
Abstract
Aims: The aim of this study was to report whether the superiority of the everolimus-eluting stent (EES) vs. the paclitaxel-eluting stent (PES) at one-year follow-up in the Taxus Element versus Xience Prime in a Diabetic Population (TUXEDO)-India trial was sustained at longer-term follow-up.
Methods and results: One thousand eight hundred and thirty (1,830) patients with diabetes mellitus and coronary artery disease were randomised to EES vs. PES. Follow-up data up to two years were available in 1,701 (92.9%) patients. The primary endpoint was target vessel failure (TVF), defined as the composite of cardiac death, target vessel myocardial infarction (TV-MI), or ischaemia-driven target vessel revascularisation (TVR). Treatment with EES had a lower two-year rate of TVF (4.3% vs. 6.6%, p=0.03). Of the secondary endpoints, EES significantly reduced any MI (1.6% vs. 3.5%, p=0.01), TV-MI (0.7% vs. 3.1%, p=0.0001), ST (0.4% vs. 2.2%, p=0.001), cardiac death or target vessel MI (2.9% vs. 4.8%, p=0.04) and TLR (1.9% vs. 3.7%, p=0.02), compared with PES. Between one year and two years, no significant differences in the clinical outcomes were observed (pinteraction >0.05).
Conclusions: In this adequately powered trial, the benefits of EES vs. PES in a diabetic population seen at one year were maintained at two years. Trial registration ctri.nic.in. Identifier: CTRI/2011/06/001830.
Abbreviations
ARC: Academic Research Consortium
EES: everolimus-eluting stent
eGFR: estimated glomerular filtration rate
MACE: major adverse cardiac events
MI: myocardial infarction
OCT: optical coherence tomographic
PCI: percutaneous coronary intervention
PES: paclitaxel-eluting stent
SD: standard deviation
ST: stent thrombosis
T2DM: type 2 diabetes mellitus
TLR: target lesion revascularisation
TUXEDO: Taxus Element versus Xience Prime in a Diabetic Population
TVF: target vessel failure
TVR: target vessel revascularisation
Introduction
Patients with type 2 diabetes mellitus (T2DM) have worse clinical outcomes with a significantly higher rate of repeat revascularisation and stent thrombosis after percutaneous coronary intervention (PCI) as compared to those without T2DM1. Clinical trials have shown lower rates of restenosis, target lesion revascularisation (TLR), and composite endpoints such as major adverse cardiac events (MACE), with everolimus-eluting stents (EES) than with paclitaxel-eluting stents (PES)2-5. However, contrary to this, a pooled analysis of four randomised trials demonstrated a marked attenuation of beneficial effects of EES, as compared with first-generation PES, in a subset of diabetic patients6. Therefore, the choice of DES for the treatment of diabetic patients remained debatable until recently.
In the Taxus Element versus Xience Prime in a Diabetic Population (TUXEDO)-India trial, 1,830 patients with diabetes mellitus and coronary artery disease undergoing PCI were randomised 1:1 to PES and EES groups. The one-year rate of the primary endpoint of target vessel failure (TVF) was significantly higher in the PES than in the EES group (p=0.005), with significant reduction in individual rates of MI (p=0.004), stent thrombosis (ST) (p=0.002), target vessel revascularisation (TVR) (p=0.002) and TLR (p=0.002)7. Whether these benefits are robust over time is not known. In the angiographic follow-up from the Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System (SPIRIT) II trial, there was an incremental late loss with EES compared with PES between six months and two years8. The present study was conducted to determine the two-year clinical outcomes from an adequately powered TUXEDO trial and to observe whether the differences in outcomes between EES and PES in the TUXEDO-India trial at one year were maintained over two years of follow-up.
Methods
STUDY DESIGN
The TUXEDO-India study was an investigator-initiated, multicentre, randomised clinical trial in which 1,830 patients with diabetes mellitus and symptomatic coronary artery disease/silent ischaemia were randomised 1:1 to PES (TAXUS Element; Boston Scientific, Marlborough, MA, USA) or EES (XIENCE Prime; Abbott Vascular, Santa Clara, CA, USA) treatment groups using an interactive web response system with a block size of 8. The study was approved by the institutional review boards of each participating centre. Written informed consent was obtained before randomisation or PCI but after angiography. The eligibility criteria of patients and main results have been reported earlier7. An independent data safety monitoring board (DSMB) periodically reviewed the study data and evaluated patients’ safety, study conduct and progress. An independent clinical events committee (CEC) adjudicated the endpoints.
All patients received oral aspirin (350 mg) and a loading dose of clopidogrel (600 mg), prasugrel (60 mg) or ticagrelor (180 mg) before the index procedure. Patients continued to take aspirin (75 to 150 mg daily), clopidogrel (at least 75 mg daily), prasugrel (10 mg daily) or ticagrelor (90 mg twice daily) for at least 12 months after stent implantation. After a 12-month period, the choice of continuing dual antiplatelet therapy was left to the discretion of the treating investigator. By necessity, the operators were aware of the stent being implanted during the procedure; however, the patient and the follow-up teams were unaware of the randomised group assignment, and a standardised follow-up protocol was implemented to reduce the risk of bias. The clinical follow-up visits were scheduled at 30 days, 180 days, one year, and two years post index procedure; follow-ups at 180 days and one year were on-site, and 30-day and two-year follow-up visits were performed telephonically.
STUDY ENDPOINTS
The primary endpoint was TVF, a composite of cardiac death, target vessel-related MI (TV-MI), or ischaemia-driven TVR at two years. The secondary endpoints included ischaemia-driven TLR; TVR; the composite of cardiac death or TV-MI; MACE; a composite of cardiac death, MI or ischaemia-driven TLR; MI (Q-wave and non-Q-wave); cardiac death; non-cardiac death; all-cause death; cardiac death or MI; all death or MI and Academic Research Consortium (ARC)-defined ST at two years. The procedural endpoints were the technical success rate and clinical procedural success rate at two years.
STATISTICAL METHODS
All the data are presented for the intention-to-treat population, which includes all patients who underwent randomisation, regardless of whether a study stent was implanted. Categorical variables were compared by the chi-square/Fisher’s exact test and the continuous variables presented as mean±SD and compared by t-test. Kaplan-Meier survival estimate curves for time-to-event variables were obtained for each group and compared by log-rank test. Hazard ratios and their 95% confidence intervals were also calculated. Logistic regression was used to calculate interaction p-values between stent type and time (between ≤1 year and >1 year). The consistency of treatment effects in pre-specified subgroups was assessed with the use of logistic regression with tests for interaction. All statistical analyses were performed with SAS software, version 9.3 (SAS Institute, Cary, NC, USA).
Results
PATIENTS
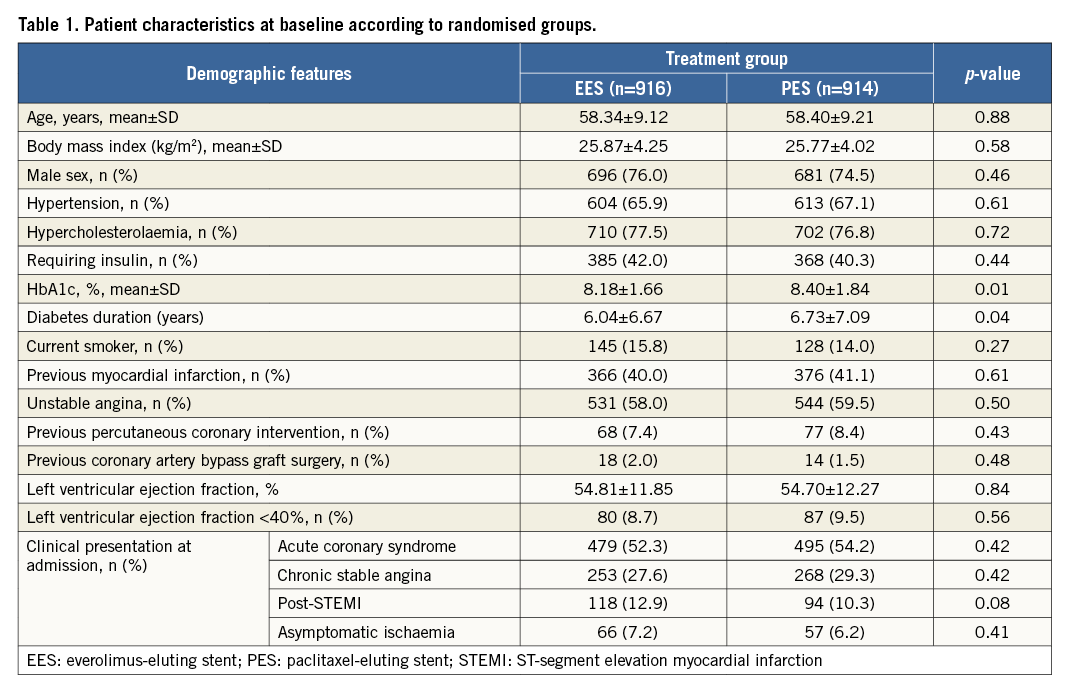
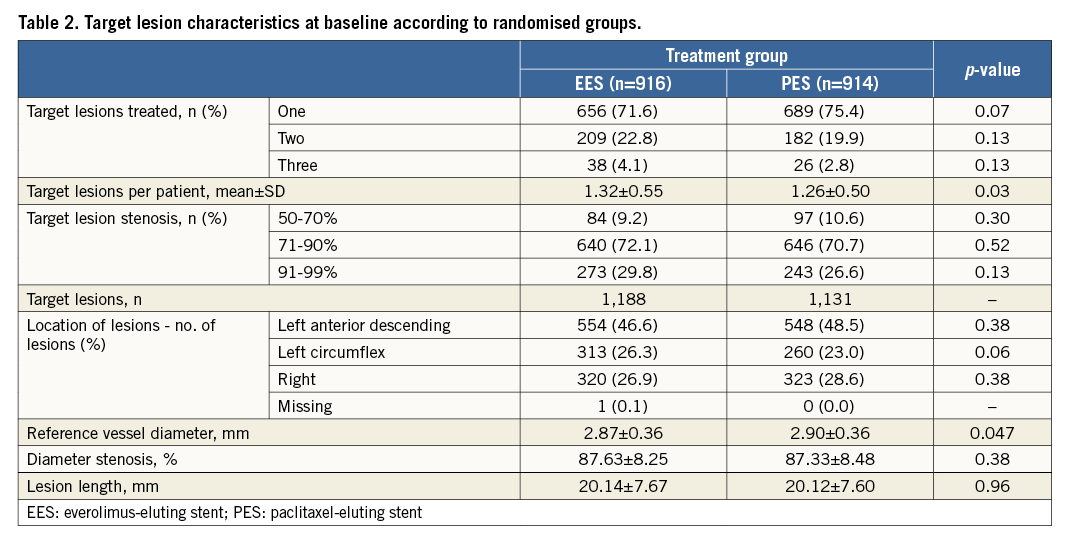
A total of 1,830 patients were randomly assigned to receive PES (914 patients) or EES (916 patients). The treatment groups were well matched with respect to baseline characteristics except for target lesions per patient, which were significantly higher in the EES-treated patients (Table 1, Table 2). All the patients were maintained on dual antiplatelet therapy for one year. Following that, they were left on aspirin, and the continuation of the second drug was left to the discretion of the local investigator.
CLINICAL OUTCOMES
Follow-up data at two years were available in 1,701 (92.9%) patients, comprising 836 in the PES group and 865 in the EES group. EES significantly reduced the two-year rate of TVF as compared to PES (4.3% vs. 6.6%; hazard ratio [HR] 0.63, 95% confidence interval [CI]: 0.42 to 0.94; p=0.03) (Table 3). The two-year reduction in TVF was driven by a significant reduction in cardiac death or TV-MI (p=0.04) and ischaemia-driven TLR (p=0.02) (Table 3). EES also significantly reduced the two-year rates of all MI (p=0.01), target vessel-related MI (p=0.0001), and non-Q-wave MI (p=0.03) (Table 3). The two-year rate of ARC definite or probable stent thrombosis was reduced by approximately 72% with EES compared with PES (0.4% vs. 2.2%; HR 0.20, 95% CI: 0.07 to 0.57; p=0.001) (Table 3). There was no statistically significant difference noted between the treatment groups with respect to the other clinical outcomes. The analysis of the data between one year and two years revealed no significant difference in the clinical outcomes between the PES and EES groups (Figure 1). Assessment of potential interaction between stent type and time (between ≤1 year and >1 year) showed no significant interaction for the majority of clinical endpoints except TVR, TLR, and the composite of death, MI or TVR, for which the outcomes were numerically higher with EES when compared with PES between one and two years of follow-up (Table 3). In addition, the logistic regression analyses with interaction testing demonstrated that two-year reduction in TVF with EES compared with PES was consistent across 12 pre-specified subgroups. However, a trend for higher TVF was found in PES compared to EES in all subgroups. The current analysis revealed a non-significant interaction in patients with an estimated glomerular filtration rate (eGFR) of ≤60 ml/min versus ˃60 ml/min, which was contrary to our one-year outcome report where a borderline interaction was noted (Figure 2)7.
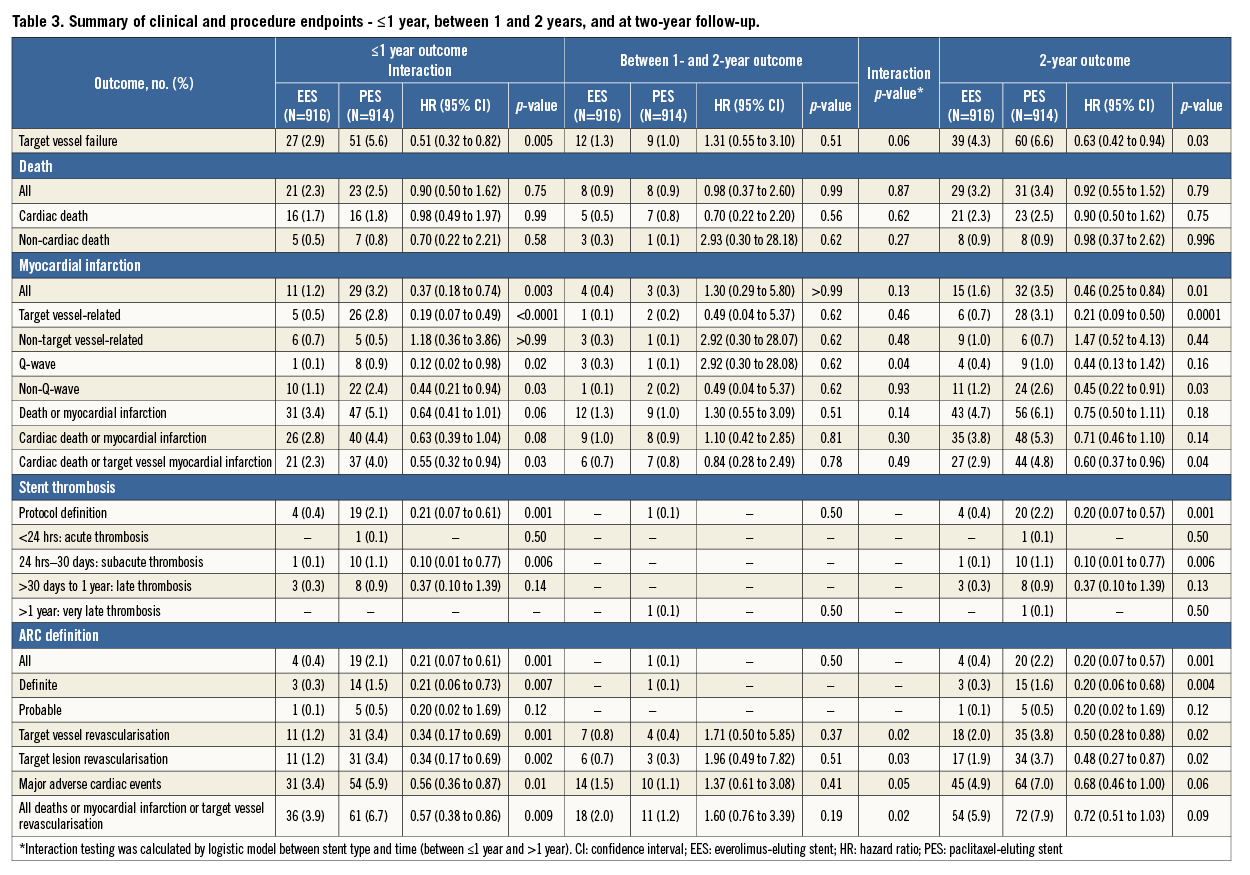
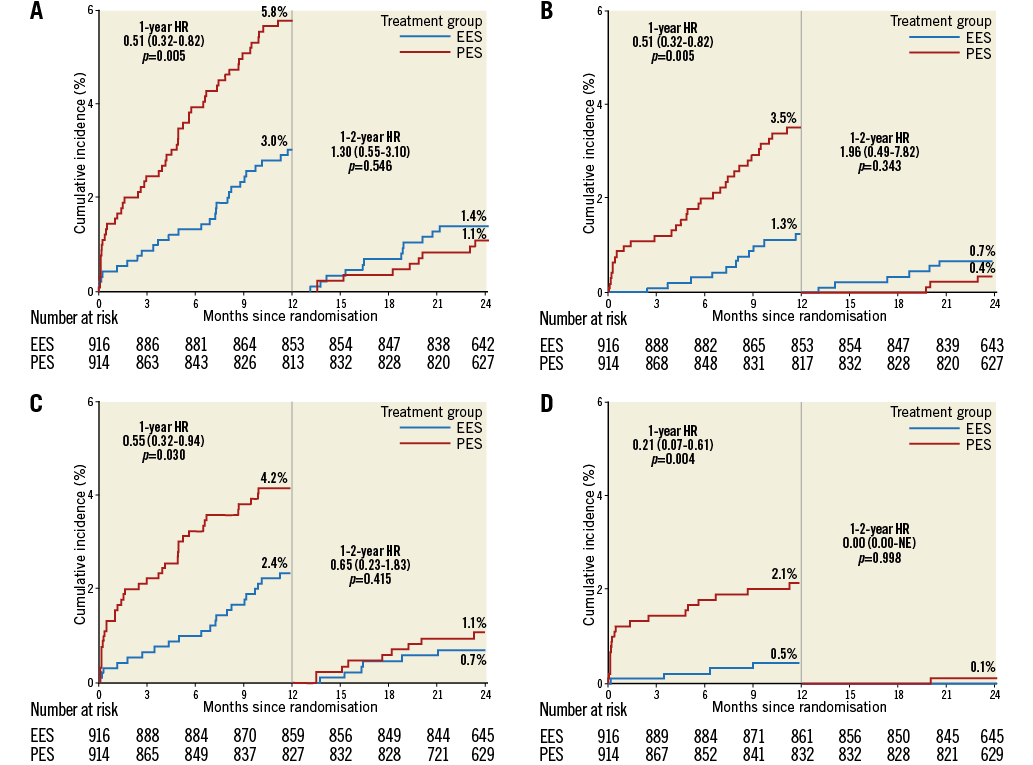
Figure 1. Time-to-event curves up to one year and between one- and two-year follow-up. Time-to-event curves for target vessel failure (A), ischaemia-driven target lesion revascularisation (B), cardiac death or target vessel myocardial infarction (C) and stent thrombosis (D). EES: everolimus-eluting stent(s); HR: hazard ratio; PES: paclitaxel-eluting stent(s)
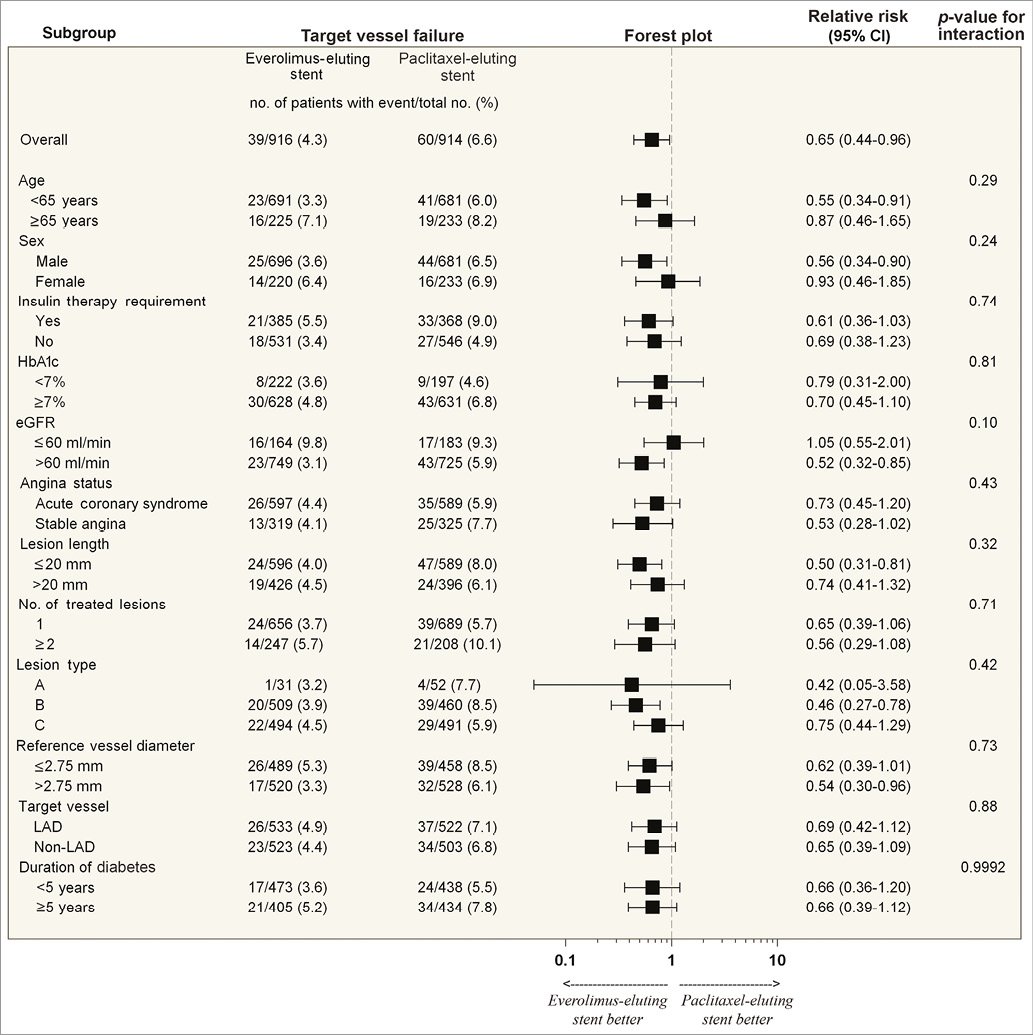
Figure 2. Two-year rates of TVF in 12 pre-specified subgroups according to stent randomisation.
Discussion
The TUXEDO-India trial demonstrated that treatment with EES, as compared with PES, resulted in significant reductions in TVF (2.9% vs. 5.6%, p=0.005), spontaneous MI (1.2% vs. 3.2%, p=0.004), ST (0.4% vs. 2.1%, p=0.002), TVR (1.2% vs. 3.4%, p=0.002), and TLR (1.2% vs. 3.4%, p=0.002) at one year. There was, however, no significant difference in all-cause or cardiac mortality. This resulted in the one-year primary endpoint of TVF being reduced by approximately 48% (absolute risk reduction 2.7%) with EES as compared to PES7. The same results were reproduced at the end of the two-year follow-up period where patients treated with the EES had an approximately 37.0% relative and 2.3% absolute reduction in the rate of the primary clinical endpoint of TVF (4.3% vs. 6.6%, HR 0.63, 95% CI: 0.42 to 0.94; p=0.03) as compared with PES. In addition, a statistically significant reduction in cardiac death or TV-MI, ST, and ischaemia-driven TLR and TVR was reported with EES. This confirms that the benefits of EES compared with PES at one year were maintained at the end of two years.
Between one and two years of follow-up after stent implantation, the clinical outcomes were comparable between the two treatment groups. This observation is in agreement with the SPIRIT IV trial but differs from that seen in the COMPARE trial, in which the advantages of EES as compared to PES continued to increase over time9,10. The follow-up finding in the present study revealed maintenance of clinical efficacy of EES and PES between one and two years.
The angiographic in-stent late loss (LL), as assessed in the SPIRIT I trial, showed a reduction of approximately 88% at six months and 71% at 12 months in the EES compared with the BMS group11,12. The findings from the SPIRIT II and SPIRIT III trials further revealed the superiority of EES over PES in reducing in-stent LL2,3. Moreover, the superiority of EES over PES, in terms of LL reduction (0.24 mm), was also expressed in the diabetic subset of the SPIRIT II trial at six months2. The SPIRIT II angiographic substudy had shown incremental late loss (in mm) between six months (0.17±0.32 vs. 0.33±0.32, p=0.01) and two years (0.33±0.37 vs. 0.34±0.34, p=0.84) with EES as compared to PES8. Angiographically assessed late loss may artificially increase the rates of TLR preferentially for stents with an increased degree of late loss13. Our study had no angiographic follow-up assessment, but only a clinical follow-up which can eliminate such bias. The target lesion failure (TLF) rate was further reported to be significantly lower for the EES (7.7% vs. 13.8%, p˂0.005), as compared to PES, in the SPIRIT III trial at two-year follow-up4. Our study in diabetics, on the other hand, did not show an increase in TVF between one and two years. The benefits of EES as compared to PES at one year could also be explained by earlier endothelialisation in EES. In a rabbit aorto-iliac model it was shown that stent re-endothelialisation occurs more rapidly with EES than with other DES14. It is therefore likely that these experimental observations translate into improved safety of EES in humans. Serial optical coherence tomographic (OCT) studies can confirm the same in humans.
Prior trials had shown sustained benefit of EES over PES in an all-comers cohort; however, whether this benefit is maintained in patients with diabetes in the long term is not known10. Patients with diabetes have rapidly progressing atherosclerosis and have higher rates of restenosis and stent thrombosis1. The results of this adequately powered trial in patients with diabetes provide reassurance about the continued clinical benefit of EES over PES not just in terms of reduction of restenosis-related endpoints, but also for the hard endpoints of cardiac death, TV-MI and spontaneous MI. Furthermore, the two-year benefits of EES compared with those of PES were consistent across 12 pre-specified subgroups. Notably, interaction of eGFR with stent type for TVF, which was statistically significant at one year, became insignificant at two years, thus reducing the relevance of this finding.
The occurrence of definite or probable stent thrombosis during the two years of follow-up was low in the EES group (four patients) compared to the PES group (20 patients), indicating greater safety of the EES up to two years. The results are in concurrence with previous studies (SPIRIT FIRST, II, and III constituting 866 patients) where only 10 (1.2%) patients developed a definite or probable stent thrombosis with EES at two-year follow-up4,15. Our results support the current worldwide practice of the use of new-generation EES in patients with diabetes mellitus.
Study limitations
The study does not report the effect of dual antiplatelet therapy after one year in either stent arm. The continuation of dual antiplatelet therapy was left to the discretion of the treating investigator after one year and has not been captured systematically. The patients included represent a selected medium risk/complexity population with only 6.9% of patients with three-vessel disease. Therefore, the results of the current study may not be applicable in patients with more extensive disease. No angiographic assessment on follow-up was carried out except for those requiring reintervention for the target vessel(s), hence data on in-segment LL are not available. With technological advances and understanding in stent design, EES have shown improvement in terms of safety, intimal hyperplasia and subsequent restenosis compared to PES, with a loss of the clinical relevance of PES in clinical practice.
Conclusions
The benefits of EES when compared to PES resulting in significantly lower rates of TVF, MI, ST, TVR, TLR, and a composite of cardiac death or TV-MI present at one year were maintained over a two-year follow-up period in diabetic patients undergoing PCI, highlighting the consistency of these differences.
| Impact on daily practice The sustained clinical benefits of EES compared with PES at two-year follow-up in diabetic patients will help clinicians in choosing the appropriate DES in clinical practice. |
Acknowledgements
The authors acknowledge the role of Nagesh Prabhu, MBBS, Santosh Kumar, MA, Gunjan Agarwal, PhD, Ali Nasir Siddiqui, M. Pharm, Harpreet Kaur, BHMS, PGDCR, Aadil Hussain, MSc, Ramanjulu Nidamanuri, M. Pharm, JSS Medical Research India Pvt. Ltd. for performing site monitoring, data management, and biostatistical analysis.
Funding
The TUXEDO-India trial was funded by research grants from Boston Scientific. The study sponsor had no role in study design and the collection, analysis, interpretation and writing of this article. The decision to submit this article for publication is that of the investigators. The researchers of this trial were independent of the funders.
Conflict of interest statement
U. Kaul reports receiving grants from Boston Scientific during the conduct of the study, grants and personal fees from Boston Scientific, and grants from Abbott Vascular, outside the submitted work. S. Bangalore reports receiving grants and personal fees from Abbott Vascular, and travel grants from Boston Scientific and Medtronic, outside the submitted work. A. Bhagwat, B. Pinto, S. Sathe and P. Jagtap report receiving grants from Fortis Escorts Heart Institute, New Delhi, during the conduct of the study. P. Jagtap also received personal fees from Medtronic Inc., outside the submitted work. The other authors have no conflicts of interest to declare.

