- drug eluting stents
- clinical outcomes
- coronary artery disease
- multivessel
Abstract
Aims: Although clinical trials have demonstrated superior clinical efficacy and improved safety of the everolimus-eluting stent (EES) compared with paclitaxel-eluting stents (PES) the clinical, angiographic and procedural factors associated with adverse clinical outcomes following drug-eluting stent (DES) deployment have not been carefully analysed.
Methods and results: We performed a patient-level pooled database analysis from the SPIRIT II, III, IV and COMPARE prospective randomised (EES versus PES) trials which enrolled 6,789 patients undergoing coronary stenting with follow-up through two years. To determine independent predictors of death, myocardial infarction (MI), ischaemia-driven revascularisation (target lesion [ID-TLR] or target vessel [ID-TVR]), and major adverse cardiovascular events ([MACE]; composite occurrence of cardiovascular death, MI, ID-TLR), we analysed clinical, angiographic and procedural variables using Cox proportional hazard stepwise regression analysis. Treatment with EES (versus PES) was a powerful, independent predictor of relative freedom from MI (HR [95% CI]= 0.54 [0.41, 0.71]; p<0.0001), cardiac death or MI (0.63 [0.49, 0.80]; p=0.0002), ID-TLR (0.59 [0.47, 0.74]; p<0.0001), ID-TVR (0.70 [0.58,0.84]; p=0.0002) and MACE (0.64 [0.54, 0.77]; p<0.0001). Both diabetes and the extent of coronary artery disease as reflected by the number of lesions treated were predictive of cardiac death, ID-TLR, ID-TVR, MI and MACE.
Conclusions: This multivariate analysis identified independent predictors of adverse outcomes to two years following DES deployment. Treatment with EES (versus PES) is an independent predictor of freedom from MI, cardiac death or MI, ID-TLR, ID-TVR and MACE.
Introduction
Randomised controlled clinical trials (RCCT) and observational registries have demonstrated improvement in clinical outcomes following percutaneous coronary intervention (PCI) with drug-eluting (DES) compared with bare metal stents (BMS)1,2. The relative benefit(s) attributed to DES have largely been confined to reductions in clinical and angiographic restenosis with little or no differences observed in the incidences of cardiovascular death and/or myocardial infarction (MI). Limitations inherent to first-generation DES, particularly suboptimal stent deliverability and risk for late stent thrombosis, prompted design iterations and the evolution of new generation DES. Furthermore, the rate of major adverse cardiovascular and cerebrovascular events (MACCE), particularly the requirement for repeat revascularisation, remains greater following PCI with DES compared with surgical coronary revascularisation in patients with complex and/or multivessel disease3.
Although recent large-scale RCCT have demonstrated superior clinical efficacy and improved safety of everolimus-eluting stents (EES) when compared with paclitaxel-eluting stents (PES)4,5, the clinical, angiographic and procedural factors associated with adverse clinical outcomes have not been carefully analysed in adequately sized cohorts. Therefore, we analysed pooled patient level data from 4 RCCT which compared EES to PES to determine independent predictors of adverse clinical events to two years following PCI.
Methods
The designs of the prospective single-blind, active controlled SPIRIT II, III, IV and COMPARE trials have previously been described4-8. These trials randomised assigned 6,789 eligible subjects undergoing PCI to treatment with either EES or PES. The PES platform utilised in the SPIRIT trials was the TAXUS Express while the TAXUS Liberte was employed in the COMPARE trial. Briefly, SPIRIT II enrolled 300 patients with ≤2 de novo native coronary stenoses, SPIRIT III enrolled 1,002 patients with similar inclusion criteria as in SPIRIT II, and SPIRIT IV enrolled 3,687 patients with ≤3 de novo native coronary stenoses involving ≤2 epicardial vessels. Whereas the SPIRIT trials excluded many high-risk patients including those with acute or recent myocardial infarction, the COMPARE trial enrolled 1,800 patients using an “all comers” design and excluded only those patients unable to comply with dual antiplatelet therapy or study procedures, who required major surgery within 30 days or who were unable to sign informed consent5. The COMPARE trial had no exclusion criteria based on symptoms or lesion type. A portion of patients enrolled in the SPIRIT II and III trials underwent protocol specified routine angiographic follow-up6,7 while only clinical follow-up was performed in the SPIRIT IV and COMPARE trials. Each study employed an independent, angiographic core laboratory as well as independent clinical events and safety monitoring committees. Clinical endpoints in all trials were adjudicated by a clinical events committee blinded to stent assignment. Follow-up is planned for five years in each trial and is complete through two years in all four studies. Patient level databases from all four trials were pooled for the present analysis.
Medications and procedure
Balloon predilatation of the target lesion was specified by protocol in the SPIRIT trials but was not required in COMPARE. Aspirin ≥75mg daily was recommended for a minimum of 1 year in SPIRIT II and indefinitely in the other trials. Clopidogrel (75mg daily) was prescribed by protocol for ≥6 months in SPIRIT II and III and for ≥12months in SPIRIT IV and COMPARE.
Clinical endpoints
Endpoint definitions for each trial have been described previously4-8 and were similar in the SPIRIT and COMPARE trials for the endpoints assessed in the current analysis. The principal endpoints for the present analysis included ischaemia-driven revascularisation defined as either target lesion (ID-TLR) or vessel (ID-TVR), MI and the composite occurrence of cardiac related death or MI, and major adverse cardiovascular events (MACE) defined as the composite occurrence of cardiac death, MI or ID-TLR. The endpoint of stent thrombosis is the focus of a separate analysis and report. Events as adjudicated in each trial were utilised for the pooled analysis and all endpoints were assessed at two years.
Statistical methods
All analyses were performed based on intention-to-treat. Binary variables are summarised as counts and percentages and were compared either using either Chi Square or Fisher Exact Test as appropriate. Continuous variables are summarised as means and standard deviations and were compared using t-tests. Outcomes from two years are displayed as time-to-event curves summarised as Kaplan Meier estimates and compared using the log-rank test and hazard ratios. To determine predictors of clinical outcomes, clinical, angiographic and procedural variables were analysed using multivariable Cox proportional hazards forward stepwise regression analysis with independent variables entered into the model at the 0.1 significance level and removed at the 0.1 level. The following variables were included in univariate analyses for each endpoint:
Demographic: age, gender, diabetes (any/insulin dependent), current smoker, hypertension or hyperlipidaemia requiring medication, prior history of CABG, MI or PCI, unstable angina, ACS; Procedural: randomised stent type (XIENCE/EES), number of lesions treated, saphenous vein graft, left main or LAD lesion treated; QCA: calcification (moderate/severe), total occlusion, thrombus, TIMI 0/1 flow, lesion length, RVD and MLD at baseline. In patients with more than one treated lesion, the most severe lesion/vessel characteristic was selected for analysis.
Results
A total of 6,789 patients were randomly assigned to treatment with EES (n=4247; 63%) or PES (n=2542; 37%), and clinical follow-up to two years was available in 96.2% EES and 96.1% PES treated patients. Baseline demographics (Table 1) were largely similar between randomly assigned stent types with several exceptions. Patients assigned to EES had a higher prevalence of hypertension and hyperlipidaemia and more often presented with stable ischaemic heart disease. Conversely, PES-assigned patients more frequently presented with acute coronary syndromes. Baseline and post-procedural angiographic variables as well as procedural variables (Table 2) demonstrated no differences in target vessel location between randomly assigned stent type. However, the presence of total occlusion, thrombus, moderate or severe calcification and modified ACC/AHA Class C lesion morphology were more frequently observed in patients assigned PES. A baseline TIMI flow of 0 was more frequent among PES-assigned patients. Although pre- and post-procedure reference vessel diameters were similar between randomly assigned stent types, PES-treated patients had longer target lesion length, less severe target lesion stenosis and had longer total stent length deployed than did EES-treated patients. Compliance with either aspirin or clopidogrel to one year and two years follow-up was similar between randomly assigned stent types (Table 3).
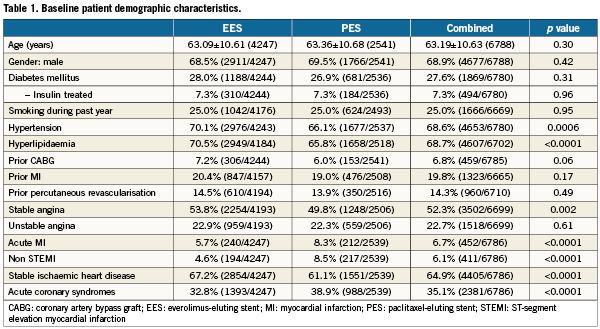
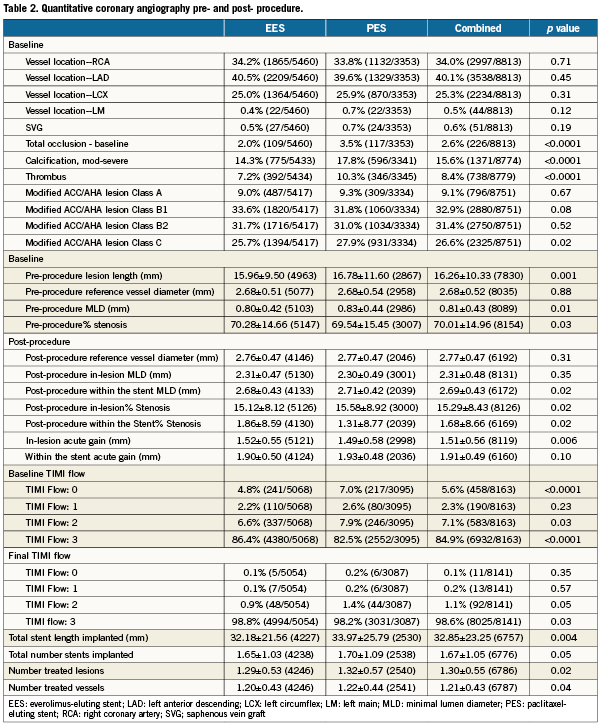
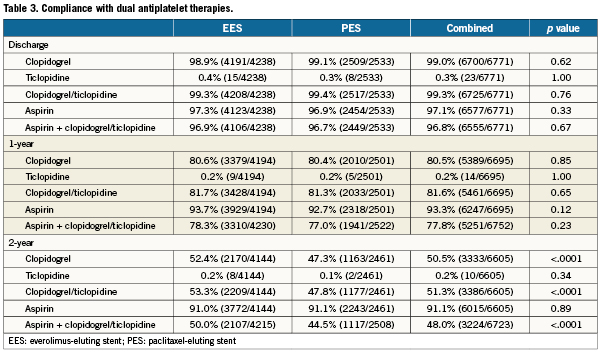
Clinical outcomes
No differences in all-cause or cardiac death were observed according to randomly assigned stent type. However, treatment with PES (versus EES) was associated with highly significantly increased relative risks for the occurrence of cardiac death, MI, the composite of cardiac death or MI, ID-TLR, ID-TVR and MACE through two years follow-up (Table 4 and Figure 1A-F). A progressive divergence in event rates over time was observed between the randomly assigned stent types.
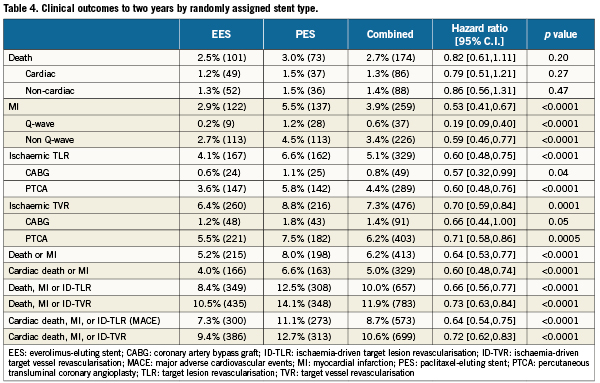
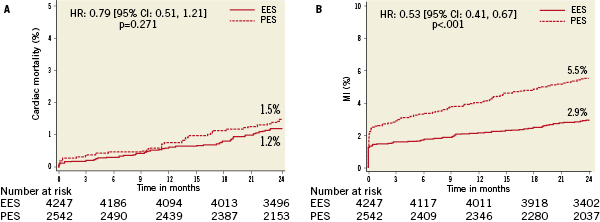
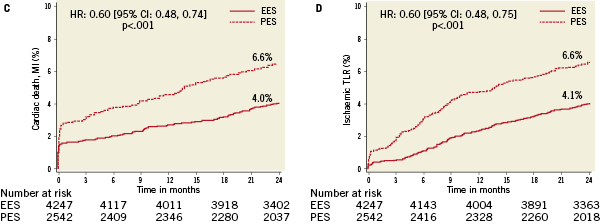
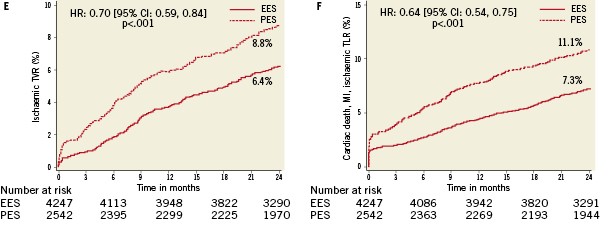
Figure 1. Kaplan-Meier curves illustrating time to event occurrence by randomly assigned stent type for (A) cardiac-related mortality, (B) myocardial infarction, (C) the composite occurrence of cardiac death or myocardial infarction, (D) ischaemia-driven target lesion revascularisation (TLR), (E) ischaemia-driven target vessel revascularisation (TVR), and (F) the composite occurrence of cardiac death, MI, and ischaemia-driven TLR.
Multivariate predictors of clinical outcomes
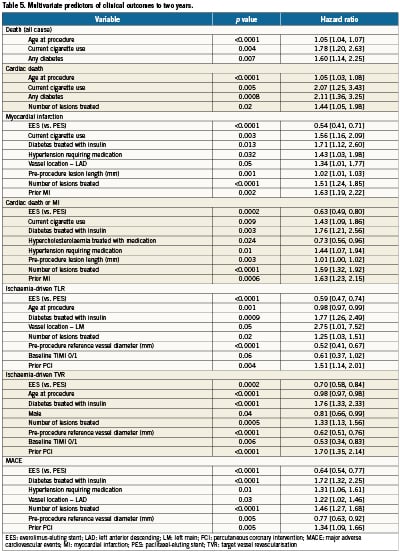
Multivariate Cox proportional hazards regression analysis identified multiple but similar independent predictors of both ID-TLR and ID-TVR (Table 5) including age, diabetes treated with insulin, number of lesions treated, baseline reference vessel diameter and TIMI flow grade, prior PCI and randomly assigned stent type (increased with PES versus EES). Target vessel location was an additional predictive variable for ID-TLR. Although randomly assigned stent type was not a predictor of all-cause or cardiac death to two years, treatment with EES (versus PES) was a powerful independent predictor of freedom from MI, cardiac death or MI, and MACE. Not surprisingly, age at procedure, current cigarette use and the presence of diabetes were predictors of death (all-cause and cardiac). The number of lesions treated was also predictive of cardiac death.
Discussion
This analysis provides insights into the clinical, angiographic and procedural factors associated with relevant clinical outcomes to two years following DES deployment in a contemporary large population of patients with coronary anatomy ranging from simple to complex undergoing PCI. Several important observations can be made. First, both the presence of diabetes (particularly requiring insulin treatment) and the extent or complexity of coronary disease as reflected by the number of lesions treated are powerful predictors of multiple clinical outcomes at two years including cardiac death, ID-TLR, ID-TVR, MI and MACE. Second, randomly assigned stent type (EES versus PES) although not predictive of mortality, was nevertheless a significant independent predictor of the 2-year occurrence of MI, the composite of cardiac death or MI, ID-TLR, ID-TVR and MACE. The observation that the risk of multiple adverse outcomes is proportional to the number of treated lesions supports a graded relationship between the extent of coronary disease (as reflected by the number of vessels and lesions treated) and outcomes following PCI3,10-12.
Despite the proven benefit of DES to reduce angiographic and clinical restenosis compared to BMS, a major limitation of PCI with DES when compared with surgical coronary revascularisation continues to be an increased requirement for repeat revascularisation procedures which is roughly proportional to the extent of disease requiring revascularisation3,13. Indeed, in the SYNergy between percutaneous coronary intervention with TAXUS and cardiac surgery (SYNTAX) trial which randomised patients with 3-vessel and/or left main coronary artery disease to either PCI with PES or surgical revascularisation, the primary endpoint of MACCE was increased at one, two and three years following PCI in direct proportion to the complexity of underlying coronary disease as reflected by the SYNTAX angiographic lesion complexity score3,14,15. The relationship between coronary disease complexity and MACCE (particularly target vessel MI and the requirement for repeat revascularisation) following PCI with DES in SYNTAX may have relevance in the context of the second major observation of the present study, i.e., that stent type (EES versus PES) is a significant independent predictor of freedom from MACCE events including MI and ischaemia-driven revascularisation. This observation may be relevant as it suggests the potential for EES (versus PES) to improve outcomes of PCI even in patients with advanced multivessel coronary disease as reflected by a recent meta-analysis16. This premise is consistent with recent observations derived from both the pooled SPIRIT III and IV as well as the SPIRIT II, III, IV and COMPARE trial patient level databases which demonstrate that the relative clinical benefit afforded by EES (compared to PES) is proportional to the complexity of coronary disease as reflected by the number of vessels or lesions treated17,18.
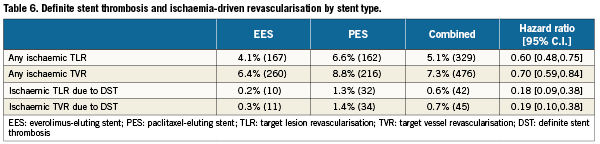
The relationship between DES type and ID-TLR/TVR may in part be explained by the greater in-stent late lumen loss and neointimal volume (demonstrated by QCA and intravascular ultrasound respectively) following PES compared with EES deployment6,19, and is supported by pooled analyses of multiple RCCT20 as well as clinical registry experiences21. Although a difference in the occurrence of stent thrombosis (increased following PES versus EES)4,5 may contribute to the observed differences in ID-TLR or ID-TVR as well, definite stent thrombosis was an infrequent contributor to either revascularisation outcome (42/329 ID-TLR events and 45/476 ID-TVR events; Table 6).
The relationship of DES type with the occurrence of MI is more complex and the pathophysiologic mechanism(s) may vary over time. The majority of target vessel MIs in the current study were observed early (≤30 days) post-PCI and were related to the PCI procedure. Target vessel MI not ascribed to stent thrombosis is usually attributed to either side branch compromise and/or distal atheroembolism22. Multiple stent specific factors including strut and/or polymer thickness, polymer viscoelastic properties, inherent polymer thrombogenicity as well as cell design (open versus closed) and have been incriminated in the genesis of periprocedural MI23. In this regard, PES (both Express and Liberté platforms) strut and polymer thickness are greater than EES, and the viscoelastic properties of translute® polymer (PES) have been associated with “webbing”24,25. Post hoc analyses of the randomised TAXUS V and VI trials which compared PES with its respective Express BMS platform demonstrated an increased incidence of periprocedural MI in patients with long lesions and overlapping PES that was ascribed to side-branch compromise26. Conversely, a pooled analysis of randomised trials comparing the sirolimus-eluting stent (SES) with its respective bare-metal (BX-Velocity) platform did not demonstrate an increased incidence of MI with SES (versus BMS) in patients with overlapping stents23. Finally, marked heterogeneity in the grade of neointimal coverage and an increased incidence of endoluminal mural thrombus has been demonstrated following PES compared with either SES or BMS treatment particularly when stent overlap is present27-30. These differences may reflect polymer-related thrombogenicity, inflammatory and/or hypersensitivity reactions which have been aetiologically incriminated in the occurrence of late and very late DES thrombosis. In this regard, both late and very late stent thrombosis were increased following PES (versus EES) in both the SPIRIT IV and COMPARE randomised trials30,31.
Study limitations
Several potential limitations of the present study should be acknowledged. First, despite the large number of total patients enrolled into these four randomised trials, sample sizes in individual subgroups may still be insufficient to derive reliable estimates of relatively low frequency events. Second, despite the randomised design of all four trials and the large number of patients enrolled, several baseline covariate imbalances were present between randomly assigned stent types that may not have been completely adjusted for by the multivariate regression methodology. Additional, unmeasured variables may have influenced the study observations as well despite the randomised nature of the pooled cohorts. However, the adjusted hazard ratios demonstrating benefit of EES vs. PES for the reduction of the 2-year rates of MI, cardiac death or MI, TLR, and MACE demonstrate a marked benefit of EES, with tight 95% CIs (upper bounds ≤0.80), making it unlikely that benefits observed with EES in the multivariable models are due to chance.
Finally, any attempt at comparison between observations made in the current analysis regarding relative benefit of EES (versus PES) and the SYNTAX trial results of PCI with PES versus surgical coronary revascularisation are conjectural and limited by marked differences in coronary disease complexity across trials. Indeed, the average number of lesions (2.2) and stents (3.0) deployed in the multivessel/lesion (≥2 vessels/lesions) cohort of the current pooled analysis are far less than in the SYNTAX trial (3.6 lesions and 4.6 stents per patient). Further studies are required to determine whether the relationship between the continuous variables which were identified as independent predictors of MACE is linear or nonlinear.
Conclusions
This present analysis has identified significant independent clinical, angiographic and procedural predictors of adverse clinical outcomes to two years following PCI with DES from a large, pooled patient level database which included the SPIRIT II, III, IV and COMPARE randomised trials. The presence of diabetes and the extent or complexity of coronary disease as reflected by the number of lesions treated were independent predictors of cardiac death, MI, the composite occurrence of cardiac death or MI, ID-TLR, ID-TVR and MACE. Importantly, treatment with EES (versus PES) was a significant independent predictor of freedom from MI, the composite occurrence of cardiac death or MI, ID-TLR, ID-TVR and MACE to two years following PCI.
Conflict of interest statement
Dr. Kereiakes reports serving on the advisory boards for Abbott Vascular and Boston Scientific. Dr. Smits reports receiving speaking and travel fees from Abbott Vascular. Dr. Kedhi reports receiving lecture fees from Abbott Vascular and Terumo Europe as well as travel costs from Boston Scientific and Medtronic. Drs. Parise and Fahy report no conflicts. Dr. Serruys has nothing to disclose. Dr. Stone reports having serving on scientific advisory boards for Boston Scientific and Abbott Vascular, and serving as a consultant for The Medicines Company, Merck, Eli Lilly, AstraZeneca and Bristol-Myers-Squibb.

