Abstract
Aims: The aim of this analysis was to compare the long-term safety and efficacy of the biodegradable polymer biolimus-eluting stent (BES) with that of the durable polymer everolimus-eluting stent (EES).
Methods and results: The COMPARE II study was a prospective, randomised, multicentre, all-comers trial in which 2,707 patients were randomly allocated (2:1) to BES or EES. The pre-specified endpoint at three years was major adverse cardiac events (MACE), a composite of cardiac death, non-fatal myocardial infarction (MI), or target vessel revascularisation (TVR). Moreover, the combined endpoint all-cause death or MI was analysed as a safety, and TVR as an efficacy measure. Three-year follow-up was available in 2,683 patients (99.1%). At three years, MACE occurred in 213 patients (11.9%) in the BES group and in 101 patients (11.1 %) in the EES group (p=0.57). The rate of the combined safety endpoint all-cause death or MI was 9.3% in the BES group vs. 8.4% (p=0.52), while the efficacy measure TVR was 7.6% in BES vs. 6.5% (p=0.27). Interestingly, definite stent thrombosis rates did not differ between groups (1.2% for BES vs. 0.8%, p=0.33).
Conclusions: At three-year follow-up, MACE as well as safety and efficacy measures including stent thrombosis were not statistically different between the biodegradable polymer-coated BES and the durable polymer-coated EES. ClinicalTrials.gov Identifier: NCT01233453
Introduction
Drug-eluting stents (DES) with biodegradable polymers have been developed to address the risk of very late adverse events such as stent thrombosis, which have been partially attributed to the durable polymer responsible for controlling drug release. Although direct evidence remains scarce, durable polymer of first-generation DES has been linked to enduring inflammatory response at the implantation site, thereby resulting in: 1) delayed re-endothelialisation and/or late-acquired malapposition, both prone to thrombotic events, as well as 2) late restenosis due to increased neointimal proliferation1-9.
DES with biodegradable polymer have been developed to mitigate this dilemma and combine the best of both worlds, i.e., the efficacy of DES and the late safety associated with bare metal stents (BMS). Recent results have underlined the safety benefits of biodegradable polymer-coated DES when compared to first-generation DES in terms of a significant reduction in very late stent thrombosis events and associated composite clinical outcomes10.
However, newer-generation durable polymer-coated DES have also been designed to improve polymer biocompatibility and, currently, the cobalt-chromium everolimus-eluting stent (EES) with durable fluoropolymer is regarded, due to its safety and efficacy profile, as the gold standard11.
The purpose of the COMPARE II trial is to compare the biodegradable polymer-coated biolimus-eluting stent (BES) (Nobori®; Terumo Corp., Tokyo, Japan) to the newer-generation durable polymer-coated everolimus-eluting stent (EES) (XIENCE V® or XIENCE PRIME®; Abbott Vascular, Santa Clara, CA, USA, or PROMUS™; Boston Scientific, Marlborough, MA, USA) in an all-comers PCI population. Initial results showed non-inferiority of BES compared to EES at one year12,13. However, potential benefits of the biodegradable polymer BES are expected beyond one year. The present analysis displays the three-year results of the COMPARE II trial.
Methods
COMPARE II (Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent; NCT01233453) is an investigator-initiated, multicentre, open-label, randomised, all-comers trial which assigned patients undergoing percutaneous coronary intervention (PCI) in a 2:1 fashion to either BES (316L stainless steel stent with 120 µm strut thickness coated abluminally with biodegradable polymer poly-lactic acid, eluting the drug Biolimus A9™ [Biosensors International, Singapore]/Nobori) or EES (cobalt or platinum chromium metallic stent with a strut thickness of 81 µm coated with a durable fluoropolymer, eluting the drug everolimus/XIENCE V or XIENCE PRIME, or PROMUS, respectively). Patients will be followed for five years after the index procedure. A detailed description of study and procedural methodologies has been published previously13.
The study protocol pre-specified composite endpoint major adverse cardiac events (MACE) was defined as cardiac death, non-fatal myocardial infarction (MI), or target vessel revascularisation (TVR). Additionally, the endpoint all-cause death or MI was analysed in a combined manner as a safety measure and TVR was used as an efficacy measure. A device-oriented endpoint was represented by the composite target lesion failure (TLF), comprising cardiac death, non-fatal target vessel-related myocardial infarction (TV-MI), and clinically driven target lesion revascularisation (CD-TLR).
Patients were evaluated at one, six, 12, 24 and 36 months at the outpatient clinic or by post, e-mail or telephone regarding medication regime and adverse events. All sites were monitored. Reportable clinical events were adjudicated by an independent committee, blinded to treatment allocation. The sponsor of this study had no involvement in the conduct of the study, data monitoring, data analysis, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for its publication. The study complied with the CONSORT 2010 Statement of the Declaration of Helsinki and was approved by all the institutional ethics committees of the participating centres.
STATISTICAL ANALYSIS
The study was designed as a non-inferiority trial at one year with a non-inferiority margin of 4%. Based on previous reports of EES and BES stent study event rates, the inclusion of 2,700 patients, including 5% loss to follow-up, would result in a 90% power to detect non-inferiority according to the Newcombe-Wilson score method. The current analysis, including subgroup analysis across clinically relevant subgroups at three-year follow-up, was pre-specified per protocol. Categorical variables are presented as numbers and percentages and were compared with the Fisher’s exact test, due to the low prevalence of some baseline variables. Continuous variables were expressed as mean±SD or medians with interquartile ranges. Continuous variables were compared using the Wilcoxon rank-sum test. All analyses were performed according to the intention-to-treat principle. Time to the respective endpoint was analysed according to the Kaplan-Meier method and the log-rank test was applied to compare the incidence of endpoints between groups. The landmark analysis used the one-year landmark. Thus, patients who had experienced the event of interest during the first year following the index procedure were excluded from analysis.
All p-values were two-sided, and a p-value of less than 0.05 was regarded as statistically significant. SAS version 8.02 (SAS Institute Inc., Cary, NC, USA) was used for analysis.
Results
In total, 2,707 patients (4,025 lesions) undergoing PCI were randomised 2:1 to BES (1,795 patients with 2,638 treated lesions) or EES (912 patients with 1,387 treated lesions). At three years, follow-up data were available in 1,780 out of 1,795 patients in the BES group (99.2%) and 903 out of 912 patients in the EES group (99.0%) (Figure 1). Detailed characteristics of baseline clinical, angiographic and procedural features have been previously presented13. Table 1 summarises the main baseline characteristics which were well balanced between groups. Briefly, the mean age was 63 years with 22% diabetic patients, 58% presenting with acute coronary syndrome (ACS), and 25% receiving multivessel PCI. Angiographic and procedural characteristics are displayed in Table 2.
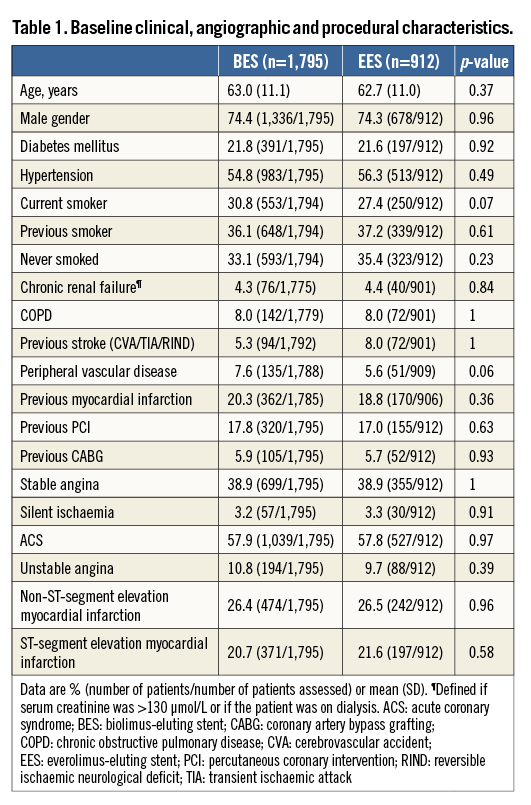
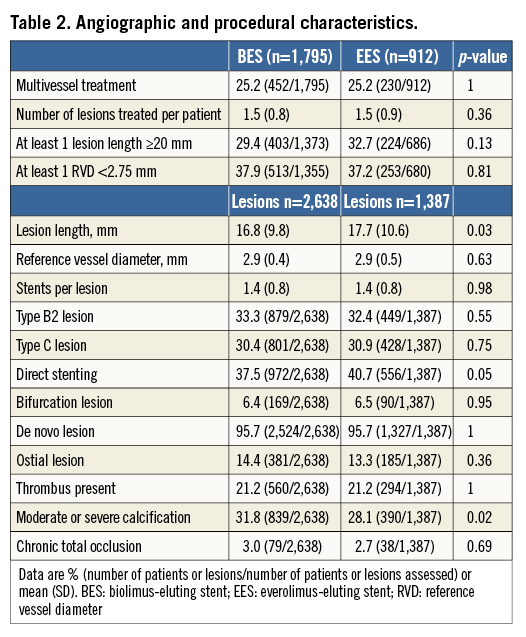
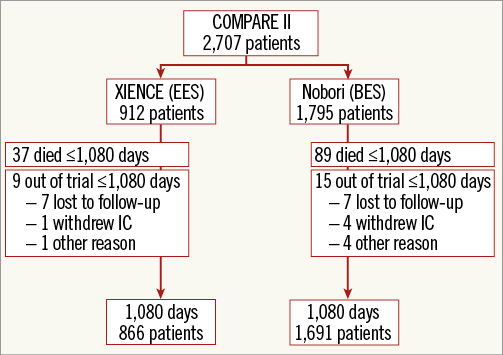
Figure 1. Study flow chart. IC: informed consent
THREE-YEAR FOLLOW-UP
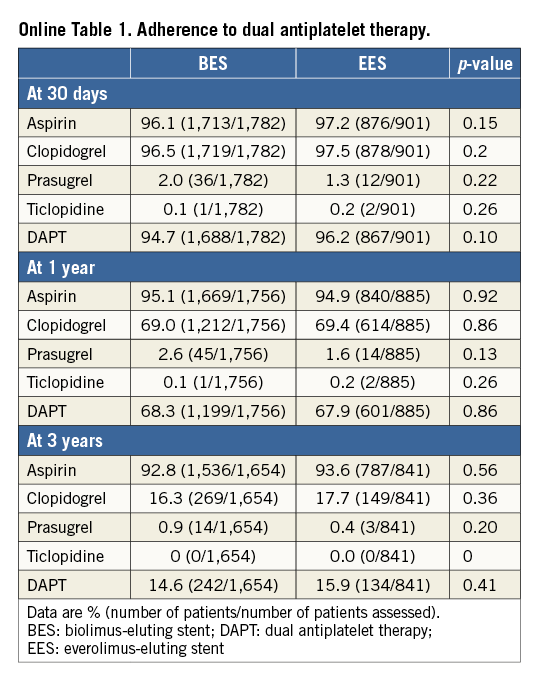
Of note, no differences were observed regarding DAPT adherence between groups through the three-year follow-up (Online Table 1).
Three-year clinical event rates are detailed in Table 3. The pre-specified composite outcome MACE (cardiac death, non-fatal MI or TVR) occurred in 213 patients (11.9%) in the BES group and in 101 patients (11.1 %) in the EES group (relative risk 1.08 [95% CI: 0.84-1.39], p=0.54). Other safety and efficacy endpoints including the device-oriented endpoint TLF as well as definite and definite/probable stent thrombosis were similar between groups.
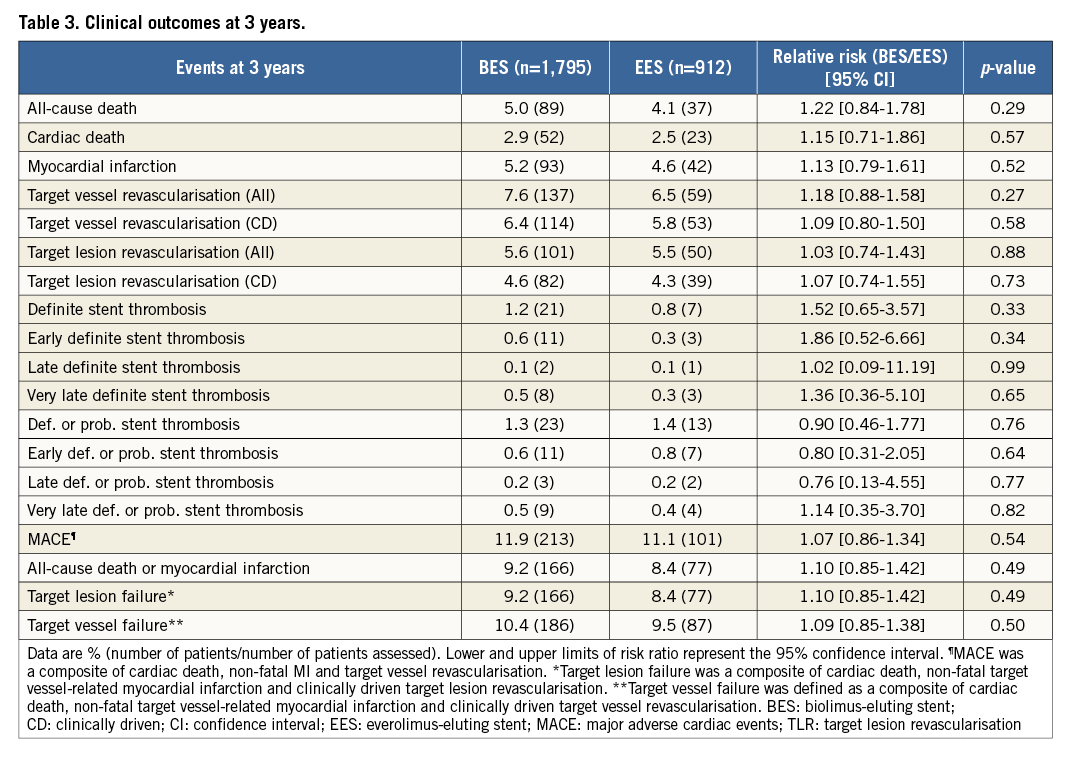
The Kaplan-Meier time-to-event curves for the endpoint MACE, as well as the individual endpoints cardiac death, MI, TVR, and stent thrombosis, are shown in Figure 2.
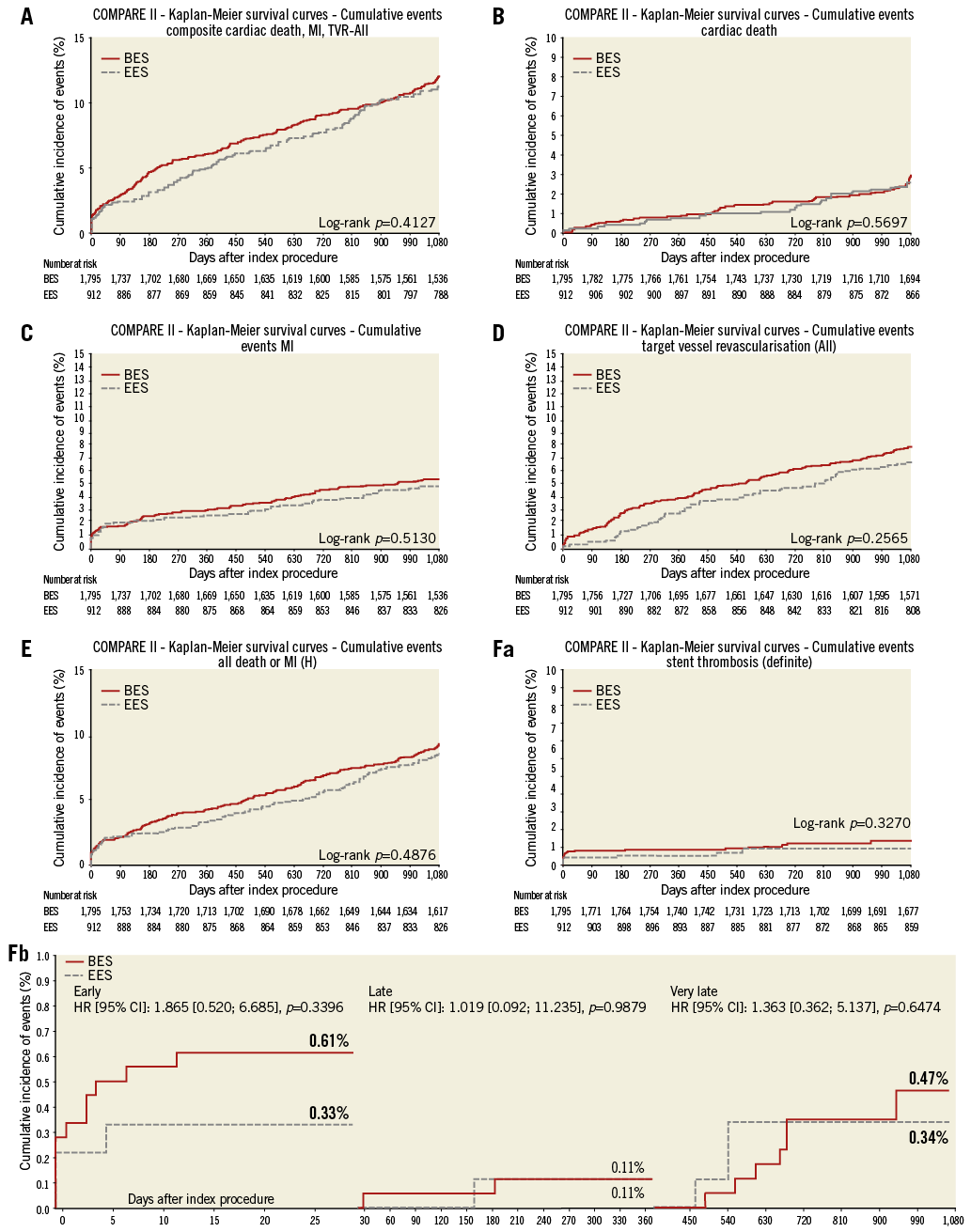
Figure 2. Kaplan-Meier cumulative event curves. A) MACE; B) cardiac death; C) myocardial infarction; D) target vessel revascularisation; E) all-cause death or myocardial infarction; F) definite stent thrombosis: (a) cumulative, (b) stratified for early, late, and very late occurrence. BES: biolimus-eluting stent; CI: confidence interval; EES: everolimus-eluting stent; HR: hazard ratio; MACE: major adverse cardiac events; MI: myocardial infarction; TVR: target vessel revascularisation
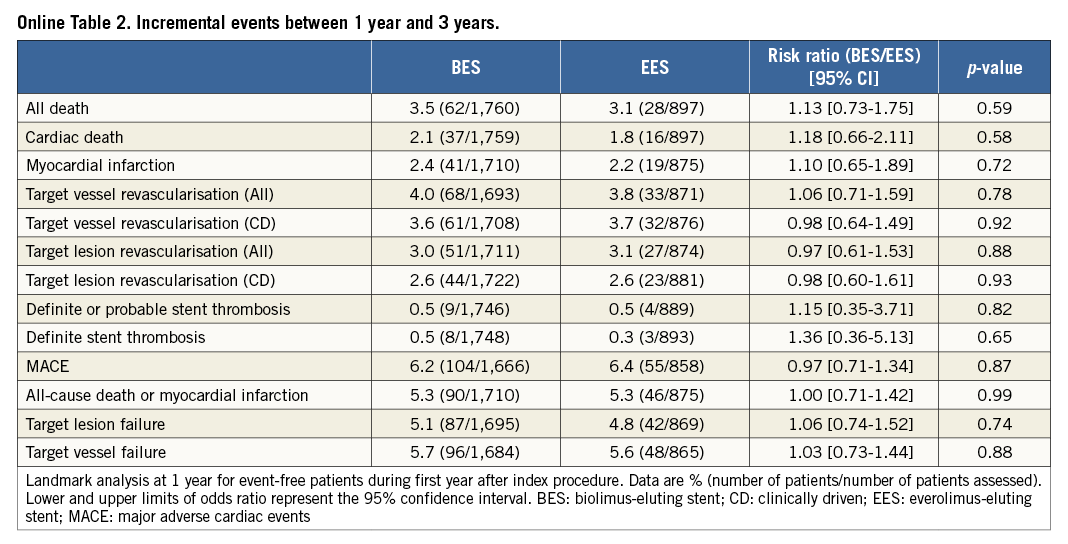
The one-year landmark analysis showed no significant differences in incremental event rates between one and three-year follow-up (Online Table 2).
The comparable clinical outcomes between stent groups held true over a wide range of subgroups (Figure 3).
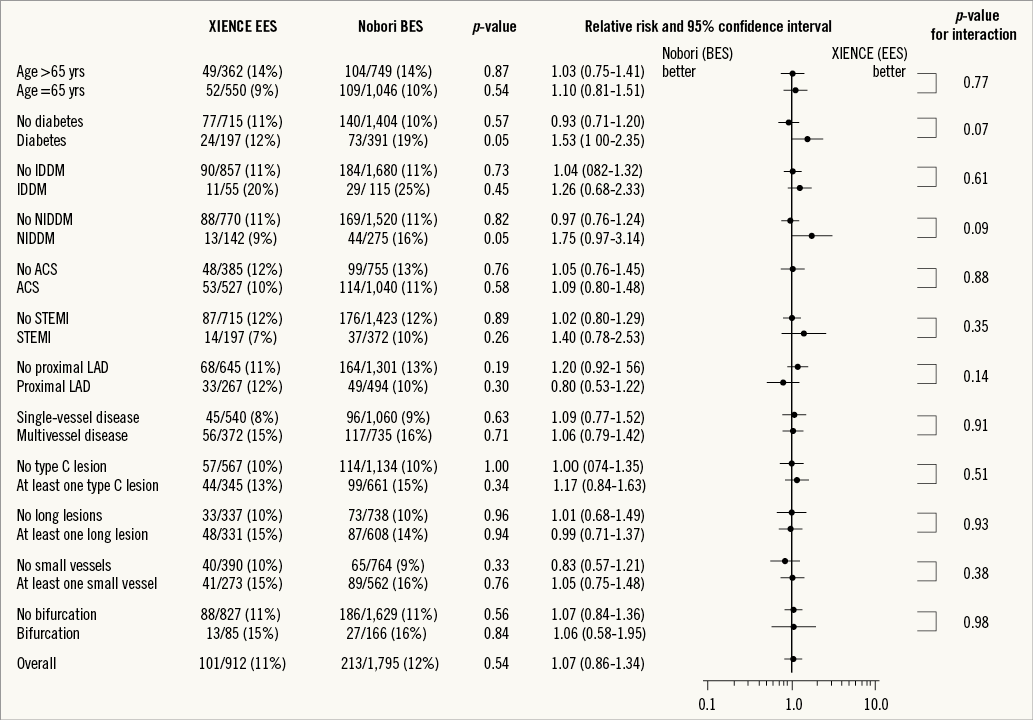
Figure 3. Subgroup analysis for the pre-specified composite endpoint MACE (cardiac death, MI, TVR). ACS: acute coronary syndrome; BES: biolimus-eluting stent; EES: everolimus-eluting stent; IDDM: insulin-dependent diabetes mellitus; LAD: left anterior descending coronary artery; MACE: major adverse cardiac events; NIDDM: non-insulin-dependent diabetes mellitus; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction
Discussion
The present trial is one of the largest randomised studies of patients treated with biodegradable polymer DES in comparison to second-generation DES stents and the first to report on three-year follow-up. The principal findings of this trial are: 1) that the biodegradable polymer-coated BES (Nobori) has a similar safety and efficacy profile at three years compared to the gold standard durable polymer-coated EES (XIENCE or PROMUS); and 2) that landmark analysis did not indicate any benefit of BES over EES in terms of safety or efficacy beyond the first treatment year. Indeed, the event curves for the cumulative pre-specified endpoint MACE started diverging at three months but were superimposed again at three years.
To date, only one other study (NEXT) has evaluated exclusively the biodegradable polymer BES versus a second-generation DES in an all-comers PCI population, and recently confirmed equivalent clinical outcomes at two years between Nobori BES and XIENCE EES in a Japanese PCI cohort14. Whilst most event rates reported are comparable to the present study, a much lower definite stent thrombosis rate was observed in the NEXT trial with regard to both stent groups, which might be attributed to fewer ACS patients being included, as well as regional differences in patient and procedural characteristics.
Accordingly, five-year follow-up data comparing biodegradable polymer with durable polymer-coated DES are available from the ISAR-TEST 4 study showing equivalent clinical outcomes between the tested stent types15. Of note, the ISAR-TEST 4 study used, partly, first-generation durable polymer sirolimus-eluting stents (SES) (CYPHER; Cordis, Johnson & Johnson, Warren, NJ, USA) in the permanent polymer DES control group. Furthermore, the biodegradable polymer DES used in the ISAR-TEST 4 study (Yukon Choice PC; Translumina GmbH, Hechingen, Germany) was different from the one used in the present study, thus limiting further inter-study comparability16,17. The long-term results from the SORT OUT V trial which compared SES (CYPHER) to BES (Nobori) are not yet published. Results at 12 months did not demonstrate any significant differences in the composite endpoint (cardiac death, myocardial infarction, definite stent thrombosis, clinically indicated target vessel revascularisation) but indicated a significant higher rate of definite stent thrombosis in the BES group18.
The current analysis did not reveal any advantages in safety and efficacy outcomes of biodegradable polymer BES over the durable polymer EES at three years, nor after landmark analysis between years one and three. In the LEADERS trial, a similar biodegradable polymer BES (BioMatrix; Biosensors Europe SA, Morges, Switzerland) to the one used in our study was compared to a first-generation durable polymer-coated SES (CYPHER; Cordis). It was reported that the biodegradable polymer DES was non-inferior to SES with a trend towards better outcomes at five years10. Interestingly, the very late definite stent thrombosis rates were significantly lower in the biodegradable polymer BES group compared to the durable polymer SES group at five years (0.6% vs. 2.2%, p=0.003). Not only did we not observe any difference in very late definite stent thrombosis rates in our study, but we also found remarkably lower very late definite stent thrombosis rates in the devices tested here when compared to the first-generation SES as reported in the LEADERS trial (0.9% at three years; 2.5% at five years). Our results are in line with the five-year results from ISAR-TEST 4, which did not find any differences between EES and the biodegradable polymer DES used in their study but found a numerically higher number of stent thromboses in the first-generation SES group compared to EES16.
In the present study, about 22% of included patients were diabetics. Subgroup analysis revealed a better outcome with regard to the composite endpoint MACE of diabetic patients treated with EES compared to BES, though the p-value for interaction was not significant (p=0.07). Limited data exist concerning diabetic patients treated with biodegradable polymer compared to newer-generation durable polymer DES. Results from the NEXT and ISAR-TEST 4 trials did not show any association of diabetic state and clinical outcomes at two and five-year follow-up, respectively. The results of the present subgroup analysis have to be regarded as hypothesis-generating, and warrant further investigation in a properly powered analysis.
Potential benefits in safety and efficacy measures of the biodegradable polymer-coated DES compared to the durable polymer DES are expected beyond the first treatment year after drug elution and polymer breakdown, leaving the bare metal stent platform in the vessel. Indeed, implantation of biodegradable polymer DES has been associated with more complete re-endothelialisation as well as preserved endothelium-dependent vasomotion when compared to first-generation SES3,19,20. However, a recent randomised comparison of BES (Nobori) and EES (XIENCE), using intravascular optical coherence tomography, reported similar stent coverage and apposition at six to eight months21. This note supports the observed similar clinical outcomes, in particular with regard to stent thrombosis rates.
The low event rates of the tested devices, in particular when compared to first-generation DES, could be related to the improved biocompatibility of the polymer coatings (durable and biodegradable) and thinner stent struts of BES and especially of EES. Recent network analyses have shown that EES have the best safety profile when compared to other durable polymer DES, biodegradable polymer BES and even BMS22-24: thus, the opportunity to demonstrate clinical benefits by BES is challenged by the excellent outcomes of the EES. In line with this, the present data suggest that little added benefit is to be expected from the current biodegradable polymer-coated DES compared to the newer-generation biocompatible durable-coated EES. What needs to be investigated further is whether longer follow-ups or newly developed biodegradable polymer-coated DES with reduced stent strut and polymer thickness can support the concept of biodegradable polymer coating.
Study limitations
Although designed as an all-comers study, only 26% of patients undergoing percutaneous interventions were enrolled in the study, so selection bias cannot be ruled out. The power of the present study was attenuated by the lower than expected event rates of the primary endpoints used for sample-size calculation13. Secondly, we report on a secondary endpoint, and testing of the primary endpoint at multiple time points other than the specified one-year primary endpoint is subject to the perils of multiple testing.
Conclusion
At three years, the combined endpoint MACE as well as the safety and efficacy endpoints were not statistically different in the Nobori biodegradable polymer-coated BES group compared with the current standard durable polymer-coated EES. Despite similar clinical outcomes, BES does not indicate any benefits, in particular towards reduction of very late adverse events, thus challenging the concept of biodegradable polymer coating. Whether differences in clinical outcome emerge beyond three years needs to be investigated.
| Impact on daily practice The present three-year results from this all-comers PCI trial show that both the biodegradable polymer-coated biolimus-eluting stent (BES) and the durable polymer-coated everolimus-eluting stents (EES) have an excellent safety and efficacy profile. Interestingly, the biodegradable polymer-coated BES does not indicate any benefit, in particular towards reduction of very late adverse events, thus challenging the concept of biodegradable polymer coating. Taken together, these results underline the improved safety and efficacy achieved with current-generation devices by enhanced biocompatibility coatings (durable and biodegradable) and thinner stent struts when compared to earlier-generation DES. |
Acknowledgements
We would like to thank all the participating centres and investigators (list of centres and investigators can be found in the Online Acknowledgements section). Moreover, we thank Erik Spaepen (SBD Analytics) and Tessa Rademaker-Havinga (Cardialysis) for statistical analysis as well as Claudia van Vliet (Maasstad Hospital) for project management.
Funding
The study was supported by a grant from Terumo Europe (Leuven, Belgium) and the Research Foundation of the Cardiology Department, Maasstad Hospital (Rotterdam, The Netherlands).
Conflict of interest statement
P. Smits has in the last three years received lecture fees from Abbott Vascular and institutional grants from Abbott Vascular, Terumo, and St. Jude. G. Vlachojannis has in the last three years received lecture and consultation fees from Abbott Vascular. The other authors have no conflicts of interest to declare.
Online data supplement
Online Acknowledgements
We would like to thank all the participating centres and investigators who are not included in the list of authors: Department of Cardiology, Maasstad Hospital, Rotterdam, The Netherlands; Department of Cardiology, Medisch Centrum Leeuwarden, Leeuwarden, The Netherlands; Department of Cardiology, Hôpital Cantonal de Fribourg, Fribourg, Switzerland; Department of Cardiology, Hospitalario Juan Canalejo, Coruña, Spain; Department of Cardiology, Hospital Clinico Virgen de la Arrixaca, Murcia, Spain; Department of Cardiology, Onassis Cardiac Surgery Centre, Athens, Greece (G. Karavolias, MD); Department of Cardiology, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands; Department of Cardiology, Kantonsspital Aarau, Aarau, Switzerland (A. Vuilliomenet, MD); Department of Cardiology, Hospital del Mar, Barcelona, Spain (A. Serra, MD); Department of Cardiology, Hospital de Santiago de Compostela, Santiago de Compostela, Spain (R. Trillo Nouche, MD); and Department of Cardiology, Amphia Ziekenhuis, Breda, The Netherlands.