BP-SES compared with PP-EES for the primary endpoint of nine-month TLF. The primary endpoint was met11 with a TLF (composite of cardiac death, target vessel-related myocardial infarction and TLR) of 4.4% for BP-SES and 4.9% for PP-EES demonstrating non-inferiority (pnon-inferiority=0.0001). The present analysis is confined to patients with small vessel coronary artery disease defined as reference diameter prior to intervention of ≤2.5 mm.
Categorical variables were compared using the χ2 statistic, Fisher’s exact test, or the Cochran-Mantel-Haenszel test. Continuous variables were compared using the Student’s t-test or non-parametric test (i.e., Mann-Whitney or Kruskal-Wallis test for multiple-group comparison). Dichotomous secondary clinical endpoints were tested using the χ2 test or Fisher’s exact method. The Kaplan-Meier method was used to estimate event rates for time-to-event outcomes, and data were compared with the log-rank test. For the continuous secondary outcome measures, the following summary statistics are presented: number, mean, median, standard deviation, and reference range (95%). The difference between randomisation arms was assessed by the Student’s t-test, analysis of variance, or non-parametric test (i.e., Mann-Whitney), as appropriate. To explore whether TLF with BP-SES versus PP-EES was consistent across clinical and angiographic subgroups, logistic regression analysis with interaction testing was performed. All analyses were performed according to intention-to-treat. All analyses were carried out using SAS software, Version 9.1 (SAS Institute, Inc., Cary, NC, USA).
Results
In the CENTURY II trial, 525 patients with 776 lesions with a reference diameter ≤2.5 mm were enrolled at 58 sites in 13 countries. This subgroup comprised 46.7% of all the patients in the trial (N=1,123) and 53.0% of all treated lesions (N=1,464). After randomisation, 277 patients were treated with BP-SES and 248 were treated with PP-EES.
Baseline characteristics did not differ significantly between the BP-SES and PP-EES groups, except for a higher frequency of history of smoking in the BP-SES group (Table 1). Most patients were male, and the prevalence of diabetes mellitus was similar between the groups (32% and 34%, respectively). Most patients had stable angina or silent ischaemia. An acute coronary syndrome (unstable angina, NSTEMI, STEMI) was the indication for percutaneous coronary intervention (PCI) in 35.3% of patients treated with BP-SES and 37.5% of patients treated with PP-EES. There was no significant difference between groups regarding treated vessels, number of lesions treated, multilesion or multivessel treatment (Table 2).
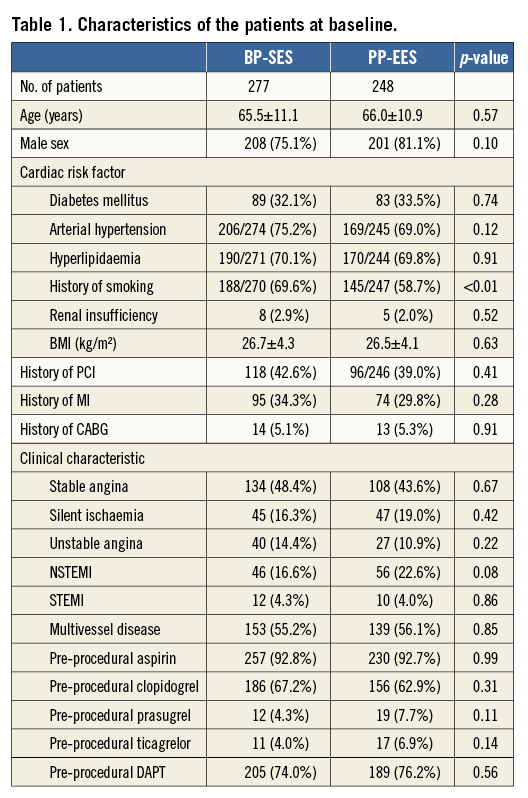
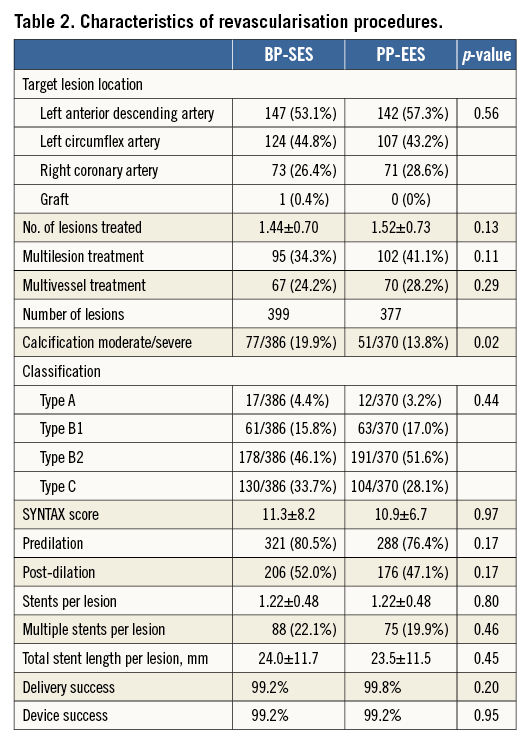
In all, 399 lesions were treated in the BP-SES group and 377 lesions in the PP-EES group. There was no significant difference regarding lesion complexity, use of predilation, need for post-dilation, number of stents, delivery, or device success. Implanted stent length was similar between the groups (mean lengths of 24.0 mm for BP-SES and 23.5 mm for PP-EES) (Table 2), as was the mean lesion length (17.2 mm and 15.7 mm, respectively, by QCA) (Table 3) and the mean pre-procedural reference diameter (2.30 mm and 2.31 mm, respectively). QCA pre- and post-PCI was comparable between the groups with respect to minimal lumen diameter, reference vessel diameter, and diameter stenosis for the in-stent and in-segment segment. Mean acute gain was also similar: 1.57 mm in the BP-SES group, and 1.55 mm in the PP-EES group for the in-stent segment (Table 3).
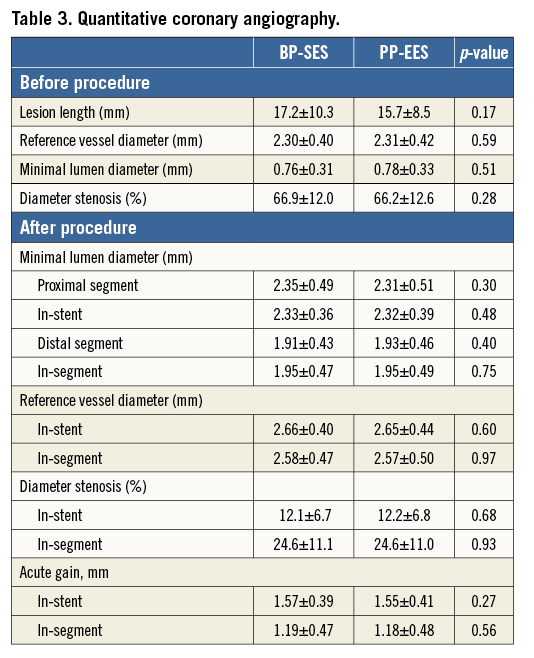
Clinical and angiographic outcome
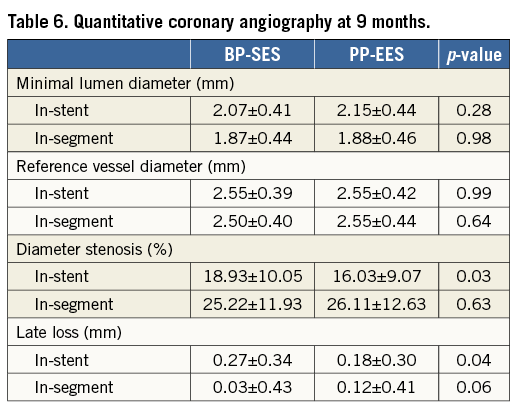
At 12 months, there was no significant difference between BP-SES and PP-EES with respect to primary and secondary outcome measures (Table 4). TLF occurred in 6.9% of patients treated with BP-SES and 7.7% of patients treated with PP-EES. Target vessel MI was documented in 1.8% vs. 3.2%, respectively, cardiac death in 1.1% vs. 1.2%, TLR in 4.0% vs. 5.7%, and definite or probable stent thrombosis in 0.7% vs. 1.2%. Use of DAPT did not differ between the BP-SES and PP-EES groups at the different time points: 97.5% vs. 97.2% at one month (p=0.83), 90.0% vs. 86.7% at nine months (p=0.24), and 67.4% vs. 63.5% at 12 months (p=0.36). Kaplan-Meier curves for TVF at 12 months (Figure 1) and definite or probable stent thrombosis at 12 months (Figure 2) were almost superimposable. In addition, there were no differences in clinical outcome at 12 months according to the vessel diameter (Table 5). Subgroup analysis demonstrated no significant interactions between treatment assignment and TLF outcomes at 12 months (Figure 3). In addition, angiographic follow-up at nine months was available for 756 lesions, including 386 treated with BP-SES and 370 lesions treated with PP-EES, and showed no difference in minimal lumen and reference diameter (Table 6). Late loss in the total segment was smaller with BP-SES, whereas late loss in the stented segment was smaller with PP-EES.
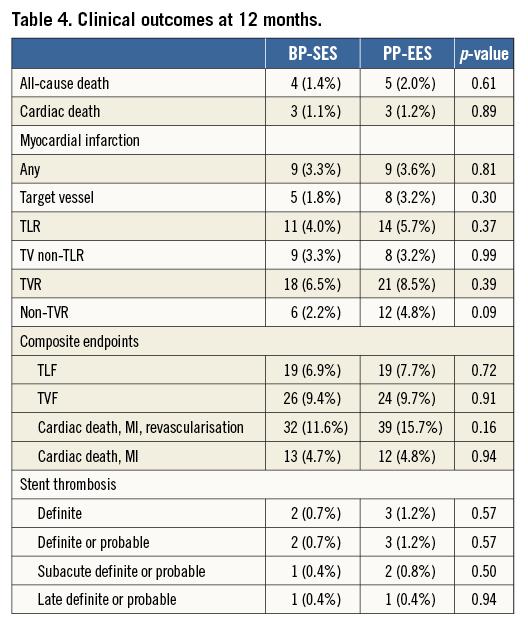
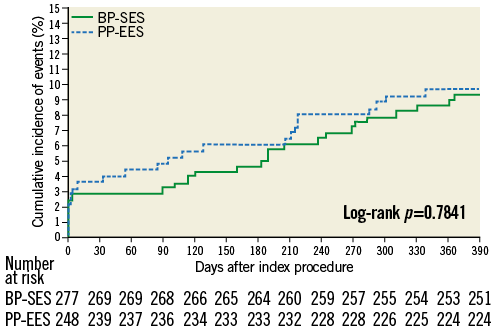
Figure 1. Cumulative frequency curves of target vessel failure for BP-SES vs. PP-EES.
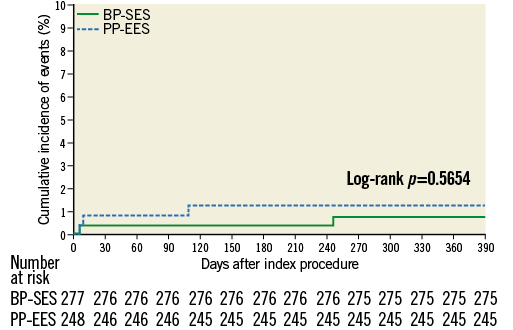
Figure 2. Cumulative frequency curves of the occurrence of definite or probable stent thrombosis for BP-SES vs. PP-EES.
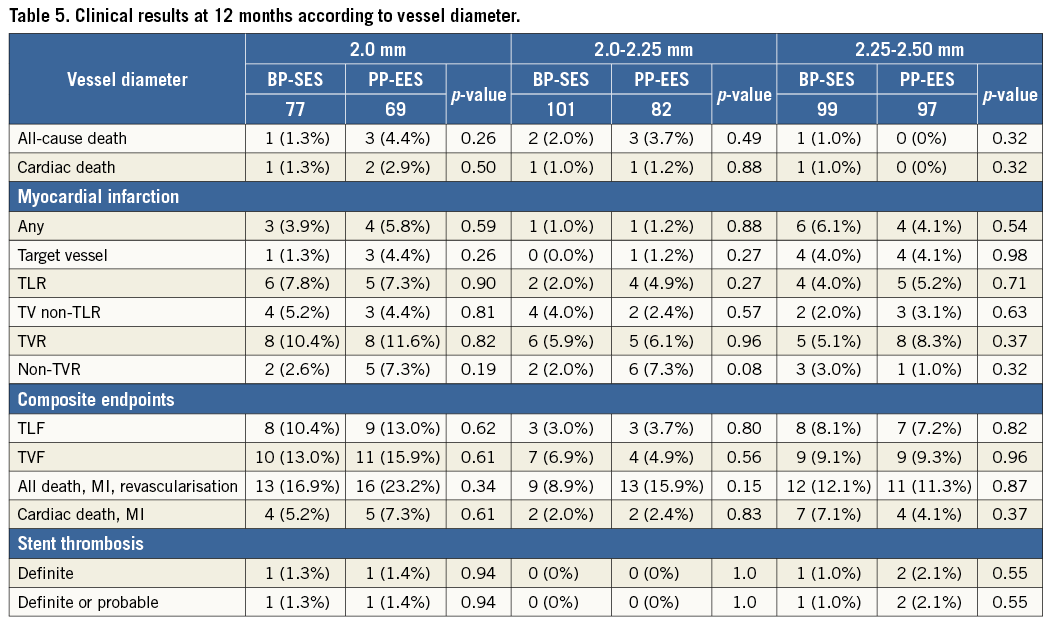
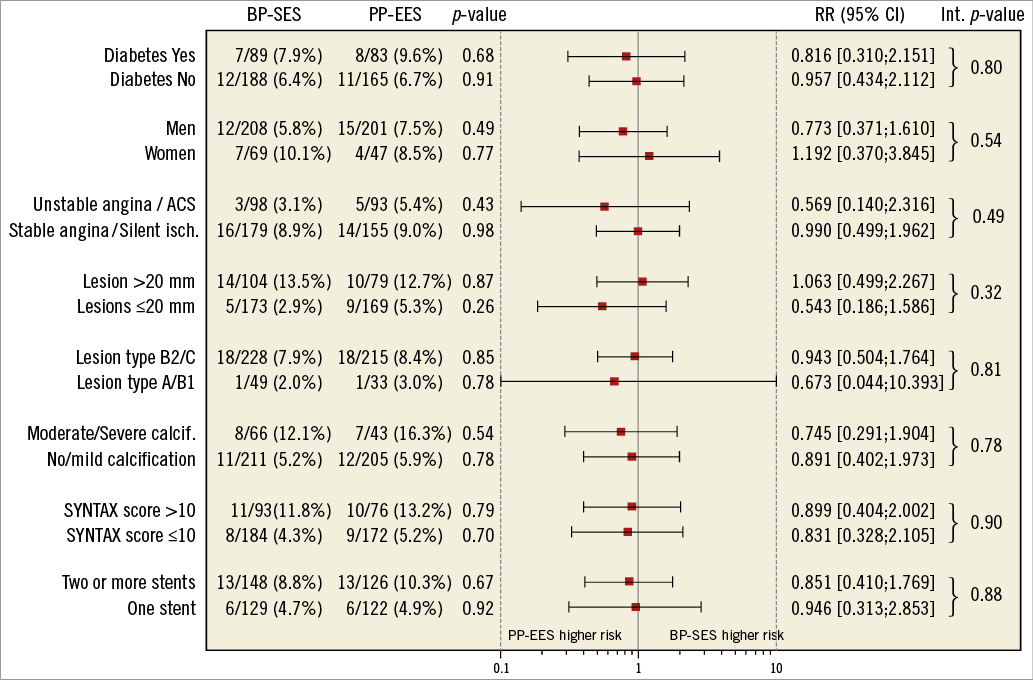
Figure 3. Subgroup analysis outcome: relative risk with 95% confidence interval of target lesion failure composite.
Discussion
In the prospective, randomised, multicentre CENTURY II trial, the treatment of stenosis in small coronary arteries with BP-SES or PP-EES resulted in clinical outcomes at 12 months that were similar between the treatment groups. The rates of TLF, TLR and stent thrombosis were low in both groups.
Our results extend the clinical safety and efficacy reported for the BP-SES11 to the treatment of lesions in small coronary arteries. The novel, thin-strut BP-SES with bioresorbable coating is designed to provide uniform drug delivery and optimal conformability. The drug is released within three to four months and the thin abluminal coating is absorbed within three to four months, leaving behind a thin-strut bare metal stent inside the vessel. In a recent network meta-analysis, the risk of stent thrombosis8-10 and need for TVR9,10 was less with PP-EES than with other DES. In this study, BP-SES demonstrated similar clinical results at 12 months to the well-proven PP-EES, with no difference in dual antiplatelet strategy. Similar to the strut thickness of XIENCE DES (81 µm), the Ultimaster DES also has very thin struts (80 µm) which are considered preferable for the treatment of small vessels. Furthermore, the Ultimaster stent has high flexibility and vessel conformability, both features being very relevant, particularly in very small vessels.
An analysis from the National Heart, Lung, and Blood Institute Dynamic Registry compared 686 patients treated with bare metal stents (BMS) and 669 patients treated with DES for small vessel coronary artery disease in routine clinical practice. Repeat revascularisation was significantly lower with DES vs. BMS (9.5% vs. 19.3%, p<0.001)13. In a network meta-analysis including 85,490 patients, the use of bioabsorbable polymer-based biolimus-eluting stents (BP-BES) was associated with lower rates of cardiac death/MI, MI and TVR than BMS14.
In the CENTURY II trial, small vessel substudy, the observed rates of TLR for BP-SES and PP-EES were in the same range as those reported with PP-EES in other trials. In the SPIRIT III small coronary artery subgroup (defined by implantation of 2.5 mm stents), patients were randomised 2:1 to receive either PP-EES or paclitaxel-eluting stents (PES). Pre-procedural reference diameters were comparable to those in our trial at a mean of 2.36±0.30 vs. 2.34±0.33 mm. TLR was significantly reduced with PP-EES compared with PES (1.3% vs. 12.5%; p=0.002)15. In a pooled analysis from SPIRIT II and SPIRIT III including 541 patients with small vessel coronary artery disease (reference diameter <2.765 mm), TLR was numerically lower with PP-EES than with PES (3.0% vs. 6.3%, p=0.091)6. These results were confirmed in the XIENCE V® USA study, where treatment with PP-EES was associated with a TLR rate of 3.8% in 838 lesions with a reference diameter of 2.55±0.36 mm, and a definite or probable stent thrombosis rate of 0.37%3.
Compared with the 12-month TLR rate for BP-SES and PP-EES reported here (4.0% and 5.7%, respectively), the TLR rate was 12.8% for 671 lesions (reference diameter ≤2.75 mm) treated with PES16. The TLR rate was also higher at 9.6% with the BioMatrix Flex™ biolimus-eluting stent (Biosensors, Morges, Switzerland) in the small vessel analysis from the LEADERS trial17. There seems to be no class effect of DES with abluminal biodegradable coating. The polylactic acid polymer coating of the BioMatrix stent is absorbed after six to nine months, leaving a stainless steel BMS platform with a strut thickness of 120 μm. The higher reported rate of TLR with the BioMatrix stent compared with BP-SES and PP-EES could be an effect of the thicker struts (120 μm vs. 80/81 μm), since thinner struts in small vessels have been associated with a lower risk for restenosis5-7,18.
Our observed definite or probable stent thrombosis rates at 12 months (0.7% and 1.2%) were well within the reported range of 0.0% to 3.0% with other DES in small vessels7,17,19,20. In contrast, in a network meta-analysis, BP-BES was associated with a higher rate of one-year stent thrombosis than cobalt-chromium PP-EES stents14. In CENTURY II, BP-SES with thin struts resulted in a similar rate of one-year stent thrombosis compared with PP-EES, which was also demonstrated with another BP-SES in the randomised I-LOVE-IT 2 study21. Long-term follow-up is needed to demonstrate a benefit regarding a potential lower risk of very late stent thrombosis with the abluminal bioresorbable coating of the BP-SES when dual antiplatelet therapy is reduced to single antiplatelet treatment.
Limitations
This study analysed a subgroup of a multicentre, international, single-blinded, randomised trial. The study was not powered to demonstrate non-inferiority of BP-SES vs. PP-EES in this subgroup. However, the primary endpoint of the overall study was met11. DAPT therapy was similar in both groups, with about 60% of patients being on DAPT at 12 months. Any differences between bioresorbable and permanent polymer-coated drug-eluting stents might become evident with a shorter DAPT period or longer follow-up.
Conclusion
In the large-scale, randomised CENTURY II trial, use of BP-SES and PP-EES resulted in similar outcomes for treatment of small vessel coronary artery disease at 12 months.
| Impact on daily practice Treatment of lesions with drug-eluting stents in small coronary arteries is associated with a higher restenosis rate and a higher risk of stent thrombosis than treating larger vessels. The new bioresorbable polymer sirolimus-eluting Ultimaster stent resulted in similar clinical outcomes at 12 months compared with the permanent polymer everolimus-eluting stent, with no difference in antiplatelet treatment strategy. Occurrence of stent thrombosis was low. The Ultimaster stent is an attractive drug-eluting stent for treatment of stenosis in small coronary arteries. The bioresorbable polymer may be associated with additional benefits during long-term follow-up with the patients being on single antiplatelet therapy. |
Funding
Grant support: CENTURY II was funded by Terumo Corporation, Tokyo, Japan.
Conflict of interest statement
S. Saito and W. Wijns report receiving grants from Terumo Corporation during the conduct of the study. The other authors have no conflicts of interest to declare.

