- drug eluting balloon
- diabetes mellitus
- coronary
- restenosis
- clinical outcome
Abstract
Aims: Coronary lesions in diabetics (DM) are associated with a high recurrence following percutaneous coronary intervention (PCI), even after drug-eluting stent (DES) deployment. Encouraging clinical data of the drug-eluting balloon catheter (DEB) SeQuent Please warrant its investigation in these patients.
Methods and results: Eighty-four diabetic patients (60.8±9.1years, 76.2% male) were randomised to either the DEB SeQuent™ Please or the DES Taxus™ Liberté™ to compare the 9-month clinical and angiographic outcome of PCI in native coronary arteries. Comparing the DEB vs. the DES the 9-month results (follow-up DEB 39/45 [86.7%], DES 36/39 [92.3%]) are statistically not different at the 0.05 level for the primary endpoint of in-segment (0.37±0.59 mm vs. 0.35 ±0.63 mm) and in-stent (0.51±0.61 mm vs. 0.53±0.67 mm) late lumen loss, overall and cardiac deaths (2/45 [4.4%] and 3/45 [6.7%] vs. 0), target lesion revascularisation (3/45 [8.9%] vs. 4/39 [10.3%]), the total MACE rate (6/45 [13.3%] vs. 6/39 [15.4%]), and the event free survival after 10.2±3.8 months (Kaplan-Meier analysis, p<0.80, log rank test).
Conclusions: The clinical and angiographic outcome of the combination of the drug-eluting balloon SeQuent Please with a cobalt chromium stent compared to the drug eluting Taxus stent are similar.
Introduction
Drug-eluting stents have been shown to improve the long- term outcome of percutaneous coronary interventions (PCI) by reducing late lumen loss on the order of 0.10±0.23 mm – 0.67±0.62 mm1,2, and the rates for in-segment restenosis and target lesion revascularisation to 6.6-11.7% and 4.8-8.3%, respectively1,2 in most of the indications in comparison to bare metal stents2-6. Patients with diabetes mellitus are typically considered at high risk for cardiovascular events and exhibit inferior outcomes following PCI compared to non-diabetics7-14. Data suggest similar clinical outcomes comparing diabetics and non-diabetics treated with the paclitaxel-eluting stent15,16, and in diabetic patients, similar clinical outcomes of paclitaxel- and sirolimus-eluting stents13,14,17 and of paclitaxel- and zotarolimus- eluting stents18. The limus eluting stents however, perform significantly better in non-diabetic patients with respect to late lumen loss and, hence, better than the paclitaxel-eluting stents17,19. Moreover, the angiographic data seem to favour sirolimus over paclitaxel. In a prospective randomised study enrolling 250 diabetics with coronary artery disease paclitaxel (P) and sirolimus (S) eluting stents were associated with nine-month in-segment late lumen losses of (P) 0.67±0.62mm and (S) 0.43±0.45mm along with in-segment restenosis rates (P) 16.5% and (S) 6.9%, respectively20. In a meta-analysis of five randomised trials in diabetic patients sirolimus-eluting stents were significantly (p<0.001) superior to paclitaxel-eluting stents in the reduction of restenosis (5.6% vs 16.4%, OR 0.30, 95% CI 0.19 to 0.48, p<0.001) and target lesion revascularisation (5.1% vs. 11.4%) without any statistically significant differences in myocardial infarction, cardiac death and stent thrombosis13.
Diabetics, with their exaggerated neointimal hyperplasia, might benefit from either a systemic application or an even distribution of an antiproliferative agent along the entire length of the device. Thus, alternative approaches such as the adjunct therapy with the glycoprotein IIb/IIIa inhibitor21-23 abciximab or brachytherapy with Ir-192 intracoronary radiation have been investigated. While the former treatment with the platelet inhibitor did not add any benefit21-23, the improvement in outcome by brachytherapy is hampered by its complex logistics and the associated precautionary measures when using a radioactive source as a routine procedure24-29.
Therefore, a therapeutic alternative that would combine the even distribution of an antiproliferative agent along the entire device and the ease of its handling might be the use of paclitaxel eluting balloon. The principle of paclitaxel eluting devices intended for intracoronary use is based on the antiproliferative mode of action of the compound. Paclitaxel is a highly lipophilic drug that stabilises cellular microtubules essential to cell function, migration and replication. It impairs arterial vascular wall contraction, retards the migration of and reduces the mitosis rate of vascular wall cells through a mitotic block. The paclitaxel eluting PTCA catheter releases about eight times the amount of the antiproliferative drug compared to a paclitaxel eluting stent within about ten seconds along the entire length of the vessel segment that is in touch with the balloon only during the time of inflation. This short time period is apparently sufficient to reduce vascular smooth muscle cell proliferation30. The studies conducted to date with the paclitaxel-eluting balloon based on the PACCOCATH technology were associated with a low in-segment late lumen loss after six months in bare metal in-stent restenosis (0.13±0.51mm31, 0.17±0.43 mm32), in small vessel disease (0.16±0.38mm33), and in the side branches of bifurcational lesions (0.12±0.47mm) when the DEB was used as a stand-alone procedure.
Following balloon angioplasty of the lesion the subsequent deployment of a cobalt chromium stent would fight the elastic recoil that occurs immediately after balloon dilatation of the lesion. In a real world registry including 2,333 patients the cobalt chromium stent that was used in this study exhibited a 6-month MACE rate of 10.5%34 thus being in the range of paclitaxel-eluting stents35-38.
Therefore, it was suggested to conduct this study to compare the clinical and angiographic outcome of the paclitaxel-eluting PTCA-balloon SeQuent™ Please (BBraun AG, Melsungen, Germany) followed by deployment of the cobalt-chromium stent Coroflex™ Blue (BBraun AG, Melsungen, Germany) versus the paclitaxel-eluting stent Taxus™ Liberté™ (Boston Scientific, Inc., Natick, MA, USA) in the treatment of stenoses in native coronary arteries of patients with diabetes mellitus in a prospective and randomised multicentre design.
Methods and results
Patients
The main patient related inclusion criteria encompassed a history of diabetes mellitus of at least three years prior to enrolment (this was later reduced after the study began to at least one year by a protocol amendment to enhance patient enrolment) with stable blood glucose levels for the six months preceding enrolment as reflected by HbA1c concentrations ≤8% (this was raised to ≤10% during the conduct to the study by a protocol amendment to enhance patient enrolment) , stable angina pectoris (CCS class 1-3) or unstable angina pectoris (Braunwald class 1-2, A-C), documented overt or silent, and eligibility for coronary revascularisation of any sort (balloon angioplasty, device-assisted balloon-angioplasty or coronary artery bypass grafting). Lesion related inclusion criteria included such as de novo native coronary artery stenosis (reference diameter: ≥2.5mm and ≤3.5mm, length of stenosis: ≥10mm and ≤20mm [increased by a protocol amendment after the study began to ≤22mm to enhance patient enrolment]), target lesion ≥3mm distant from a major side branch (>2.0mm in diameter) and ≥3mm from a previously deployed stent of any type (including active and passive coatings, bare metal stents), diameter stenosis preprocedure either ≥70% or ≥50% if ischaemia corresponding to the target lesion was documented either by exercise stress ECG, stress echocardiography, scintigraphy, magnetic resonance imaging, or suspected based on angina pectoris, and target lesion had to be eligible for treatment with a single stent only (patients with multiple stents at the target lesion were analysed in the intention-to-treat group).
The main patient related exclusion criteria encompassed such as acute (< 24 h) or recent (≥24 hours and ≤48hours) myocardial infarction, unstable angina pectoris (Braunwald class 3), severe congestive heart failure, severe heart failure New York Heart Association IV, cardiogenic shock at the time of the procedure (systolic blood pressure of less than 80mmHg requiring inotropic support, intra-aortic balloon pump and/or fluid challenge), severe valvular heart disease, life expectancy of less than five years (study is scheduled for a 3-year clinical follow-up) or other factors making clinical follow-up difficult. The main lesion related exclusion criteria comprised another coronary stent implanted previously into the target vessel, drug-eluting stents less than nine months and bare metal stents or stents with passive coatings less than three months before the PEPCAD IV DM PCI, target lesion located in any type of coronary bypass (i.e., venous graft, arterial bypass) or graft/native artery connection, coronary artery occlusions of any type (e.g., acute or chronic), in-stent restenosis, and another stent within the same vessel less than 3mm distant from target lesion, while procedure related exclusion criteria included such as evidence of extensive thrombosis within target vessel before the intervention, multi-lesion percutaneous coronary intervention within one vessel (PCI of a total of two lesions one each in a different vessels was permitted).
Study design
The PEPCAD IV DM study is a prospective, multicentre, randomised, two-armed phase-II pilot study in Malaysia and Thailand assessing the acute, 9-month and 3-year outcome (will be reported upon completion of that period) of the paclitaxel eluting PTCA balloon angioplasty preceding cobalt-chromium stent deployment versus conventional balloon angioplasty preceding paclitaxel eluting stent application in the treatment of native coronary artery stenoses (clinicaltrials.gov: NCT00462631).
Ethical considerations
The study was approved by the independent Institutional Review Boards of the respective centres.
Statistics
The Kolmogoroff-Smirnoff-test was used to prove Gaussian distribution allowing for calculation of the mean and standard deviation. Non-gaussian samples are described by median and range. Discriminant variables are evaluated with the two-sided Fisher’s exact test. Continuous variables are compared with student’s unpaired t-test. Additionally, a confidence interval for the difference of the late lumen loss was computed in order to compare the respective limits with the medical relevant differences of the late lumen loss. For all tests the significance level α is 0.05.The null hypothesis (H0) is: The late lumen loss in the paclitaxel eluting PTCA balloon catheter/stent group after nine months is equal to the late lumen loss in the conventional angioplasty/paclitaxel-eluting stent group.
The sample size estimate for the primary endpoint “late lumen loss” is based on the assumption of a late loss of DEB combined with the Coroflex™ Blue of 0.32±0.62mm and of 0.67±0.62mm with the Taxus stent20 (alpha error: 5%, beta-error: 20%, follow-up rate 80%) resulting in a sample size of 64 patients per group.
Primary variable
The primary variable is in-segment late lumen loss.
Secondary variables
The secondary variables include late lumen loss index, acute gain, the binary restenosis rate, minimal lumen diameter pre- and post- procedure and at follow-up, major adverse cardiac events, and the incidence of thrombotic events according to Academic Research Consortium (ARC) criteria.
Quantitative angiography
For quantitative coronary angiographic analysis the computer assisted CAAS II (Pie Medical, Maastricht, The Netherlands) was used.
QCA was performed for the projection showing the highest degree of stenosis (lesion length according to the “shoulder-to-shoulder” criterion) and the orthogonal view if available. The maximum of both of the values determines the severity of the stenosis. This evaluation was carried out centrally by the angiographic corelab of the Clinical Research Institute, Rotenburg an der Fulda, by two independent operators. A difference of ±3% of the relative stenosis (%) between the two readings was accepted. If the discrepancy exceeded this value, the third operator decided upon the result of the assessment.
For detailed evaluation purposes, parameters were calculated such as acute lumen gain, late lumen loss and late loss index.
Study products
Characteristics of the paclitaxel eluting PTCA balloon catheter
The certified SeQuent™ Please PTCA balloon catheter (B.Braun, Melsungen, Germany) serves as the basis for the paclitaxel eluting PTCA balloon catheter. The latter is coated with a paclitaxel nominal dose of 3µg/mm2 balloon surface area. The device features a gentle distal transition from shaft to balloon and a highly flexible tip that should allow for crossing even severe lesions (material: COMAX®, lesion entry profile [tip]: 0.67mm (0.017in.), nominal pressure: 6bar, maximum inflation pressure 15 bar (2.5/3.0/3.5 mm) and 12 bar (4.0mm). The catheter was available in 20, 26 and 30mm length and 2.5, 3.0 and 3.5mm diameter for this study.
Characteristics of the Coroflex™ Blue
The Coroflex™ Blue stent featuring a L-605 cobalt chromium core is a new generation coronary scaffolding device as characterised by a stent surface of 86mm2 (13mm length) and 111mm2 (16mm length), strut dimensions of 0.065mm by 0.096mm, crossing profiles of 0.89mm (0.035 inches) for ∅2.5mm stents, 0.94mm (0.037 inches) for ∅3.0mm stents, 0.99mm (0.039 inches) for ∅3.5mm stents, 1.02mm (0.04 inches) for ∅4.0mm stents, by ametal coverage area of 20% at ∅2.5mm, 13% at ∅4.0mm, by a maximum recoil of 5.6%, by foreshortening of –3%, flexibility (crimped) of 0.205mN, by a nominal pressure of 10bar, by a maximum inflation pressure of 18bar (≤4.0mm) and 20bar (≤3.5mm), and by a balloon / stent overhang of <0.5mm.
The Coroflex™ Blue stent has proven its efficacy and reliability in experimental animal39 and human studies34.
The stent is premounted on a SeQuent™ PTCA-balloon catheter (B.Braun Melsungen AG, Melsungen, Germany) in the present trial used with nominal diameters of 2.5, 2.75, 3.0, 3.5, and 4mm and lengths of 8, 12, 16, 19 and 25mm.
Characteristics of the paclitaxel eluting Taxus™ stent
The paclitaxel-eluting Taxus™ stent featuring a 316 LVM (low carbon vacuum melted) stainless steel core is a coronary device characterised by the antiproliferative drug being eluted from a polymer coating. Characteristics include such as a stent surface of 86mm2 (13mm length) and 111mm2 (16mm length), paclitaxel dose of 86µg (13mm length) and 111µg (16mm length) into 1µg of paclitaxel per mm2 of stent surface, a strut thickness of 0.137mm, a crossing profile of 1.15mm (0.045 inches), a metal coverage area 17.4% (∅2.5mm), 14.5% (∅3.0mm), 12.8% (∅3.5mm), 11.2% (∅4.0mm), by a maximum recoil of 4.6%, by a foreshortening of 1%, by a conformability of 0.309Nmm, by a nominal pressure of 9bar, and by a maximal inflation pressure of 18bar (≤4.0mm), 16bar >4.5mm).
The paclitaxel eluting Taxus™ stent series has proven its efficacy and reliability in experimental animal40 and numerous human4,35,41,42 studies by significantly reducing arterial wall inflammation and proliferation compared to bare metal stents.
The paclitaxel eluting Taxus™ stent was used in the present trial diameters of 2.5, 3.0 and 3.5mm and lengths between 12, and 24mm.
Procedure
All procedures had to be performed according to common interventional practices including the administration of intracoronary nitroglycerin 0.2mg of glycerol trinitrate or isosorbide dinitrate) and intra-arterial heparin (50-100U/kg body weight). Predilation with a conventional balloon catheter was recommended before drug-eluting stent deployment and using the drug-eluting balloon according to the manufacturer’s recommendations. The cobalt chromium stent deployed had to be shorter by 4-6mm compared to the drug-eluting balloon in order to avoid geographic mismatch33.
Concomitant medication
Aspirin was administered ≥100mg/d orally for ≥2days pre-intervention. A clopidogrel loading dose of 300mg of clopidogrel was applied at least six hours before the procedure. Aspirin 100mg/d orally was scheduled for life while 75mg/daily of clopidogrel were given in the drug-eluting balloon group for three months and in the drug-eluting stent group for six months, respectively. Patients intolerant to aspirin continued clopidogrel therapy throughout the study.
Results
Eighty-four of the targeted 128 (65.6%) patients were enrolled between May 2007 and January 2009 before the study was prematurely terminated for slow enrolment jointly by the Lead Investigator and the Medical Monitor. The nine month follow-up was completed by 39/45 (86.7%) of the drug-eluting balloon patients and by 36/39 (92.3%) in the drug-eluting stent group.
The baseline clinical and angiographic data are not statistically different between both treatment groups with the exception of older age and the higher prevalence of hypertension in the drug-eluting balloon group (p<0.03 each) (Table 1).
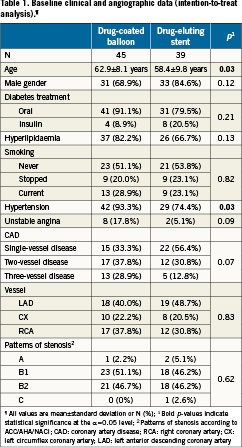
The angiographic data show no statistical significant difference in lesion characteristics (Table2). The minimal lumen diameter post-procedure tends to be larger in the drug-eluting stent group (p<0.06). There was no statistical significant difference in the angiographic follow-up data at nine months (Figures 1a, 1b). The Kaplan-Meier analysis of the event free survival of the ongoing clinical follow-up does not show a statistical difference between the treatment groups after 10.2±3.8 months (Figure 2).
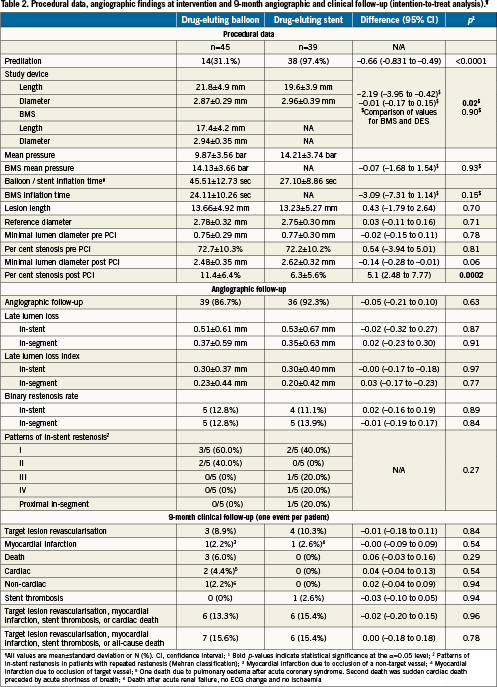
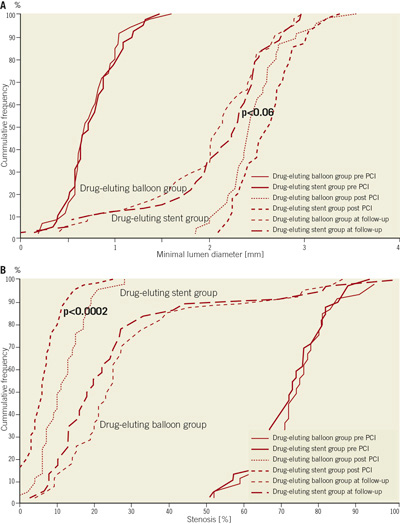
Figure 1. A) The cumulative frequency of the minimal lumen diameter reflects the trend towards a larger lumen diameter post-procedure in the drug eluting stent group as compared to the drug eluting balloon group (p<0.06). B). The cumulative frequency of per cent stenosis reflects alesser stenosis post-procedure in the drug eluting stent group compared to the drug eluting balloon group (p<0.0002).
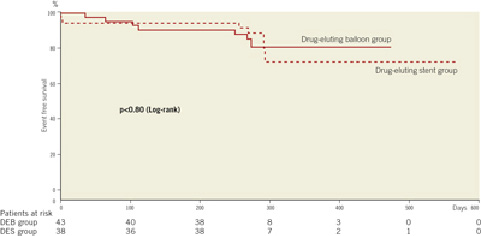
Figure 2. The Kaplan-Meier plot demonstrates no statistical significant difference (p<0.80) in event free survival between the two treatment groups.
Discussion
The percutaneous interventional and surgical treatment of significant coronary artery lesions in patients with diabetes mellitus has remained a challenge for the past decades due to the association with higher recurrence rates than in non-diabetics7-14.
There is little doubt about the superiority of drug-eluting stents compared to stand alone angioplasty and bare metal stent deployment2-6. Comparative studies between the stents that elute drugs such as sirolimus and paclitaxel have suggested no clinically significant differences in favour of any of the two, with still unsatisfactory recurrence rates13,14,17,43,44 or any benefit compared to stents eluting zotarolimus18. A possible reason may be the fact that drug-eluting stent deployment delivers the drug to only about 15% of the vessel area. Hence, application of the antiproliferative drug using adrug-eluting balloon catheter in combination with the scaffolding properties of a new bare metal cobalt-chromium stent was compared to administering the same drug (i.e., paclitaxel) by a drug-eluting stent. Overall, there were no statistically significant differences in the clinical and angiographic outcomes after nine months. Detailed analysis reveals a trend (p<0.06) towards a higher minimal lumen diameter post-procedure in the drug-eluting stent group. This may be in part attributed to the higher recoil of the cobalt-chromium compared to the drug-eluting stent despite similar inflation pressures. Late lumen loss in-stent and in-segment showed a statistically insignificant mean difference of 0.02mm (95% C.I. 0.32 to 0.27mm) between both treatments despite a more favourable minimal lumen diameter post-procedure in the drug-eluting stent group. The in-segment late lumen loss is somewhat smaller than the 0.62±0.73mm observed in the PEPCAD I study, in which stents were deployed as a bail out measure33 and within the range of the paclitaxel-eluting stent (0.50±0.6mm44. The in-segment late lumen loss is smaller than in the two studies with the paclitaxel eluting stent of 125 (0.67±0.62mm)20 and 200 patients with diabetes (0.67±0.53mm), respectively45. The late lumen loss of the newer generation zotarolimus eluting stents in diabetics is on the order of 0.66±0.44 mm46 to 0.87mm (in-stent)18 and 0.37±0.52mm (in-segment)46 and, thus, not superior to the data presented in this study.
The event free survival showed no statistical difference between both treatment groups throughout the entire observation period, nor did the rate of major adverse cardiac events (MACE) with 6/45 (13.3%) for drug-eluting balloon patients and 6/39 (15.4%) for the drug-eluting stent group. MACE rates for paclitaxel eluting stent deployment in diabetics have been reported on the order of 7.2%-8%18,45, for sirolimus on the order of 2%45, and for zotarolimus of 6.9%18. The more detailed analysis of the current study reveals 3/45 (8.9%) target lesion revascularisations in the drug-eluting balloon group and 4/39 (10.3%) in the drug-eluting stent group. In diabetics, after one year target vessel failure rates range between 6.9% and 10.8%18,45,47 in patients following paclitaxel eluting stent procedures, and have been reported to be 6.4% in everolimus eluting stent deployment47 and 8.6% following zotarolimus eluting stents18. Target lesion revascularisation may be as high as 12% in diabetics treated with the paclitaxel eluting stent20. One myocardial infarction occurred in each treatment group, non-target lesion related in the drug-eluting balloon group and target lesion related in the drug-eluting stent group. Two cardiac deaths occurred in the drug-eluting balloon group, with no identification of the culprit vessel. These incidences are in the range of the above mentioned studies.
Overall, there was no difference either in the clinical or in the angiographic outcome between the two treatments despite the fact that some biases were not in favour of the drug-eluting balloon: in the drug-eluting balloon group, clopidogrel was administered for three months as opposed to six months in the drug-eluting stent groups, there were more hypertensive patients in the drug-eluting balloon groups as the post-procedural minimal lumen diameter tended to be smaller by a mean of 0.14mm (p<0.06). Moreover, in comparison to other stents eluting paclitaxel or zotarolimus, there does not seem to be a difference in clinical outcome compared to the drug-eluting balloon catheter used in this study. Data on everolimus eluting stents are too scant to date for a conclusive statement and warrant randomised controlled studies.
The study is limited by its premature termination owing to slow patient enrolment. A longer clopidogrel administration for twelve instead of six months in the paclitaxel eluting stent according to the guidelines published after the initiation of this study may have altered the outcome. Given that its initiation was in 2006, the newest generation of drug-eluting stents could not have served for comparison. In addition, the data may not be transferred to any other drug-eluting balloon technologies, since data with these devices on other indications such as in-stent restenosis, small vessel disease48 and bifurcations49 are limited and/or seem to be less favourable when compared to those of the balloon used in this study32,33,50,51. Moreover, it is also unclear as to whether the outcome of the drug-eluting balloon in combination with other bare metal stents might be different. The study results may have been influenced, as well, by the significantly (p<0.0001) higher rate (97.4%) of predilation prior to drug-eluting stent implantation as compared to the drug-eluting balloon (31.1%) procedures. This reflects the thinking of the investigators at the point of formulating the study protocol that there was no need for predilation in drug-eluting balloon procedures. The current recommendation mandates the use of a smaller and shorter balloon to ensure the deliverability of the drug-eluting balloon to the site of treated segment.
The data emerging during the time of PEPCAD IV suggests the use of a drug-eluting balloon as a stand-alone procedure whenever possible based on the in-segment late lumen loss being consistently below 0.2mm32,33,50,51. This might be possible in more than 70% of patients according to the bail-out stent rate of 28% in the PEPCADI small vessel disease study33.
In summary, the current data suggest that the drug-eluting balloon SeQuent™ Please in combination with the cobalt-chromium stent Coroflex Blue is a valuable therapeutic alternative for the treatment of native coronary artery stenosis in patients with diabetes mellitus.
Conflict of interest statement
The Clinical Research Institute was compensated for data management and QCA by B.Braun Melsungen, Germany. M. Boxberger and M. Unverdorben are employees of B.Braun. The other authors have no conflict of interest to declare.
References

