
Abstract
Aims: Although several studies have shown positive outcomes after the use of drug-coated balloons (DCB) for in-stent restenosis (ISR), data on randomised controlled trials versus latest-generation drug-eluting stents (DES) are limited. Therefore, in this randomised trial, we sought to evaluate whether a butyryl-tri-hexyl citrate (BTHC)-based paclitaxel DCB is non-inferior to a biodegradable polymer sirolimus-eluting stent (BP-SES) therapy in patients with ISR in either a bare metal stent (BMS) or DES.
Methods and results: A total of 229 patients with ISR in BMS or DES from 13 German centres and one Latvian centre were 2:1 randomly allocated to DCB (n=157) or DES (n=72). The primary efficacy endpoint was defined as in-stent late lumen loss (LLL) at six months, and the primary safety endpoint was target lesion failure (TLF) at 12 months. LLL in the DCB arm was 0.03±0.40 mm compared to 0.20±0.70 mm in the DES arm (p=0.40). DCB proved to be non-inferior to DES (Δ = –0.17±0.52 mm, 97.5% CI –∞; –0.01]; p<0.0001). At 12 months, Kaplan-Meier TLF estimates were 16.7% in the DCB arm and 14.2% in the DES arm (p=0.65) and remained similar at 18 months (DCB versus DES: 17.4% versus 19.5%, p=0.88).
Conclusions: In patients with DES or BMS ISR, treatment with a paclitaxel DCB showed similar LLL at six months and TLF rates up to 18 months compared to a second-generation sirolimus DES.
Abbreviations
ARC: Academic Research Consortium
BMS: bare metal stent
BP-SES: biodegradable polymer sirolimus-eluting stent
BTHC: butyryl-tri-hexyl citrate
DAPT: dual antiplatelet therapy
DCB: drug-coated balloon
DES: drug-eluting stent
DP-EES: durable polymer everolimus-eluting stent
DS: diameter stenosis
ISR: in-stent restenosis
LLL: late lumen loss
MI: myocardial infarction
MLD: minimum lumen diameter
RVD: reference vessel diameter
TLF: target lesion failure
TLR: target lesion revascularisation
Introduction
In-stent restenosis (ISR) remains a clinical challenge despite the use of newest-generation drug-eluting stents (DES)1. In up to 10% of patients the need for revascularisation of in-stent restenosis occurs2. Both drug-coated balloons (DCB) and DES are recommended by current guidelines to treat ISR3; however, the optimal treatment of ISR remains unknown. DCB therapy has the advantage of avoiding additional stent layers1 and has been shown to be non-inferior compared to first-generation DES in previous studies4,5. Data that compare DCB with second-generation DES for ISR treatment are sparse6-9. Additionally, a class effect for different DCB cannot be assumed3.
Therefore, in this randomised trial, we sought to evaluate whether a butyryl-tri-hexyl citrate (BTHC)-based paclitaxel DCB is non-inferior to a biodegradable polymer sirolimus-eluting stent (BP-SES) therapy in patients with ISR in either a bare metal stent (BMS) or DES.
Methods
PATIENT SELECTION AND STUDY DESIGN
The BIOLUX RCT (BIOtronik – Clinical performance of the Pantera LUX paclitaxel releasing balloon versus the drug eluting Orsiro hybrid stent system in patients with in-stent restenosis – a Randomized Controlled Trial) was a prospective, multicentre, randomised controlled trial, which compared the efficacy of a DCB-based treatment strategy to a DES-based treatment strategy for in-stent restenosis in both BMS and DES. The treatment allocation followed a block randomisation with a factor of 2:1 (DCB versus DES), stratified according to diabetic status at screening. Randomisation was performed on a per patient basis and done in the electronic case report form after the guidewire crossed the target lesion.
Investigational devices were a BTHC-based paclitaxel DCB (Pantera Lux; Biotronik AG, Bülach, Switzerland10) and a BP-SES with ultra-thin struts (Orsiro; Biotronik AG11). Both devices were CE marked at the start of the study and used within the approved indications.
Figure 1 shows a study flow chart with details of randomisation, and angiographic and clinical follow-up. Ethics committee approval was obtained prior to the start of the study for all centres. The study was performed according to the Declaration of Helsinki and Good Clinical Practice. All patients provided written informed consent. Further information on patient selection and study design is available at clinicaltrials.gov using the following identifier: NCT01651390.
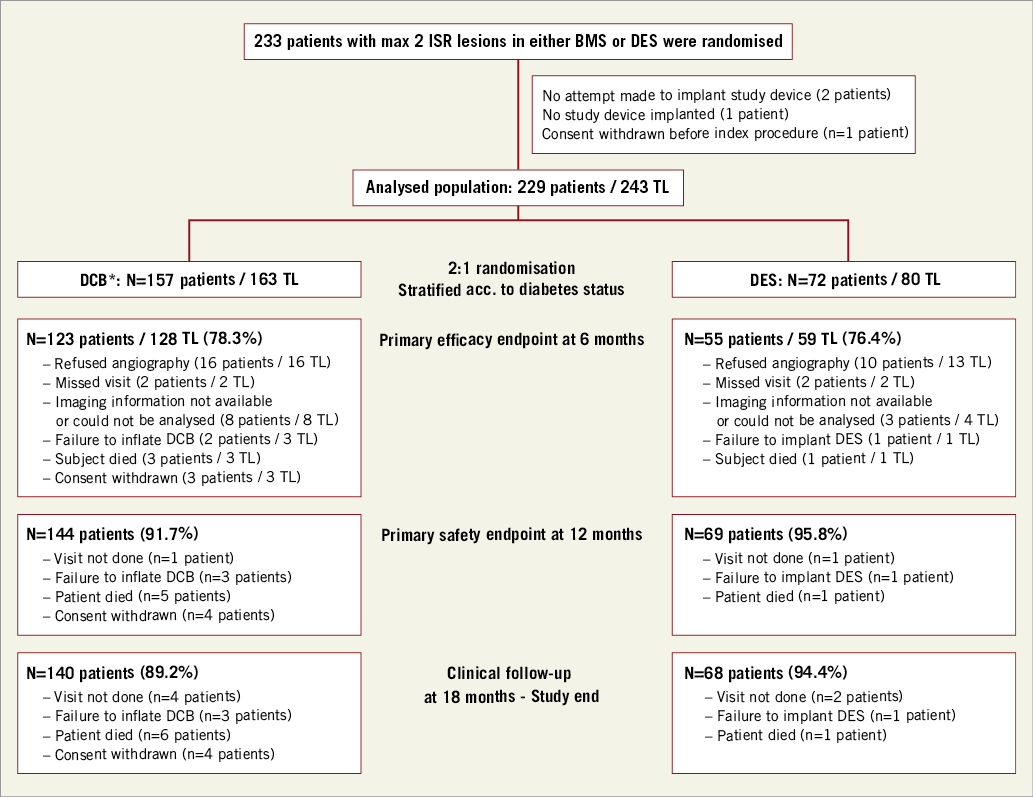
Figure 1. Study flow chart with details of randomisation, angiographic and clinical follow-up. *including crossover patient (n=1). BMS: bare metal stent; DCB: drug-coated balloon; DES: drug-eluting stent; ISR: in-stent restenosis; n: number; TL: target lesion
INCLUSION CRITERIA
Patients presenting with clinical evidence of ischaemic heart disease and/or a positive functional study, stable or unstable angina pectoris or documented silent ischaemia and a maximum of two restenoses (>50% stenosis on visual assessment) in either BMS or DES were eligible. In case of two target lesions, both lesions had to be treated during the same session and with the same type of device.
EXCLUSION CRITERIA
Patients with ST-segment elevation myocardial infarction within 72 hours prior to the index procedure, acute cardiac decompensation or cardiogenic shock, severe impaired left ventricular function (ejection fraction <30%), target lesion located in the left main coronary artery, thrombus in the target vessel, known allergies to acetylsalicylic acid, clopidogrel, prasugrel, ticagrelor, heparin or similar drugs, severely impaired renal function (need for dialysis or creatinine >2.5 mg/dl) or a life expectancy of less than 18 months were excluded. Additionally, patients with ISR in small vessels (<2 mm diameter) or large vessels (>4 mm) as well as patients with very short (<6 mm length) or diffuse lesions (>28 mm length) were excluded.
PROCEDURE
All patients were pre-treated with aspirin and a P2Y12 inhibitor following local standard practice. Anticoagulation during the procedure was obtained with unfractionated heparin according to the local protocol. Predilation using uncoated or cutting/scoring balloons was mandated, but selection of the balloon-artery ratio was left to the operator’s discretion. The study protocol recommended selecting a DCB/DES covering the predilated segment and at least 2-3 mm proximal and distal. In case of long lesions, use of two or more subsequent drug-eluting balloons (DEB)/DES was allowed with a small overlap. Post-dilatation was left to the operator’s discretion. In case of dissections requiring treatment, the study protocol mandated implantation of BMS (DCB arm) or an Orsiro stent (DES arm). At post procedure, dual antiplatelet therapy (DAPT) was given as per local standard.
QUANTITATIVE CORONARY ANGIOGRAPHY
Angiograms were analysed by an independent core laboratory not blinded to treatment allocation. Lesion morphology was assessed using the Mehran classification12. Following administration of intracoronary nitroglycerine, two orthogonal projections were selected presenting the target lesion free of foreshortening or vessel overlap, but presenting the stenosis appearing most severe. Matched projections were repeated after intervention and at follow-up. An automatic edge-detection system (CAAS II System; Pie Medical Imaging, Maastricht, the Netherlands) was used for quantitative analysis. Analyses were perfomed in-stent and in-segment (area treated and 5 mm margins proximal and distal). Reference vessel diameter (RVD) was defined as the computed estimation of the original diameter of the artery at the level of the obstruction. Acute gain was calculated from the minimum lumen diameter (MLD) difference between post and pre procedure. Late lumen loss (LLL) was calculated from the MLD difference between post procedure and follow-up or prior to a target lesion revascularisation (TLR). Diameter stenosis (DS) was defined as ([1-MLD]/RVDx100).
STUDY ENDPOINTS
The primary efficacy endpoint was in-stent LLL at six months post procedure. The primary safety endpoint was defined as target lesion failure (TLF, a composite of cardiac death, target vessel myocardial infarction [MI] and clinically driven TLR) at 12 months. Secondary endpoints included angiographic endpoints (MLD and DS, in-segment LLL, MLD and DS) and clinical endpoints (TLF at 6 and 18 months, and stent thrombosis [ST] rate after 6, 12 and 18 months). Device success was defined as successful delivery, application and removal of the balloon or stent to the target lesion site in the coronary artery.
Any device effect or serious adverse event was adjudicated by an independent clinical events committee (CEC) that was not blinded to treatment allocation. Academic Research Consortium (ARC) definitions were used to adjudicate clinical events including ST13. Briefly, MI was defined as: A) recurrent thoracic chest pain or ischaemic equivalent and new pathologic Q-waves in ≥2 contiguous ECG leads and cardiac marker >1*URL (preferably creatine kinase-myocardial band [CKMB]) or in the absence of any marker on CEC decision upon clinical scenario; B) appropriate cardiac enzymes, b1) CK ≥2*URL confirmed by a second marker >1*URL (preferably CKMB) or in the absence of marker data on CEC decision upon clinical scenario, or b2) in the absence of CK: CKMB >3*URL, or b3) in the absence of CKMB: troponin >3*URL, or b4) in the absence of cardiac enzyme data, clinical decision based upon clinical scenario14.
STATISTICAL ANALYSIS
Categorical data are presented as counts and percentages and were compared using the chi-square or Fisher’s exact test. Continuous variables were analysed for data distribution and are either presented as mean±standard deviation compared using the Student’s t-test, or are presented as median with interquartile range compared using the rank-sum Mann-Whitney-Wilcoxon test. Failure estimates were assessed with Kaplan-Meier analysis and compared with the log-rank test. Analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA). A p-value <0.05 was considered statistically significant.
Sample size calculation was based on the primary endpoint with the following assumptions: i) LLL of 0.07±0.31 mm after DCB treatment10; ii) LLL of 0.07±0.22 mm after DES implantation11; iii) a non-inferiority margin of 0.15 mm equal to the upper limit of the 95% CI of the mean LLL of the Orsiro stent11; and iv) an attrition rate of 23%. Accordingly, an overall sample size of 210 randomised patients (140 patients treated with DCB and 70 patients treated with DES) was required to prove non-inferiority of DCB in ISR with an alpha of 0.025 and 95% power.
Results
PATIENTS AND PROCEDURES
Between August 2012 and January 2015, 233 patients were enrolled in 13 centres in Germany and one centre in Latvia (Figure 1). One hundred and fifty-seven patients were randomised to the DCB arm and 72 patients to the DES arm. In one patient a crossover between the DCB arm and the DES arm occurred (n=1, 0.6%).
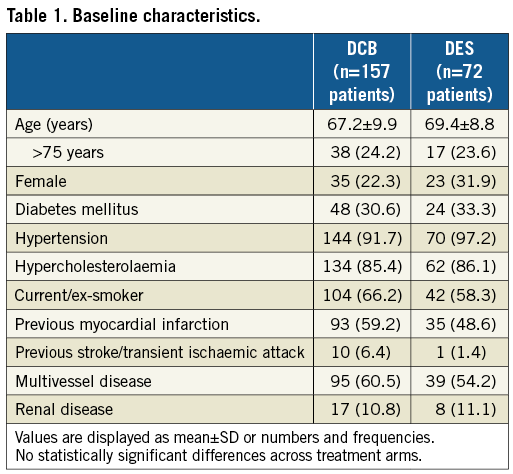
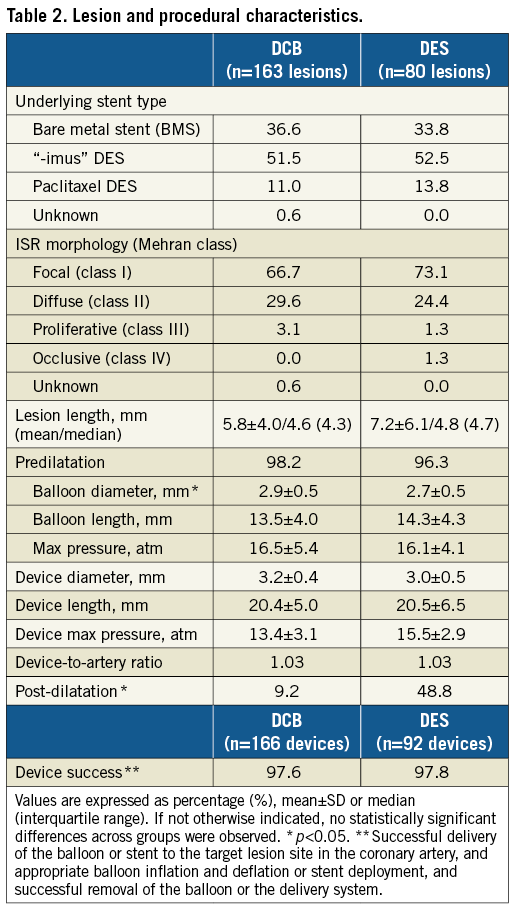
Clinical characteristics and baseline angiographic findings were similar in both groups (Table 1, Table 2). If not stated otherwise, no significant difference was observed. In both groups, a similar number of lesions had more than one underlying stent (DCB: 18 lesions [11.0%]; DES: 9 lesions [11.3%]). Post-dilatation was more frequent in the DES group (DCB: 9.2%; DES: 48.8%, p<0.001) (Table 2). The DES group obtained a significantly larger acute lumen gain and final MLD, and a lower residual stenosis (Table 3).

PRIMARY ENDPOINTS
PRIMARY EFFICACY ENDPOINT
The angiographic endpoint was based on 123 patients (78.3%) in the DCB arm and 55 patients (76.4%) in the DES arm (Figure 1). Mean in-stent LLL was evaluated at 0.03±0.40 mm (DCB arm) and 0.20±0.70 mm (DES arm), resulting in a difference of Δ = –0.17±0.52 mm. Based on the one-sided non-inferiority p-value p<0.0001 and the one-sided 97.5% confidence interval (CI) upper limit = –0.01, DCB proved to be non-inferior to DES in the treatment of ISR (Figure 2). Consistent findings for mean in-stent LLL were observed in BMS-ISR (-0.02±0.31 mm versus 0.09±0.55 mm, p=0.8856) and DES-ISR (0.06±0.44 mm versus 0.26±0.77 mm, p=0.3288), as well as in the diabetic and non-diabetic subgroups.
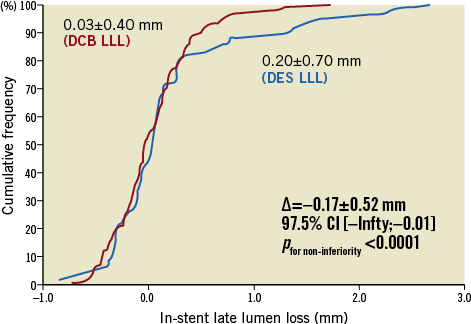
Figure 2. Angiographic primary endpoints. DCB: drug-coated balloon; DES: drug-eluting stent; LLL: late lumen loss
PRIMARY SAFETY ENDPOINT
Kaplan-Meier TLF estimates at 12 months were 16.7% (95% Cl: 11.6, 23.7) in the DCB arm versus 14.2% (95% Cl: 7.9, 24.7) in the DES arm (p=0.65).
SECONDARY ENDPOINTS
After six months (Table 3), the difference in in-stent DS in both arms was significant (DCB 28.5±15.7% versus DES 23.7±25.8%, p<0.001), though the MLD in both arms was similar (2.2±0.5 mm versus 2.2±0.8 mm, p=0.47).
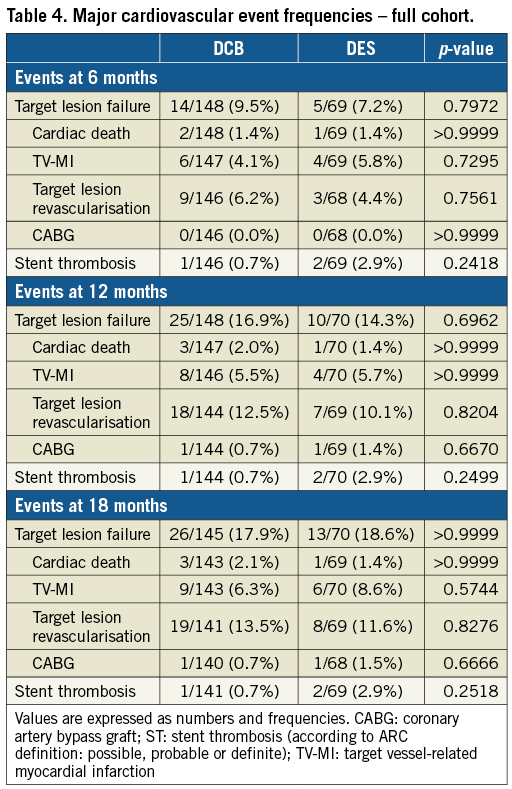
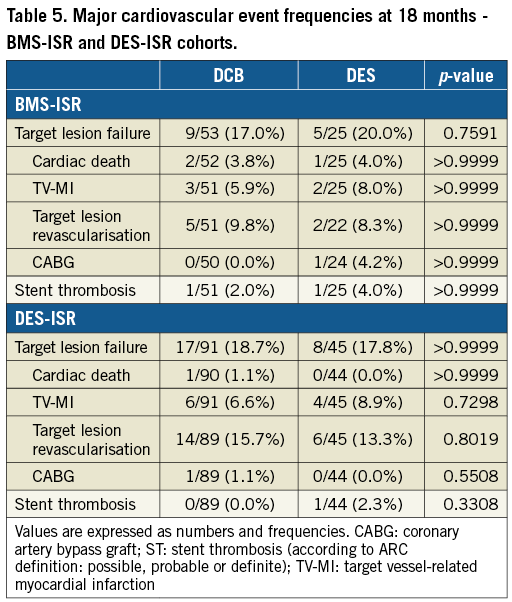
Clinical secondary endpoints were assessed at 6, 12 and 18 months. TLF and ST occurred at a similar rate in both treatment groups in the full cohort (Table 4), as well as in the BMS-ISR and the DES-ISR cohorts (Table 5). One patient in each arm had a definite ST under DAPT treatment. The patient in the DCB arm experienced the thrombosis at day 2 post procedure and the patient in the DES arm at day 122 post procedure. In addition, one patient in the DES arm, also under DAPT treatment, experienced a possible ST at day 144 post procedure.
Discussion
Treatment of ISR is challenging; current guidelines on revascularisation4 recommend both DCB and DES. However, data comparing DCB to second-generation DES in ISR are sparse6-9. The concept of DCB treatment avoiding an additional stent layer is intrinsically attractive. Shorter DAPT duration after DCB6 compared to DES could favour DEB in patients at high risk for bleeding.
In a mixed population of patients presenting with ISR in either BMS or DES, this multicentre RCT demonstrated that treatment with a BTHC-based paclitaxel DCB has comparable angiographic outcomes at six months and clinical outcomes up to 18 months post procedure compared to treatment with a biodegradable polymer sirolimus-eluting stent.
In our study, LLL after DCB was non-inferior compared to DES (0.03±0.40 mm versus 0.20±0.70 mm; p=0.40). Unlike the DARE study7 and RIBS IV/V6 (two other RCTs comparing DCB and second-generation DES in a mixed ISR population, mandating predilation and leaving post-dilation to the operator’s discretion), in-segment LLL in our study (-0.02±0.38 mm versus 0.13±0.67; p=0.94) was comparable after DCB and DES. In the DARE study, DCB treatment resulted in a significantly lower LLL compared to DES (0.17±0.41 mm versus 0.45±0.47 mm; p<0.001). In RIBS IV/V, DCB treatment resulted in an increased LLL (0.24±0.6 mm versus 0.12±0.6 mm; p=0.02). There is an ongoing discussion as to whether LLL compared to DS or MLD is an appropriate angiographic endpoint in studies comparing DCB and DES5. However, even in studies comparing DES, angiographic endpoints such as LLL or DS are challenged15.
In-segment DS pre-procedure was comparable between our study, DARE and RIBS IV/V. Compared to our study and DARE, an increased lumen gain post procedure was observed in the RIBS IV/V study. Different predilatation strategies and maximum pressure to deploy the device balloon may explain the observed results. In our study and DARE, similar pressures to deploy the device balloon were reported but a higher pressure was reported in RIBS IV/V (DEB arm: 13 atm versus 14 atm versus 18 atm; DES arm: 16 atm versus 16 atm versus 20 atm, respectively). At follow-up, lumen area in the DCB arm remained stable in our study; however, in DARE and RIBS IV/V, lumen narrowing was observed. In the DES arm, we observed a reduced lumen loss in our study compared to DARE and RIBS IV/V.
In the BIOLUX RCT we used a BTHC-based paclitaxel DCB, which is in contrast to the iopromide-based paclitaxel DCB in DARE and RIBS IV/V. It remains speculative whether the excipient (BTHC versus iopromide) influences long-term angiographic outcomes. Despite comparable pressure to inflate the DCB and similar DS, lumen area over time remained stable in our study compared to DARE (32% DS at follow-up and 31% DS post procedure in our study versus 36% DS and 30% DS in DARE). In both DARE and RIBS IV/V a durable polymer everolimus-eluting stent (DP-EES) was implanted. Compared to DP-EES, use of BP-SES resulted in reasonable long-term angiographic outcomes, even if moderate maximum pressure to inflate the device balloon was applied. Similar outcomes with DP-EES could be obtained by applying high maximum pressure only. Regarding in-stent stenosis, similar to the DARE trial we observed that the post-procedural in-stent diameter stenosis was lower in the DES arm than in the DCB arm. Although in-stent diameter stenosis increased over time in the DES arm and remained stable in the DCB arm, at six-month follow-up the diameter stenosis in the DES arm remained significantly lower compared to the DCB arm. The high SD indicates a wide scatter of measurements that prevents drawing definite conclusions from this finding. Further studies are needed to elucidate this finding.
We found consistent clinical outcomes between treatment arms in the full cohort and the BMS-ISR and DES-ISR subgroups. TLF was mainly driven by TLR, and TLR frequencies were somewhat higher than previously reported7. This might be explained by the high proportion of target lesions with more than one previously implanted stent. However, the basis for this observation is small and may hamper careful interpretation. Similar to DARE and RIBS IV/V, our study is underpowered to prove the significance of the observed clinical outcomes. Inconsistent reporting in respect of Kaplan-Meier failure estimates versus event frequencies between studies further impedes a careful discussion. These findings urge an RCT powered for a clinical endpoint such as TLF at 12 months post procedure.
Limitations
– Follow-up limited to 18 months. Extension to five years will provide important insights on long-term treatment effect and cost-effectiveness.
– CEC and core lab were not blinded to treatment allocation.
– Data may be not be generalisable since a specific DCB was used and a class effect has not yet been proven.
– Intracoronary imaging such as intravascular ultrasound or optical coherence tomography was not mandated but may provide information on both substrata underlying lesions and expansion of the underlying stent.
Conclusions
Treatment of patients with ISR in BMS or DES with a BTHC-based paclitaxel DCB is safe and a valuable option to treat this challenging patient cohort. Angiographic results at six months and clinical outcomes up to 18 months post procedure are comparable to those with a biodegradable polymer sirolimus-eluting stent. Further RCT head-to-head studies are warranted since there might not be an overall class effect for DCB.
| Impact on daily practice Treatment of in-stent restenosis in BMS or DES is a frequent clinical challenge. Treatment with a BTHC-coated paclitaxel DCB is a valuable and safe option without adding a layer of metal to the vessel wall. |
Acknowledgements
The authors thank Dr Ulrich Dietz (Wiesbaden, Germany) for providing the angiographic core laboratory assessments as well as Dr Helmut Schühlen (Berlin, Germany), Dr Jan Kähler (Herford, Germany) and Dr Hubertus Degen (Neuss, Germany) for serving as the clinical events committee. Additionally, the authors thank Lidia Mukina (Biotronik AG) for her assistance with the statistical analysis.
Funding
The study was sponsored by Biotronik AG, Bülach, Switzerland. The sponsor was involved in the design of the study, data collection, monitoring and data analysis and interpretation.
Conflict of interest statement
C. Jensen reports personal fees from Abbott and Neovasc. G. Richardt reports personal fees from Abbott, Biotronik, Boston Scientific, Novartis and Bayer, outside the submitted work. R. Tölg reports personal fees from Abbott, Biotronik, Boston Scientific, Novartis and Bayer. A. Erglis reports grants and personal fees from Abbott Vascular, personal fees from Biotronik, personal fees from Biosensors, grants and personal fees from Boston Scientific, outside the submitted work. D. Fischer reports personal fees from AstraZeneca, Bristol Myers Squibb, Novartis, B. Braun, Daiichi Sankyo and Bayer, as well as grants from Novartis and B. Braun. J. Mehilli reports grants and personal fees from Abbott and Edwards, and personal fees from Biotronik, Boston Scientific, BMS and Terumo. M. Wiemer reports grants and personal fees from Biotronik. C. Naber reports personal fees from Abbott, Biosensors, Biotronik, Medtronic, Elixir, REVA and MicroPort. The other authors have no conflicts of interest to declare.

