Abstract
Aims: Although large clinical trials have shown that everolimus-eluting stents (EES) significantly reduce target vessel revascularisation (TVR), myocardial infarction (MI) and stent thrombosis (ST) compared to paclitaxel-eluting stents (PES) in diverse populations, there is a paucity of data comparing EES and PES in patients presenting with MI.
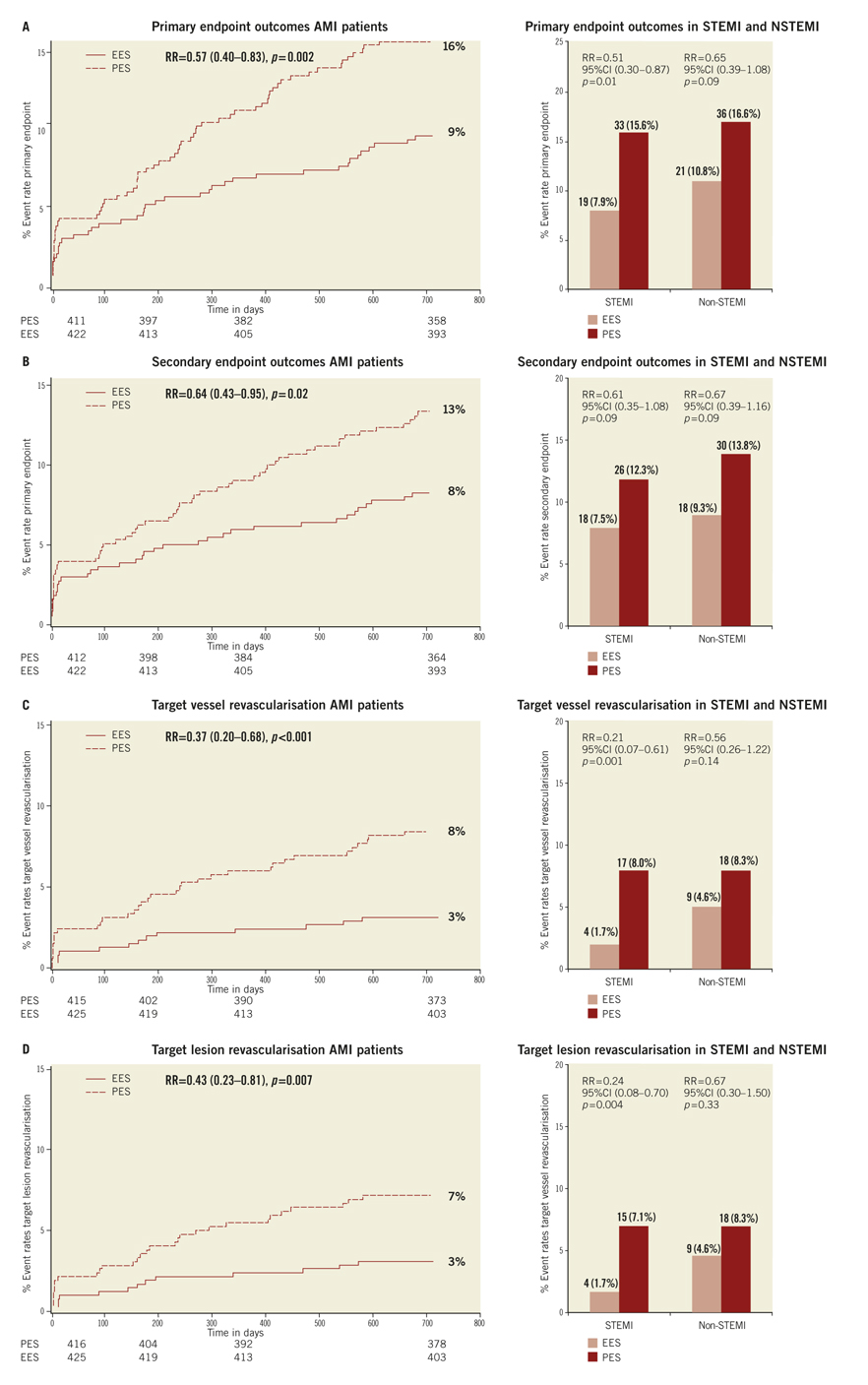
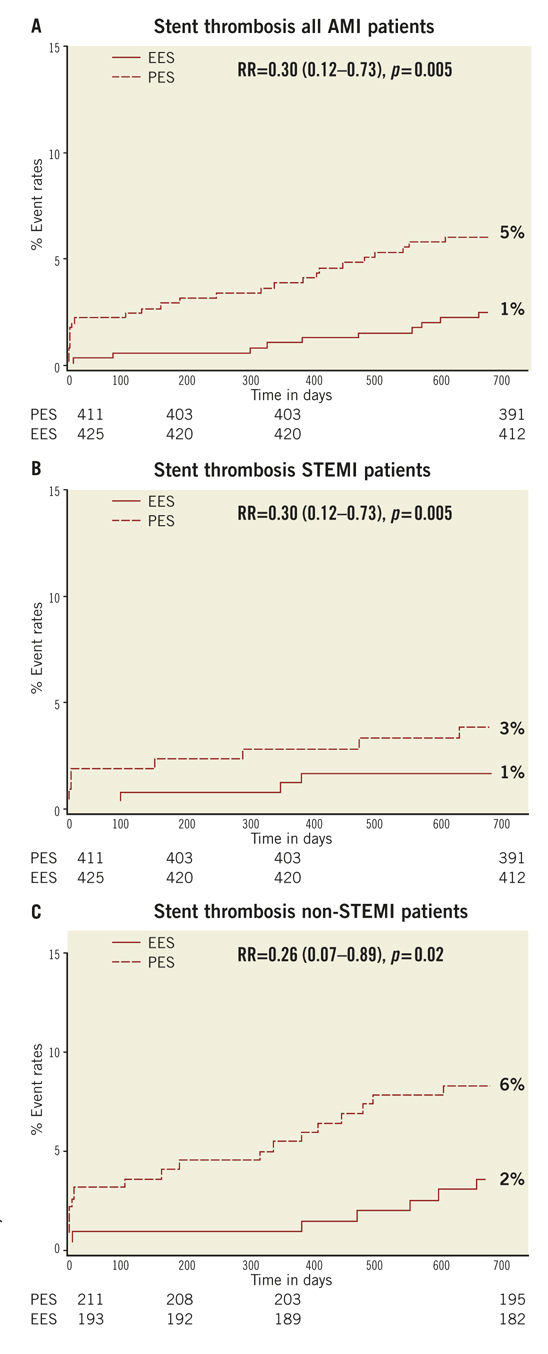
Methods and results: We performed a post hoc subgroup analysis on COMPARE, an all-comer trial comparing EES to PES. We identified 863 patients (EES=434, PES=429 treated for MI: 452 ST-elevation MI (STEMI) and 411 non ST-elevation MI (NSTEMI). EES was associated with a significant reduction in the primary endpoint, a composite of all-cause mortality, MI, and TVR, at two years (RR=0.57; 95% CI: 0.40-0.83, p=0.002). While the effect was more marked in the STEMI (RR=0.51; 95% CI: 0.30-0.87, p=0.01) than the NSTEMI subgroup (RR=0.65; 95% CI: 0.39-1.08, p=0.09), the interaction p-value (0.5) suggests that a difference in treatment effect between presentations is unlikely. ST rates were significantly lower with EES (RR=0.30; 95% CI: 0.12-0.73, p=0.005).
Conclusions: At two years, EES results are superior to PES in terms of safety and efficacy endpoints in treatment of MI.
Introduction
Paclitaxel-eluting stents (PES) and sirolimus-eluting stents (SES) substantially reduce the need for re-intervention compared to bare metal stents.1,2 However, increased rates and ongoing propensity for stent thrombosis (ST) remain a matter of concern.3-7 Specific concerns have been raised with respect to the unrestricted use of first generation DES in patient populations presenting with AMI.8
Second generation drug-eluting stents were designed to improve safety, efficacy and procedural success rates. Specifically, a second generation, thin-strut, cobalt-chromium, everolimus-eluting stent (EES) demonstrated significant improvements in angiographic and clinical outcomes when compared to PES in two early randomised trials.9,10 Thereafter, two large clinical trials, SPIRIT IV and COMPARE, independently demonstrated that the EES was superior to the PES with regard to broadly similar primary composite safety and efficacy endpoints in less selected populations.11-14
Both trials showed strikingly similar relative risk reductions in thrombotic events with EES compared with PES at 30-day, 1- and 2-year follow-up. No study has compared EES with PES in patients presenting with myocardial infarction (MI). To investigate whether these promising results with EES can be replicated in this high risk category of patients, we performed a post hoc subgroup analysis comparing the outcomes of EES and PES in patients treated for MI in the COMPARE trial.
Methods
We performed a subgroup analysis of patients presenting with myocardial infarction from the population of the COMPARE trial.12 The methodology of the COMPARE trial has been published previously.12 In summary, consecutive patients, between 18 and 85 years, referred to the Maasstad Ziekenhuis (Hospital) Cardiology Centre for elective or emergent percutaneous coronary intervention, were eligible to participate in the study. There were no limitations on the number of lesions or vessels, on the location of lesions, or on their length. Exclusion criteria were contraindications or expected non-adherence to dual antiplatelet drug therapy in the 12 months after the procedure; planned major surgery within 30 days; inability or refusal to comply with follow-up procedures; participation in other coronary-device trials; and inability to give informed consent. For the present analysis, the same primary and secondary endpoints as well as the same definitions as for the COMPARE trial were used12.
As the majority of patients were treated in the setting of an evolving MI, the periprocedural infarctions were adjudicated in the following manner:
If the peak total CK (or CK-MB) from the index infarction had not yet been reached: recurrent chest pain lasting >20 minutes (or new ECG changes consistent with MI) AND the peak CK (or CK-MB in absence of CK) level measured within 24 hours after the event is elevated by at least 50% above the previous level.
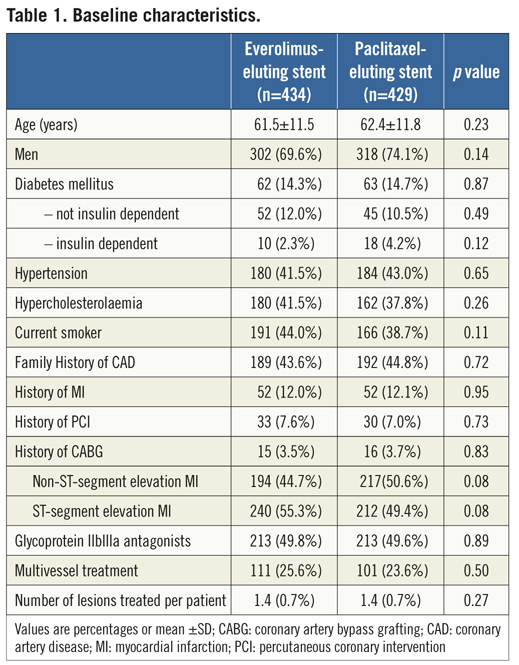
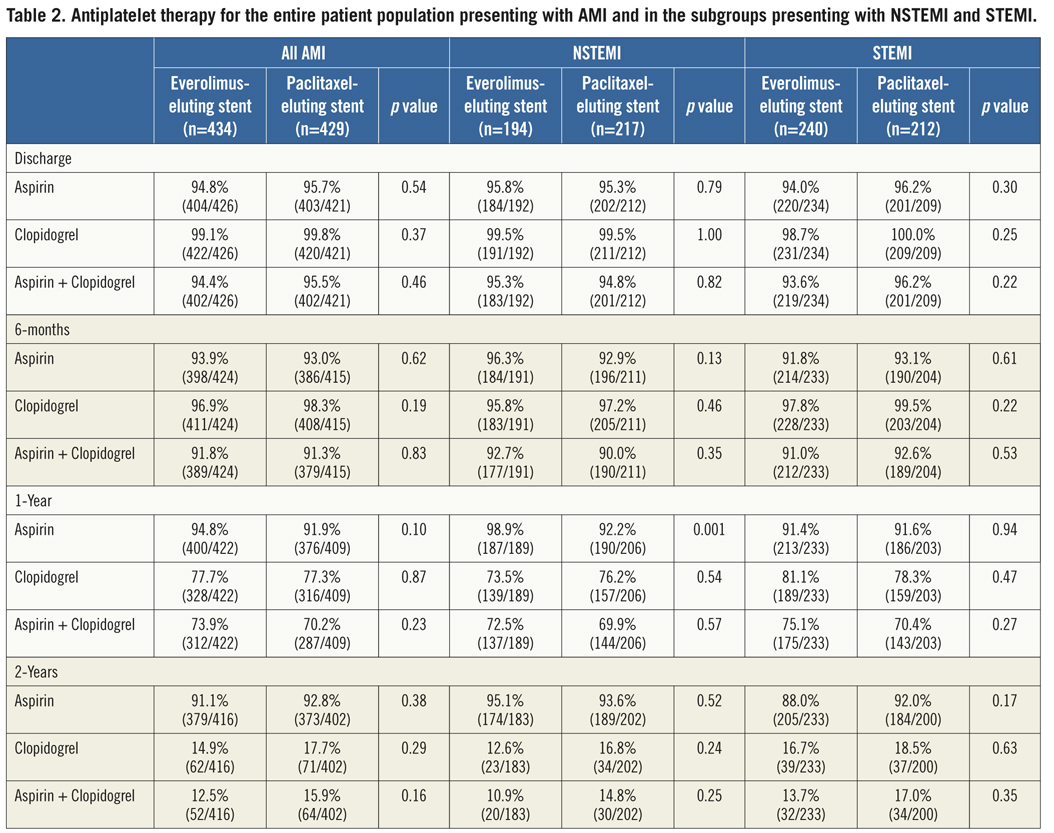
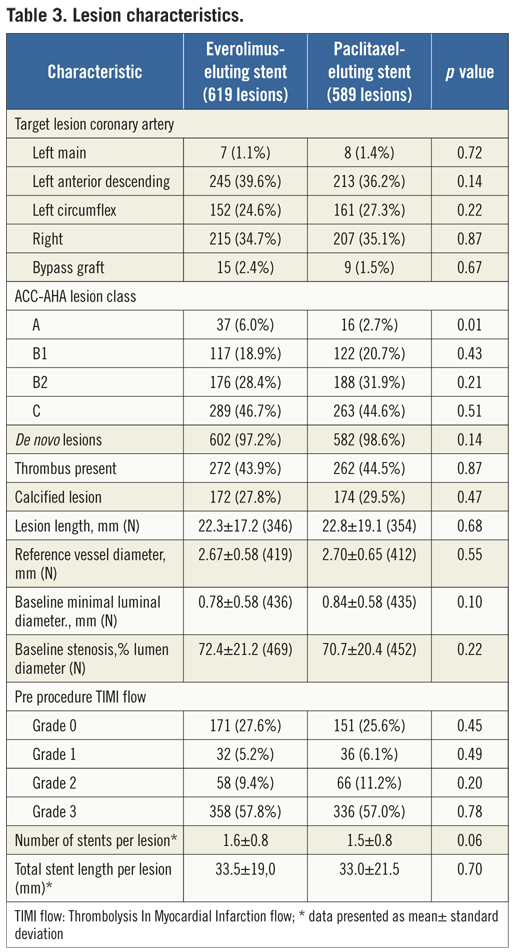
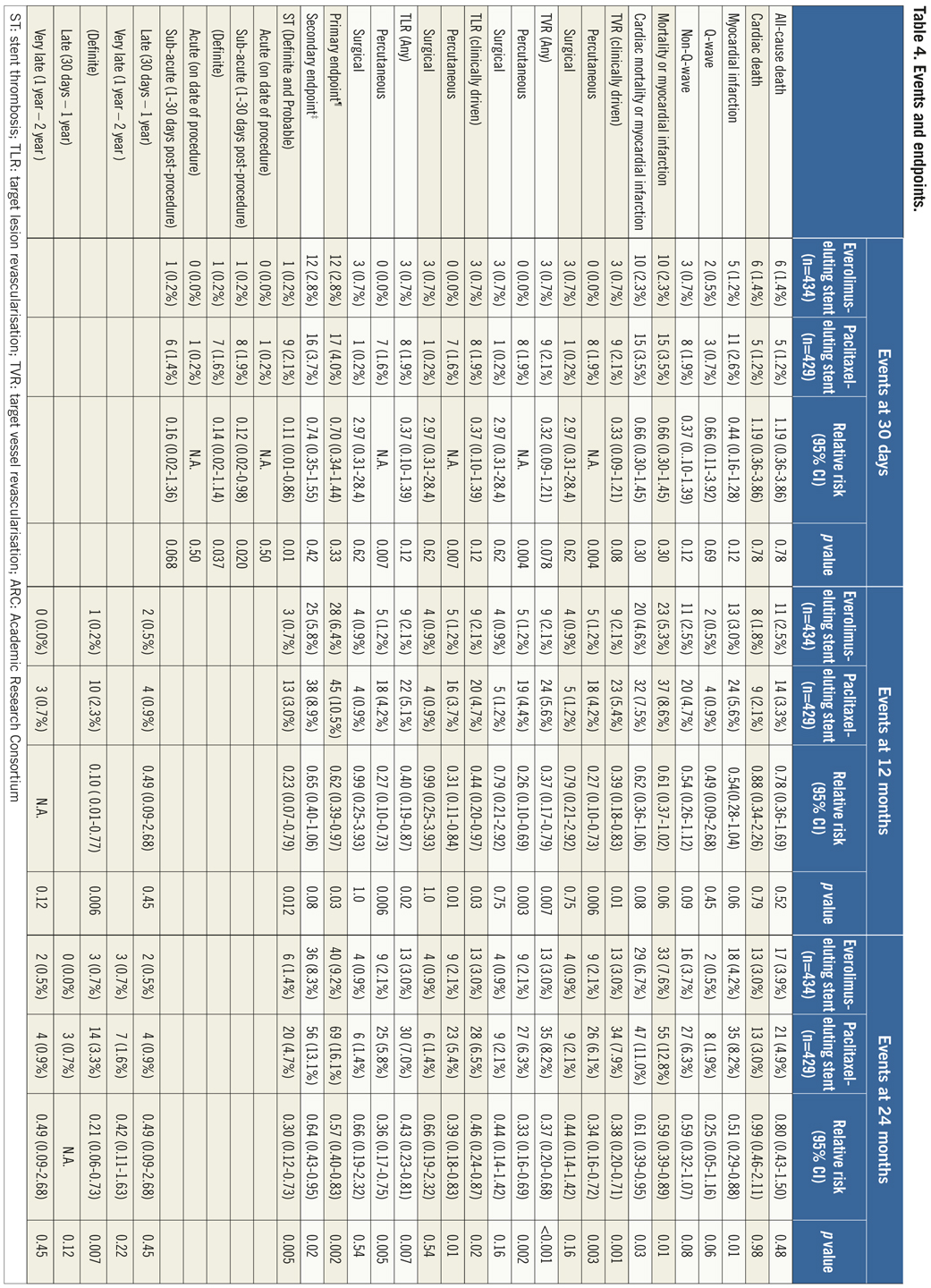
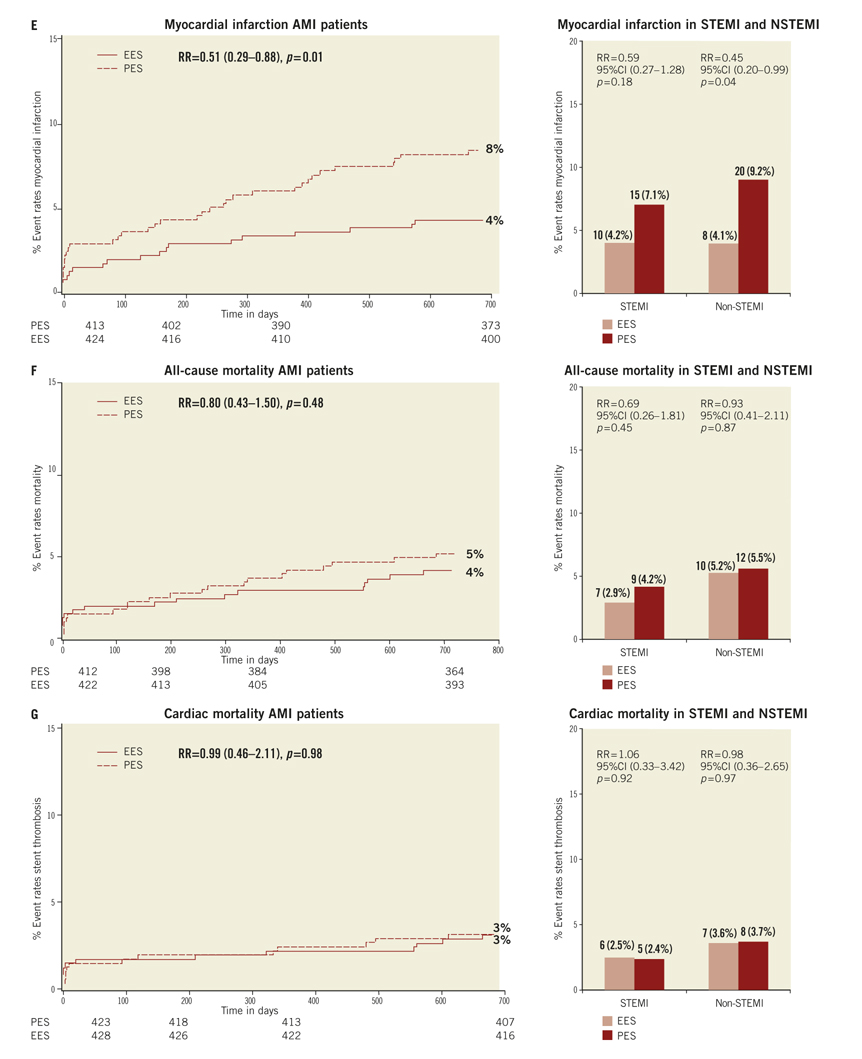
If the elevated CK (or CK-MB) levels from the index infarction are falling or have returned to normal within 24 hours post-index PCI: either a new elevation of CK >2 x ULN within 24 hours post-index PCI if the CK level has returned to Randomisation was performed by means of sealed, opaque, sequentially numbered allocation envelopes after passage of the guidewire. The allocation schedule was based on computer-generated random numbers. (SAS, release 8.02; Cary, NC, USA). Patients were assigned on a 1:1 basis to treatment with a polymer-based, everolimus-eluting stent (XIENCE V®, Abbott Vascular, Santa Clara, CA, USA) or a polymer-based, paclitaxel-eluting stent (Taxus Liberté™, Boston Scientific, Natick, MA, USA). Staged procedures were permitted, and the same stent type, allocated at initial randomisation, was used. All patients provided written informed consent. The study was investigator-initiated. Funding for the study was provided by unrestricted research grants from Abbott Vascular and Boston Scientific, who had no involvement in the design, conduct or analysis of the study. The study complied with the Declaration of Helsinki regarding investigation in humans and was approved by the Institutional Ethics committee of the Maasstad Ziekenhuis, Rotterdam, Netherlands and the Dutch Central Committee on Research Involving Human Subjects (CCMO trial nr: NL15206.101.06). Medication All patients not on dual antiplatelet therapy received a dose of 100 or 300 mg aspirin and 300 or 600 mg of clopidogrel before the procedure. The higher doses of aspirin and clopidogrel were given to patients in acute settings. An initial bolus of unfractionated heparin (70 to 100 IU/kg) was given to all patients, and additional boluses given to achieve and maintain an activated clotting time of >250 seconds, which was checked every 30 minutes. The use of bivalirudin or low-molecular heparin was prohibited. The use of glycoprotein IIb/IIIa antagonists was at the discretion of the operator, even in the setting of primary PCI. At the time of discharge, all patients were receiving 100 mg of aspirin once daily for an indefinite period, as well as 75 mg of clopidogrel daily for 12 months. Study endpoints and definitions Adverse events were assessed in the hospital, and at 1, 12 and 24 months. Data were collected by study monitors who visited the hospitals where follow-up was undertaken, reviewed the patients’ clinical notes, and collected the source documents. Furthermore, medical questionnaires were posted to all patients at 1, 6, 12 and 24 months to check for adverse events and establish current antiplatelet medication. In case of no response, information was obtained by telephone contact. Data were stored in our institution. Data processing and adjudication of adverse events, including stent thrombosis, were done by an independent contract research organisation and corelab (Cardialysis, Rotterdam, The Netherlands). The pre-specified primary endpoint was a composite of all death, non-fatal myocardial infarction and target vessel revascularisation at 12 months. The secondary endpoints were the primary endpoint at two year follow-up and the composite of major adverse cardiac events (cardiac death, non-fatal myocardial infarction and clinically driven target-lesion revascularisation at two year follow-up). The same endpoints and definitions were used as well in this current study. Definitions of endpoints are presented elsewhere.12 Acute myocardial infarction was defined as a typical rise and fall in concentrations of troponin or creatinine kinase-MB with at least one of the following: ischaemic symptoms, development of pathological Q-waves, ischaemic electrocardiographic changes or pathological findings of an acute myocardial infarction. ST-elevation MI was defined as patients with ST-segment elevation: new or presumed new ST-segment elevation at the J point in two or more contiguous leads with the cut-off points ≥0.2 mV in leads V1, V2, or V3 and ≥0.1 mV in other leads (contiguity in the frontal plane is defined by the lead sequence aVL, I, inverted aVR, II, aVF, III).15 Statistical analysis A new database with only patients presenting with MI was created. Categorical variables of all AMI patients were evaluated using the chi-square or Fisher’s exact tests, whereas continuous variables were evaluated using the Wilcoxon rank-sum test. The time to the pre-specified endpoints was evaluated according to the Kaplan-Meier method and the log-rank test was applied to compare the incidence of the endpoints between groups. Relative risks with 95% confidence intervals, were calculated using the delta method for binomially distributed data. This analysis was performed for all MI patients as well as for patients presenting with non-ST-elevation myocardial infarction (NSTEMI) or ST-elevation myocardial infarction (STEMI) separately. Formal interaction testing was performed to determine whether treatment for NSTEMI or STEMI influenced the relative risk of EES versus PES for the occurrence of primary and secondary endpoints at two years. The statistical analysis was performed according to the intention to treat principle. All p-values were two-sided, and a p-value of less than 0.05 was considered to indicate statistical significance. Analyses were performed using SAS version 8.02 (SAS Institute Inc. Cary, NC, USA). Results From the COMPARE database we identified 863 patients (EES=434, PES=429) that were treated with PCI for MI. From these patients 452 (EES=240, PES=212) had a STEMI at presentation and 411 patients were treated for NSTEMI (EES=217, PES=194). Baseline demographic and angiographic data The baseline demographic data is presented in Table 1. Baseline characteristics did not differ significantly between groups. The use of DAPT was also similarly distributed in both groups (Table 2). The use of glycoprotein IIbIIIa blockers did not differ significantly between groups in the overall population (49.8% vs. 49.6% in EES and PES, respectively) or in the subgroup undergoing primary PCI for STEMI (62.6% vs. 61.1% in EES and PES, respectively). In addition, the angiographic findings were similar between groups. No significant differences were observed in lesion numbers and distribution, lesions quantified coronary analysis results, TIMI flow at baseline and presence of thrombus. Although the total stent length was similar between the two groups, a slight, but not significantly, higher number of stents was used in the EES group (Table 3). This dissimilarity can be attributed to the difference in the maximal device length available for use (32 mm for PES and 28 mm for EES) during the trial. TIMI flow 0 (60% vs. 55.2%, p=0.3) and the use of thrombosuction catheters (47.3% vs. 46.2% p=0.6) during primary PCI for STEMI did not differ significantly in EES and PES respectively. There were no statistical differences in angiographic baseline characteristics between EES and PES groups in either STEMI or NSTEMI subgroups. Clinical outcomes Table 4 shows the clinical outcomes at 30 days, one year and two years follow-up. At one year, the primary endpoint, a patient oriented composite of all-cause mortality, non-fatal MI and TVR, occurred in 28 (6.4%) of patients in the EES group versus 45 (10.5%) in the PES (RR=0.62; 95% CI:0.39-0.97, p=0.03). At the two year follow-up, the primary endpoint occurred in 40 (9.2%) of patients assigned to EES and 69 (16.1%) of patients assigned to PES (RR=0.57; 95% CI: 0.40-0.83, p=<0.002). This difference was mainly driven by a significantly lower rate of both non-fatal myocardial infarction and target vessel revascularisation at one and two years in patients with EES; all-cause mortality did not differ significantly between treatment groups (Figure 1). Figure 1. Kaplan-Meier cumulative event curves for all MI patients and event rates split between STEMI and non-STEMI patients (bars) for (A) the primary endpoint, (B) secondary endpoint, (C) target vessel revascularisation (TVR), (D) target lesion revascularisation (TLR). Figure 1. Kaplan-Meier cumulative event curves for all MI patients and event rates split between STEMI and non-STEMI patients (bars) for (E) myocardial infarction (MI) and (F) All-cause mortality, (G) Cardiac mortality. Dotted lines indicate paclitaxel-eluting stent (PES); Continuous lines indicate everolimus-eluting stent (EES). At two years, the primary endpoint remained significantly lower for EES treated patients in STEMI (EES: 19 (7.9%) vs. PES: 33 (15.6%); RR=0.51; 95% CI: 0.30-0.87, p=0.01) where it was mainly driven by a significant reduction in TVR and a trend towards less non-fatal MI. The primary endpoint was also lower for EES treated patients in NSTEMI (EES: 21 (10.8%) vs. PES 36 (16.6%); RR=0.65; 95% CI: 0.39-1.08, p=0.09) but failed to reach significance. The same trend was observed also for the TVR and non-fatal MI in this subgroup whereas no clinically relevant differences were observed in all-cause mortality. However, the results of formal interaction testing for the primary and secondary endpoint resulted in a RR for interaction of 1.13; 95% CI: 0.79-1.63, interaction p=0.5 and 1.05; 95% CI: 0.70-1.56 interaction p=0.8, respectively, suggesting that a difference in treatment effect in the NSTEMI subgroup and the remaining patients from the MI population (STEMI subgroup) is unlikely. The secondary endpoint, a device-oriented composite of cardiac-death, non-fatal MI and TLR, at two years, occurred in 36 (8.3%) of patients assigned to EES and in 56 (13.1%) assigned to PES, (RR=0.64; 95% CI: 0.43-0.95, p=0.02). Similar to the primary endpoint, this difference was driven by a reduction in rates of both non-fatal MI and TLR, while no differences were observed in the rates of cardiac death (Figure 1 and Table 4). The stent thrombosis (definite and probable) rate at two years was significantly lower in the EES group: six (1.4%) compared to PES group: 20 (4.7%); RR=0.30; 95%CI: 0.12-0.73, p=0.005). As was the case in the general COMPARE population, a significant reduction in ST (definite and probable) was already evident at 30 days (EES: 1 (0.2%) vs. PES: 9 (2.1%); RR=0.11; 95%CI: 0.01-0.86, p=0.01) and the curves continued to diverge to the end of the second year (Figure 2A). A significant reduction in ST (definite and probable) was also observed (Figure 2B) also in the NSTEMI group at one year (EES: one (0.5%) vs. PES:nine (4.2%); RR=0.12; 95%CI: 0.02-0.97, p=0.02) and two years (EES: three (1.6%) vs. PES 13(6.0%); RR=0.26; 95%CI: 0.07-0.89, p=0.02). In the STEMI subgroup, the ST rates were lower in the EES group but the observed reduction in ST did not reach statistical significance (Figure 2C). Figure 2. Kaplan-Meier cumulative event curves for stent thrombosis (ST) for (A) all MI patients, (B) STEMI patients, (C) non-STEMI patients. Dotted lines indicate paclitaxel-eluting stent (PES); Continuous lines indicate everolimus-eluting stent (EES). The lower rates of ST in the overall MI population treated with EES paralleled significantly better outcomes in the safety endpoint of all-cause mortality and/or MI, with differences that continued to enhance during the two-year follow-up (Table 4). Discussion The major finding of this study is that the use of the second generation EES, compared to PES, during percutaneous coronary intervention for myocardial infarction was associated with a significant reduction in the risk of both the primary endpoint –a patient-oriented composite of all-cause mortality, non-fatal MI and TVR– and the principal secondary endpoint –a device-oriented composite of cardiac mortality, non-fatal MI and TLR. The improvement in event-free survival with the EES was driven by a reduction in repeat TVR, reflecting superiority in terms of efficacy, as well as a reduction in non-fatal MI, a safety component of the primary and secondary endpoint. There were no significant differences in rates of all-cause or cardiac mortality. With respect to the primary and secondary endpoints, the curves separated early and the effect grew in magnitude over the 2-year follow-up period. Another notable finding of this study is that the use of EES was associated with a significant reduction in ST, when compared to PES. The difference in ST outcomes between devices was apparent early and persisted at the end of the second year. As no significant differences were observed in baseline demographic or angiographic outcomes, the pathophysiologic mechanism(s) underlying the marked reduction in ST following EES, although speculative, may relate to specific design features of this stent. The combination of thin fracture-resistant struts, the low dose of everolimus, and the thromboresistant non-inflammatory proprieties of the fluorinated polymer, may contribute to the lower rates of early ST with EES.16,17 The lower rates of ST following EES at 30 days, one year and two years may be explained by more rapid and complete stent re-endothelialisation as observed in pre-clinical animal models.18 As expected, the reduction in ST was paralleled by a significant reduction in the safety composite of cardiac death or MI. The use of EES was associated with a reduction in major adverse cardiac events in the subgroup of patients presenting with STEMI. This is the first study in which the outcome with EES has been shown to be superior to PES for a composite of all-cause mortality, non-fatal myocardial infarction and target vessel revascularisation, in the setting of STEMI. However, our findings are consistent with the results of other studies that used the same stents in this setting. The PES subgroup of the HORIZONS-AMI trial had similar clinical outcomes to the PES subgroup of the present trial.3 Similarly, the outcomes with EES in our study are consistent with those observed in the STEMI subgroup of the RESOLUTE ALL-COMER trial.19 In the NSTEMI subgroup, a clear trend towards significance, with respect to the primary and secondary endpoint, was observed with EES. As the interaction p-values for the primary and secondary endpoints, were not significant, it appears reasonable to conclude that a difference in the treatment effect tendency between STEMI and NSTEMI is unlikely. Taken together, these findings suggest that the previously documented superiority, with regards to efficacy and safety endpoints, observed with EES can be extended to a high risk patient population presenting with AMI11,12. The ST rates with EES in the current study are comparable and numerically superior to those reported with BMS in historical comparisons20-22. This conclusion is also supported by the findings of the recently presented EXAMINATION trail, where EES is associated with significantly lower rates of definite as well as definite and probable ST compared to identically designed BMS, in setting of STEMI.23 More widespread use of DES for treatment of AMI should be guided by cost-effectiveness trials now that safety concerns have been largely resolved. This study has several limitations. This study presents a subgroup analysis from the randomised COMPARE study. However, randomisation was not stratified for the current subgroups, and therefore the number of patients in each treatment arm is not completely balanced. Nevertheless, the baseline characteristics were well matched between groups. The study was not powered for any of the individual endpoints therefore, for certain endpoint components, this may result in beta-errors, especially when data are further divided and analysed in STEMI and NSTEMI subgroups. Therefore, larger randomised trials will be needed in the setting of MI. Finally, these results reflect the outcomes of PCI for treatment of MI from a single tertiary centre. In conclusion, we have shown that the use of second-generation EES as compared to PES, is associated with significantly better outcomes, in terms of safety and efficacy endpoints at two year follow-up in patients presenting with MI. The use of EES compared to PES, is also associated with a significant reduction in rates of ST in MI patients. However, larger randomised trials, as well as cost-effectiveness analysis are required to further guide device choice in this setting. Conflict of interest statement E. Kedhi reports having received lecture fees from Abbott Vascular, Saint Jude Medical and Terumo Europe. P.C. Smits reports having received lecture fees from Abbott Vascular and Terumo Europe. All the other authors report no disclosures.