
Abstract
Aims: Both biodegradable polymer sirolimus-eluting stents and permanent polymer everolimus-eluting stents offer potential for enhanced late outcomes in comparison with earlier-generation permanent polymer sirolimus-eluting stents. However, long-term comparative efficacy data among these devices remain a scientific gap. We aimed to compare the efficacy and safety of biodegradable polymer sirolimus-eluting stents (Yukon Choice PC) versus permanent polymer everolimus-eluting stents (XIENCE) versus permanent polymer sirolimus-eluting stents (CYPHER) at five-year follow-up.
Methods and results: Overall, 2,603 patients were randomised to treatment with the Yukon Choice PC (n=1,299), XIENCE (n=652) or CYPHER (n=652) stents. The primary endpoint was the device-oriented composite of cardiac death, target vessel-related myocardial infarction (MI), or target lesion revascularisation (TLR). The main secondary endpoint was definite/probable stent thrombosis (ST). Follow-up was performed up to five years. Concerning the primary endpoint, there was no significant difference between Yukon Choice PC and XIENCE stents (20.5% vs. 19.5%, HR=1.04, 95% CI: 0.84-1.29; p=0.71) or between CYPHER and XIENCE stents (23.5% vs. 19.5%, HR=1.21, 95% CI: 0.95-1.53; p=0.12). In terms of safety, rates of ST were similar with both Yukon Choice PC and XIENCE (1.2% vs. 1.4%; HR=0.83, 95% CI: 0.37-1.91; p=0.67) but numerically higher with CYPHER as compared to XIENCE (2.4% vs. 1.4%, HR=1.67, 95% CI: 0.73-3.82; p=0.22).
Conclusions: Biodegradable polymer Yukon Choice PC and permanent polymer XIENCE stents showed comparable clinical outcomes at five years. Permanent polymer CYPHER stents showed numerically higher rates of device-related adverse events. Trials registration: ClinicalTrials.gov (identifier: NCT00598676).
Introduction
The development of drug-eluting stents (DES) represented a significant victory in the battle against coronary restenosis1,2. However, the improvement in efficacy with early-generation DES occurred at the collateral cost of a delay in healing of the stented arterial segment3. This condition underlies a spectrum of clinical events including late stent thrombosis, late luminal loss creep and in-stent neoatherosclerosis4,5. Although undoubtedly multifactorial in origin, inflammatory reaction to permanent polymer coatings seems to play a central role6.
Both biodegradable polymer sirolimus-eluting stents (Yukon Choice PC; Translumina GmbH, Hechingen, Germany and Dehradun, India) and permanent polymer everolimus-eluting stents (XIENCE; Abbott Vascular, Abbott Park, IL, USA) offer potential for enhanced late outcomes in comparison with early-generation permanent polymer DES. Preclinical data with these devices show evidence of improved vascular healing7,8. Moreover, in relation to biodegradable polymer DES, long-term data from clinical trials have shown improvement in late outcomes versus early-generation DES9,10. On the other hand, long-term follow-up data with the XIENCE stent remain scant and direct comparison of five-year outcomes versus biodegradable polymer DES is an important scientific gap.
In the present analysis, we report the final five-year outcomes from patients enrolled in the Intracoronary Stenting and Angiographic Results: Test Efficacy of Three Limus-Eluting STents (ISAR-TEST 4) trial and randomly allocated to treatment with Yukon Choice PC, XIENCE or permanent polymer sirolimus-eluting stents (CYPHER).
Methods
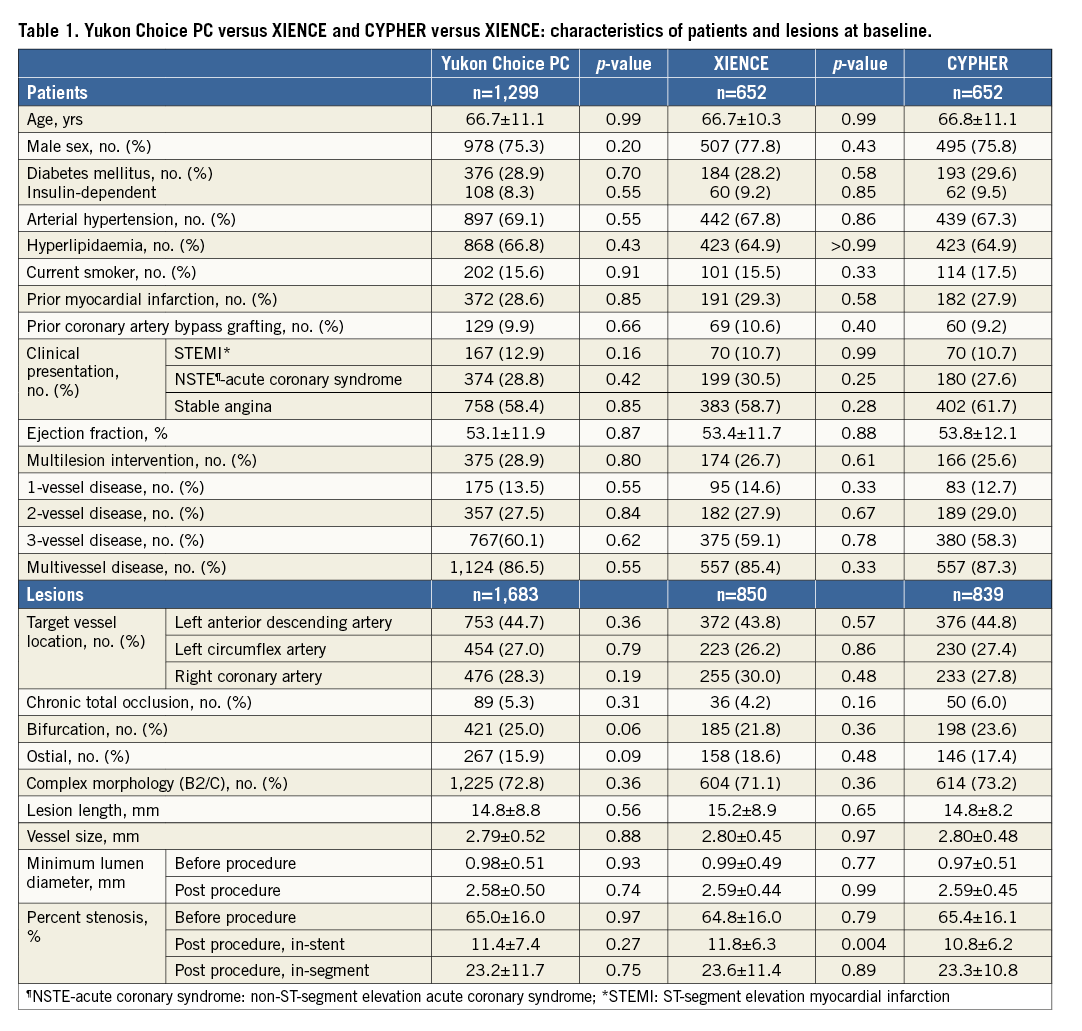
Between September 2007 and August 2008, patients were enrolled at two centres in Munich, Germany. The primary study comparison was between outcomes of patients treated with biodegradable polymer versus permanent polymer DES. Full details of the study population, methods, endpoints and primary analysis have been previously reported11. In brief, patients were randomly allocated to receive biodegradable polymer sirolimus-eluting stents (Yukon Choice PC) or permanent polymer DES (either everolimus-eluting [XIENCE] or sirolimus-eluting stents [CYPHER; Cordis Corporation, Miami Lakes, FL, USA]) in a 2:1:1 allocation. Description of stent platforms and elution characteristics are reported elsewhere11-13. The aim of the current study was to compare outcomes of patients treated with Yukon Choice PC versus XIENCE and CYPHER versus XIENCE stents after five years of clinical follow-up.
The primary outcome of the ISAR-TEST 4 study was a device-oriented composite of cardiac death, myocardial infarction (MI) related to the target vessel, or revascularisation related to the target lesion (TLR). The main secondary endpoint was definite/probable stent thrombosis. Stent thrombosis was classified according to the Academic Research Consortium (ARC) criteria14. Patients were systematically evaluated at one, 12, 24, 36 and 60 months by telephone call or office visit. All events were adjudicated and classified by an event adjudication committee blinded to the treatment groups.
Statistical analysis
Continuous data are presented as mean (±SD) or median (25th-75th percentiles). Categorical data are presented as counts and proportions (%). Unless otherwise stated, differences between groups were checked for significance, using Tukey’s multiple comparison test (continuous data) and chi-squared or Fisher’s exact test (categorical variables). Survival was analysed according to Kaplan-Meier methods and hazard ratios were calculated using Cox proportional hazards methods. The proportional hazards assumption was checked by the method of Grambsch and Therneau15 and was fulfilled in all cases in which we used Cox proportional hazards models. Analysis of the primary outcome was also performed for pre-specified subsets of interest, and interaction between treatment effect and these covariates was assessed with Cox proportional hazards models. All analyses were by intention-to-treat using all patients randomised in the study, regardless of the treatment actually received. Statistical software S-PLUS, version 4.5 (S-PLUS; Insightful Corp., Seattle, WA, USA) was used for analysis.
Results
A total of 2,603 patients were randomised to receive either Yukon Choice PC (n=1,299), XIENCE (n=652) or CYPHER (n=652) stents. Study flow chart and treatment allocation are shown in Figure 1. Baseline patient and lesion characteristics were well balanced across all groups (Table 1). Five-year follow-up was complete in all but 229 patients (8.8%), and three-year follow-up was complete in all but 110 patients (4.2%).
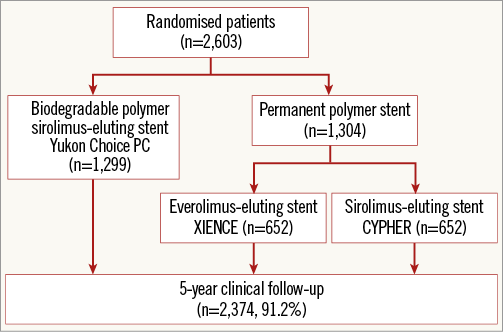
Figure 1. ISAR-TEST 4 study flow chart. Participant flow through the study.
YUKON CHOICE PC VERSUS XIENCE: FIVE-YEAR CLINICAL FOLLOW-UP
The results of follow-up are summarised in Table 2 and the landmark analysis from one to five years is shown in Table 3. At five years, the incidence of the primary endpoint was not significantly different between Yukon Choice PC and XIENCE stents (20.5% vs. 19.5%, respectively, hazard ratio [HR]=1.04, 95% CI: 0.84-1.29; p=0.71) (Figure 2A). The incidence of the primary endpoint between one and five years was comparable and low in both groups: 8.1% with Yukon Choice PC vs. 6.9% with XIENCE stents (HR=1.17, 95% CI: 0.80-1.72; p=0.42) (Figure 3A). The comparability between the two study devices regarding the primary endpoint was observed across pre-specified subgroups of age, sex, diabetes status and vessel size as well as myocardial infarction at presentation with no significant interaction (Figure 4A).
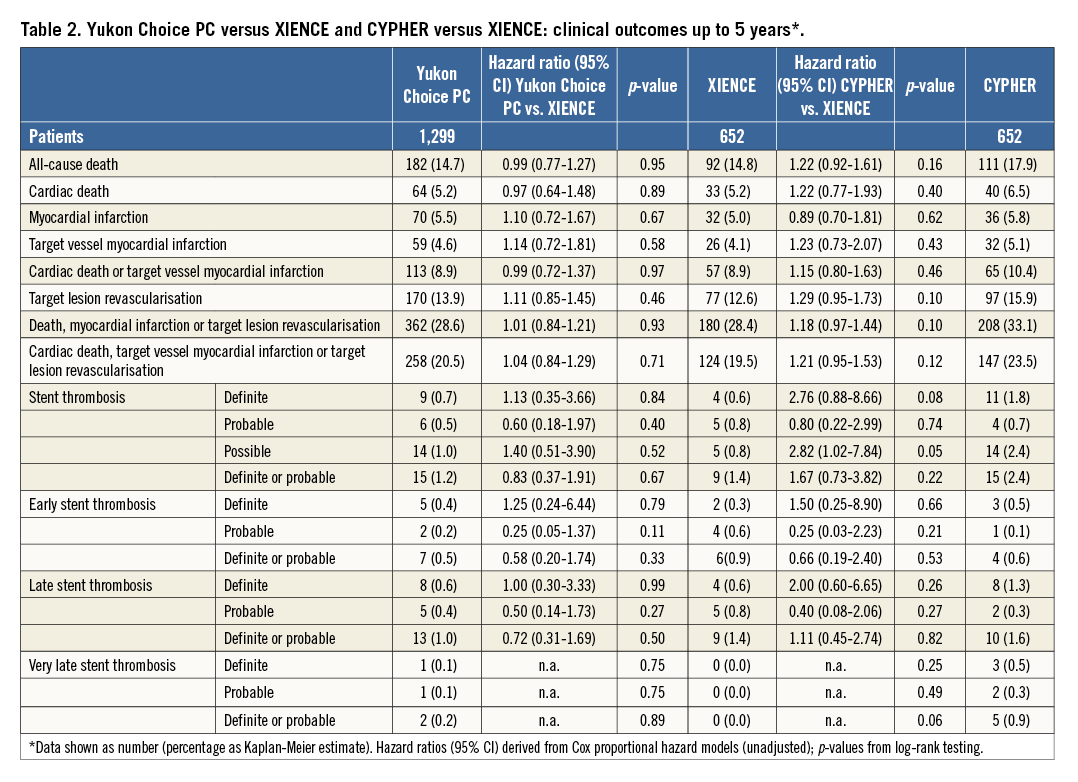
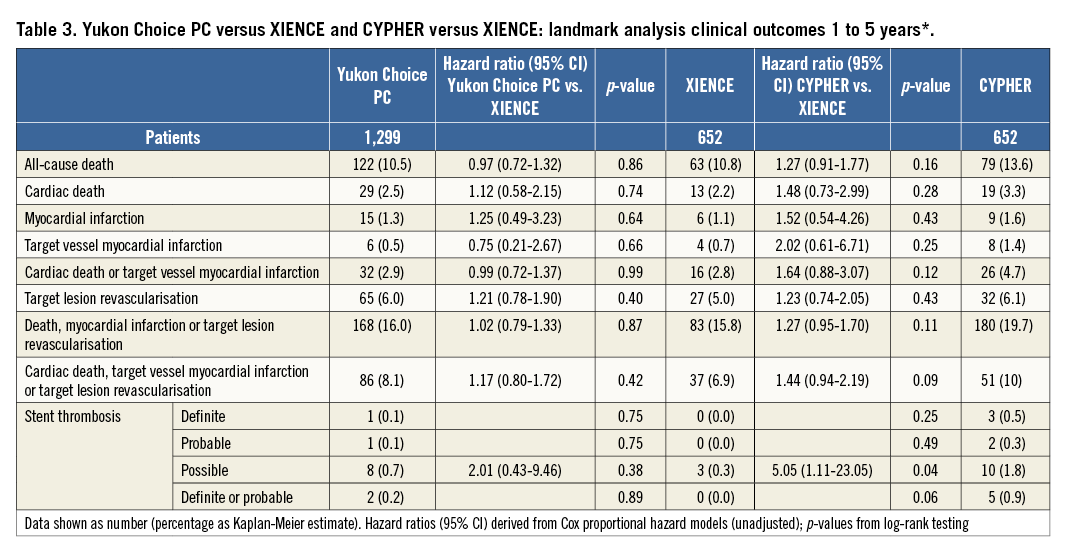
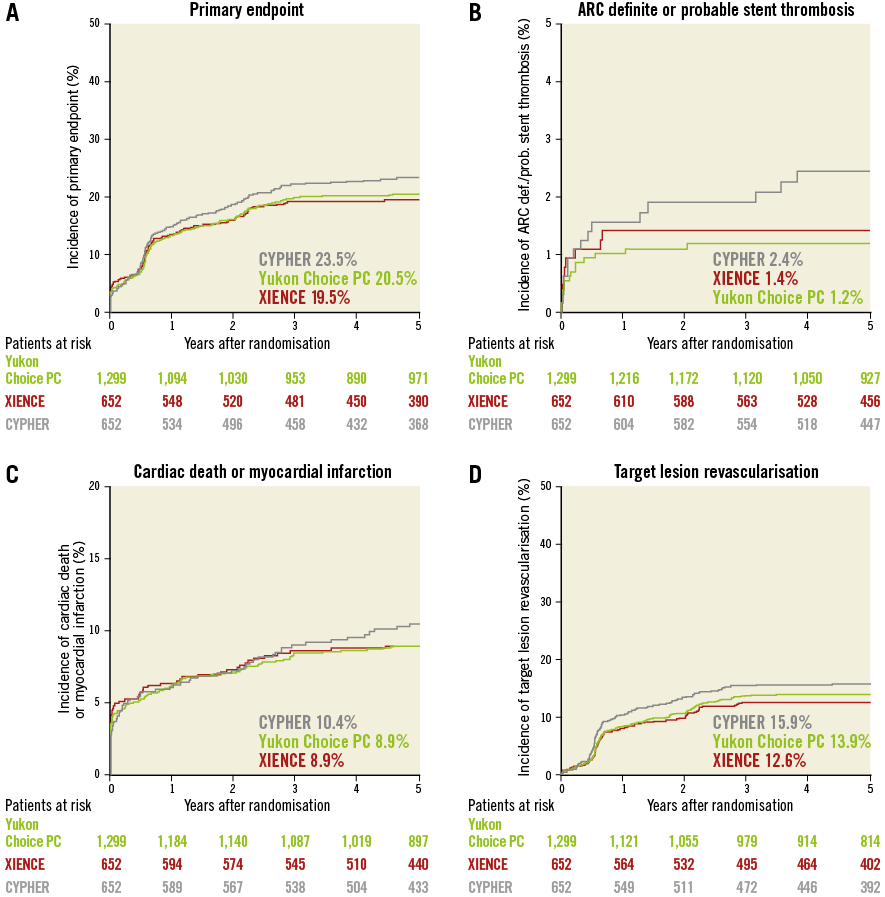
Figure 2. Comparison of outcomes in patients treated with Yukon Choice PC versus XIENCE versus CYPHER. Kaplan-Meier curves for primary endpoint (A), definite/probable stent thrombosis (B), composite of cardiac death and myocardial infarction related to the target vessel (C), and target lesion revascularisation (D).
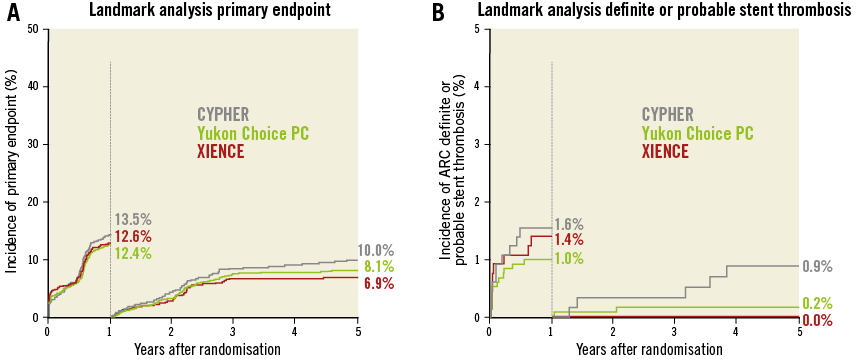
Figure 3. Landmark analysis from one to five years: comparison of outcomes in patients treated with Yukon Choice PC versus XIENCE versus CYPHER. Kaplan-Meier curves for primary endpoint (A), and definite/probable stent thrombosis (B).
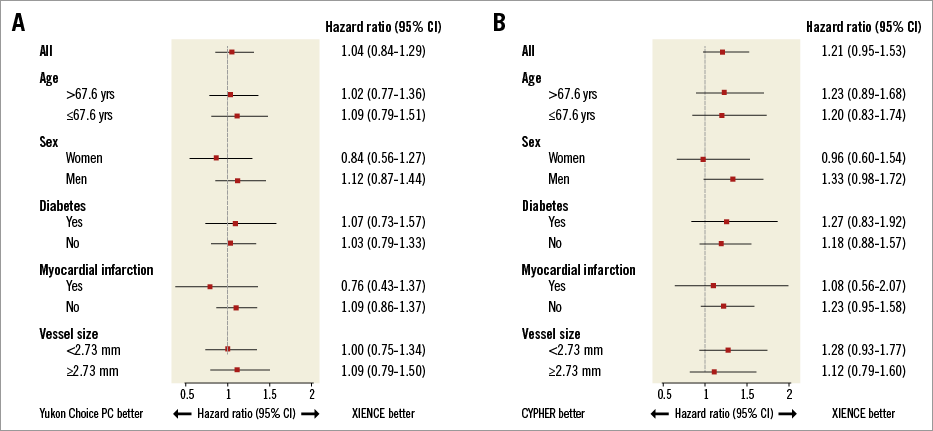
Figure 4. Comparison of incidence of primary endpoint according to pre-specified subgroups. Subgroup analysis in patients treated with Yukon Choice PC versus XIENCE (A), and CYPHER versus XIENCE (B).
Regarding safety outcomes, definite/probable stent thrombosis was low in both groups: 1.2% with Yukon Choice PC vs. 1.4% with XIENCE stents (HR=0.83, 95% CI: 0.37-1.91; p=0.67) (Figure 2B). Full results of stent thrombosis adjudication are presented in Table 2. The incidence of definite/probable stent thrombosis between one and five years was comparable and low in both groups: two events (0.2%) with Yukon Choice PC and no event with XIENCE (p=0.89) (Figure 3B). The composite of cardiac death or MI related to the target vessel was also similar in both groups at five years (Figure 2C) as well as between one and five years (Table 3). Regarding antirestenotic efficacy, TLR at five years was also similar in both groups (Figure 2D) with comparable rates in both groups between one and five years (Table 3).
CYPHER VERSUS XIENCE: FIVE-YEAR CLINICAL FOLLOW-UP
At five years, the incidence of the primary endpoint was not significantly different between CYPHER and XIENCE stents (23.5% vs. 19.5%, respectively, HR=1.21, 95% CI: 0.95-1.53; p=0.12) (Figure 2A). The incidence of the primary endpoint between one and five years was numerically higher in the CYPHER group (10.0%) as compared to the XIENCE group (6.9%) (HR=1.44, 95% CI: 0.94-2.19; p=0.09) (Figure 3A). The comparability between the two study devices regarding the primary endpoint was observed across all subgroups with no significant interaction (Figure 4B).
Regarding safety outcomes, definite/probable stent thrombosis was numerically higher in the CYPHER (2.4%) as compared to the XIENCE group (1.4%) (HR=1.67, 95% CI: 0.73-3.82; p=0.22) (Figure 2B). The incidence of definite/probable stent thrombosis between one and five years was numerically higher in the CYPHER group (five events, 0.9%) as compared with the XIENCE group (no event) (p=0.06) (Figure 3B). The composite of cardiac death or MI related to the target vessel was comparable in both groups at five
years (Figure 2C) though was numerically higher with CYPHER as compared with XIENCE between one and five years (Table 3). Regarding antirestenotic efficacy, TLR at five years was numerically higher in the CYPHER as compared with the XIENCE group (Figure 2D) though the incidence of TLR between one and five years was comparable in both groups (Table 3).
Discussion
The current manuscript represents the first report of long-term randomised trial data comparing treatment with XIENCE versus Yukon Choice PC and CYPHER stents. The major findings of this study are the following. (i) Both Yukon Choice PC and XIENCE stents are associated with similar clinical outcomes at five years in terms of efficacy and safety, with low rates of device-related events between one and five years. Moreover, although this study was not powered to detect differences in the incidence of stent thrombosis, the incidence of this endpoint was low and similar in both groups. (ii) Both CYPHER and XIENCE stents are associated with similar clinical outcomes at five years. Although not statistically significant, the incidence of stent thrombosis was numerically higher with CYPHER stents, especially between one and five years.
Biodegradable polymer DES are an intuitively attractive technology, combining the acute beneficial effects of polymer coating –control of drug-release kinetics16 and possible reduction in acute thrombogenicity17– with the long-term benefit of uncoated bare metal stents. Randomised control trial data show generally comparable one-year results versus high efficacy early and new-generation permanent polymer stents at 12 months11,18-21. In fact, the demonstration of comparable efficacy against standard DES is an important first step in the proof-of-concept chain of investigation. The next step is the evaluation of potential benefit late (>12 months) after device implantation. For this reason, long-term follow-up of clinical trials with this technology is important. The three-year results of ISAR-TEST 4 showed similar clinical outcomes with biodegradable polymer and permanent polymer DES22. Final five-year results from the LEADERS trial showed durable long-term efficacy with a trend towards lower rates of stent thrombosis compared with the CYPHER stent9. Moreover, pooled long-term follow-up of three randomised trials showed a significant reduction in stent thrombosis with biodegradable polymer DES versus the CYPHER stent driven by a statistically significant and probably clinically important 78% reduction in stent thrombosis events between years one and four10.
A limitation of available long-term follow-up data with biodegradable polymer DES is that the early-generation CYPHER stent was the comparator stent9,10. However, newer-generation stents such as the XIENCE stent were shown to be more effective and safer than early-generation stents in a number of large-scale registries23,24. Moreover, meta-analyses of direct comparison clinical trials also demonstrated some evidence of improved clinical outcomes with the XIENCE stent when compared with the CYPHER stent25. These findings are in line with preclinical data showing improved vascular healing with XIENCE stents7,26. In this respect, in the present analysis we focused initially on the comparison between patients treated with Yukon Choice PC and XIENCE stents. In addition, however, although the sirolimus-eluting CYPHER stent is no longer in routine clinical use, comparison of five-year outcomes between the widely used XIENCE stent and this benchmark early-generation stent remains an important scientific issue.
The principal findings of the current report are that device-related events were low and comparable with both Yukon Choice PC and XIENCE stents up to five years. The low incidence of events with biodegradable polymer at five years is in line with the late performance observed in other reports9,10. These results should be confirmed by long-term follow-up of randomised trials recently reporting primary outcome results19-21. Indeed, recently reported results from the NEXT trial showed comparable clinical outcomes between newer biodegradable polymer stents and the XIENCE stent up to two years26. In addition, the low incidence of events with the XIENCE stent is important, as this is the first report of five-year follow-up from a large-scale randomised trial with this stent. These data build on the favourable efficacy and safety profile of the device during short- to medium-term follow-up and confirm that this stent should be a benchmark for evaluation of emerging stent technologies.
On the other hand, although not statistically significant, the higher incidence of device-oriented endpoints with the sirolimus-eluting CYPHER stent lends further support to concerns about delayed vascular healing and late adverse events with this stent. Although certainly not powered to detect differences in the incidence of stent thrombosis, the numerically higher rate of stent thrombosis between one and five years with this stent is notable and consistent with prior reports9.
Limitations
The current report has some important limitations. First, the primary design of the ISAR-TEST 4 trial was a non-inferiority comparison of biodegradable versus permanent polymer DES at 12 months. Accordingly, additional comparisons at five years are post hoc. Second, the trial was not specifically powered for a comparison between Yukon Choice PC versus XIENCE and CYPHER versus XIENCE stents. Although overall event rates were comparable in all groups, comparisons must be interpreted with caution. Third, in terms of the comparison between biodegradable polymer and permanent polymer stents, caution must be exercised in directly attributing any observed outcome differences to the polymer coatings alone, as the stent platforms studied also differ in relation to stent backbone and drug type and dose. Fourth, this study was not powered for the detection of differences according to stent thrombosis. Fifth, the study protocol included angiographic follow-up, and the influence of planned invasive surveillance on the individual components of the primary endpoint should be considered. Sixth, although both treatment groups received the same recommendation for duration of treatment after stenting, complete data relating to compliance or actual duration of dual antiplatelet therapy received were not available.
Conclusions
Biodegradable polymer Yukon Choice PC and permanent polymer XIENCE stents showed comparable clinical outcomes at five years. On the other hand, permanent polymer CYPHER stents showed numerically higher rates of device-related adverse events driven primarily by higher incidences of device-oriented endpoints between one and five years.
| Impact on daily practice Both new-generation biodegradable polymer sirolimus-eluting stents and permanent polymer everolimus-eluting stents offer potential for enhanced late outcomes in comparison with earlier-generation drug-eluting stents. The five-year data from ISAR-TEST 4 build on the favourable efficacy and safety profile of both newer-generation devices during short- to medium-term follow-up and confirms their comparable safety and efficacy during long-term follow-up. The incidence of stent thrombosis events observed with both devices at five years was low, lending further support to the favourable late safety profile of newer-generation DES. |
Conflict of interest statement
A. Kastrati holds a patent related to biodegradable polymer coating, and reports having received lecture fees from Abbott, Biosensors and Biotronik. R. Byrne reports having received lecture fees from Biotronik. The other authors have no conflicts of interest to declare.

