Abstract
Aims: Little is known about the respective healing responses and clinical efficacy and safety of drug-eluting balloons (DEB) and the second generation of drug-eluting stents (DES) when used to treat in-stent restenosis (ISR). In this study, we set out to compare prospectively the healing characteristics, as assessed by optical coherence tomography (OCT), of DEB versus DES after treatment of ISR in bare metal stents (BMS).
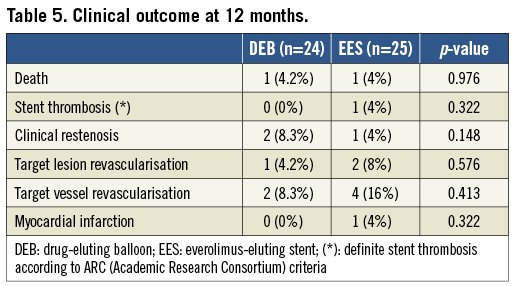
Methods and results: Fifty patients with BMS ISR were randomised to treatment with a paclitaxel-eluting balloon vs. an everolimus-eluting stent (EES). The primary endpoint was the percentage of uncovered struts, assessed with OCT at nine months, as a marker of vessel wall healing. A mean of 366±135 and 636±184 struts were analysed per patient in the DEB and EES groups, respectively. The percentage of uncovered struts per patient was significantly lower with DEB vs. EES (1.4% vs. 3.1%, p=0.025). Mean neointimal hyperplasia area was 2.4±1.08 mm² in DEB vs. 1.92±0.67 mm² in EES (p=0.1806), while the percentage of malapposed struts per patient was very low in both groups (0.2% vs. 0.3%, p=0.699). At nine months, angiographic in-stent MLD (minimum lumen diameter) was lower (2.13 vs. 2.54 mm, p=0.006), while diameter stenosis (26.4 vs. 11.4%, p=0.002), and LLL (0.28 vs. 0.07 mm, p=0.1) were higher after DEB compared to EES. During one-year follow-up, we did not observe differences in the rates of death, TLR (target lesion revascularisation) or stent thrombosis.
Conclusions: DEB appears to be associated with better healing characteristics, as assessed by stent strut coverage with OCT, but tended to be slightly less effective compared to EES. These findings give support to the use of either DEB or EES as valuable treatment options for ISR. Clinical trial registration: http://www.clinicaltrials.gov. Unique identifier: NCT 01065532
Introduction
Despite the improved safety of the latest generation of drug-eluting stents (DES), a substantial proportion of patients undergoing percutaneous coronary intervention (PCI) are still being treated with bare metal stents (BMS)1. BMS in-stent restenosis (ISR) remains therefore the most common presentation of stent failure. However, the optimal treatment strategy for ISR remains unclear. DES have been widely adopted as the best available treatment for ISR, with improved midterm outcomes as compared to balloon angioplasty, repeat BMS implantation or vascular brachytherapy (VBT)2,3. Nevertheless, late catch-up of the rate of target lesion revascularisation (TLR) and stent thrombosis (ST) remain important concerns with respect to their long-term safety and efficacy when used in the setting of ISR4.
Recently, drug-eluting balloons (DEB) have been proposed as an attractive treatment alternative to DES for ISR5. DEB have shown comparable clinical efficacy to DES in this setting, while avoiding the implantation of a second layer of metal in the diseased coronary artery segment, possibly leading to better vessel wall healing and a better safety profile6. As a consequence, the use of DEB for treatment of BMS ISR received a class IIa (level of evidence B) recommendation in the most recent ESC guidelines on myocardial revascularisation7. Despite this endorsement, the promising results of the use of DEB in ISR lesions could not be repeated in the setting of de novo lesions in the PEPCAD III trial, where the use of BMS mounted on a DEB was found to be inferior to DES implantation8. In the de novo study, the first DEB study using a primary OCT endpoint, the same strategy of DEB combined with BMS in de novo lesions resulted in acceptable inhibition of neointimal hyperplasia, although late lumen loss was larger than seen in current trials using next-generation DES9. In a recent registry of ISR lesions treated with DEB, OCT findings immediately after treatment and at follow-up provided important insights into the underlying mechanism through which these devices exert their effect. Balloon predilation and DEB application increase lumen and stent volumes during the initial procedure by optimisation of stent expansion and compression of neointimal hyperplasia. The local effect of paclitaxel is responsible for the long-term antirestenotic effect and induces further decrease in neointimal volume10.
In parallel, DES technology has evolved with the development of next-generation DES, with preserved or improved efficacy compared to the first-generation DES, and an enhanced safety profile, translating into better healing characteristics in optical coherence tomography (OCT) studies and improved clinical outcome in large studies and registries1,11-13.
In the present study, we set out to compare DEB with the latest generation of DES in the setting of BMS ISR, by evaluating their respective healing characteristics at midterm using OCT.
Methods
STUDY DESIGN
The design and methodology of the SEDUCE (Safety and Efficacy of a Drug elUting balloon in Coronary artery rEstenosis) study have been previously described14. It is a prospective, two-centre (University Hospitals Leuven and ZOL Hospital Genk, Belgium) randomised clinical trial with angiographic and OCT follow-up at nine months and yearly clinical follow-up up to five years. Patients with symptomatic BMS ISR were randomised to treatment with a paclitaxel-eluting balloon (SeQuent® Please; B. Braun Melsungen AG, Berlin, Germany) versus a XIENCE V®/Prime™ everolimus-eluting stent (EES) (Abbott Vascular, Santa Clara, CA, USA) in order to evaluate the healing response of the vessel wall after balloon angioplasty with a paclitaxel-eluting balloon as compared to implantation of an EES in patients with coronary in-stent restenosis. The primary endpoint of the study was the percentage of uncovered stent struts per patient at nine-month follow-up, as assessed with OCT. Secondary endpoints were stent apposition and neointimal thickness, assessed with OCT, nine-month angiographic in-stent and in-segment late lumen loss (LLL), percentage diameter stenosis at follow-up, binary in-stent and in-segment restenosis and in-stent and in-segment minimum lumen diameter (MLD). Clinical outcome was assessed at one month, eight months and one year and included the separate endpoints as well as the cumulative composite rate of major adverse cardiac events (MACE).
The study (NCT01065532) complies with the Declaration of Helsinki and was approved by the institutional ethics committee. All patients gave written informed consent for participation in this trial.
STUDY PROCEDURE AND RANDOMISATION
Patients fulfilling inclusion/exclusion criteria were screened for participation in the trial. Patients with bare metal stent in-stent restenotic lesions with a target lesion length <24 mm and a reference vessel diameter between 2 and 4 mm were considered candidates if they were eligible for percutaneous coronary intervention and if the target vessel was judged suitable to be examined with OCT. The exclusion criteria were a left ventricular ejection fraction <30%, impaired renal function (serum creatinine >2.0 mg/dl), lesions in the left main trunk, bifurcation lesions, previous brachytherapy of the target vessel and a life expectancy of less than one year. Definite inclusion in the trial and randomisation were performed whenever extensive predilation of the ISR with a semi- or non-compliant balloon resulted in an acceptable angiographic result in the absence of significant recoil or dissection. After a satisfactory angiography had been performed and patients were deemed eligible to receive either of the two study procedures, patients were randomised. The randomisation process was organised via an independent entity (Leuven Coordinating Centre [LCC], Leuven, Belgium), using an interactive voice response system (IVRS) and a centralised computer-generated random sequence. Randomisation was stratified by centre, and block sizes of four were used to guarantee a balanced assignment throughout the course of the study. For practical reasons, the operator and the patient were not blinded to the treatment allocation. All patients were treated according to their allocated treatment.
For patients assigned to DEB, a SeQuent Please balloon of appropriate size (balloon-artery ratio ≥1) and length was inflated for 60 seconds and then retrieved from the coronary artery. For patients randomised to DES, an appropriately sized XIENCE EES was implanted at high pressure (14-18 atm for at least 20 seconds). Although the protocol encouraged full coverage of the previously stented segment with the newly allocated treatment (DEB or EES) extending longitudinally at least 3 mm beyond both stent edges, the ultimate decision was made by the operator, who could also decide to treat only a part of the stented segment, if this was felt to be in the patient’s interest.
QCA measurements
Digital coronary angiograms were analysed offline by the local core laboratory, using a validated automated edge detection system (ACOM.PC 5.0; Siemens, Munich, Germany). The MLD and diameter stenosis were evaluated at the end of the procedure and at follow-up both in the stent and in the stented segment (defined as the whole stented tract plus the 5 mm edges proximally and distally to the stent). LLL was calculated as the difference in MLD between measurements immediately after the procedure and at follow-up. Binary angiographic restenosis was defined as diameter stenosis >50% by quantitative coronary angiography (QCA) on the follow-up angiogram.
OCT IMAGE ACQUISITION
The OCT study was performed using the frequency-domain OCT system (C7-XR OCT Intravascular Imaging System and Dragonfly™ OCT catheter; St. Jude Medical, St. Paul, MN, USA) with a pullback speed of 20 mm/s and rotation speed of 100 frames/s using a non-occlusive technique. OCT examination was only performed during the nine-month control angiography. Intracoronary administration of nitrates (100 to 300 µg) was performed before starting the intracoronary imaging procedure. After advancing the OCT catheter distally to the stented segment, an automatic pullback was started approximately 5 mm distally from the most distal stent struts as soon as optimal blood clearance was obtained using an automated flush with contrast medium.
OCT ANALYSIS METHODOLOGY
A manual check of image quality and completeness of data was performed prior to the core lab analysis. Pullbacks or frames with poor image quality (mainly caused by residual blood, artefact or reverberation, or with portions of the stent out of the image) were excluded from further analysis.
Lumen and stent areas were calculated every three frames (i.e., approximately every 0.6 mm) along the entire stented segment. Quantitative strut level analysis was performed at 0.6 mm intervals along the entire target segment.
The centre of the luminal surface of the strut was determined for each strut, and its distance to the lumen contour was calculated automatically to determine strut-level intimal thickness. The number of struts without coverage was counted for each frame analysed.
Quantitative and qualitative OCT analyses were performed offline by the local core laboratory. The validated OCT Detecting Instrument for StEnt Reendothelialisation aNd Apposition (ODIERNA; Leuven, Belgium) was used for automatic detection of struts, strut coverage at follow-up and quantification of lumen area and stent area15,16. Automatic measurements were supervised by expert analysts and manually edited if needed. Particular attention was given to struts presenting a very thin intimal layer. In the EES arm, a double layer of struts was present in the follow-up OCT images. For the calculation of the stent and neointimal area, results are reported for both the inner (most luminal) and the outer layer of struts. The OCT core lab assessment procedure is described in Figure 1 and Figure 2. Assessment of late stent malapposition was performed manually. Arbitrarily, a cut-off of 110 µm (thickness of the XIENCE V everolimus stent + half of the blooming artefact) was applied for all strut apposition assessments17.
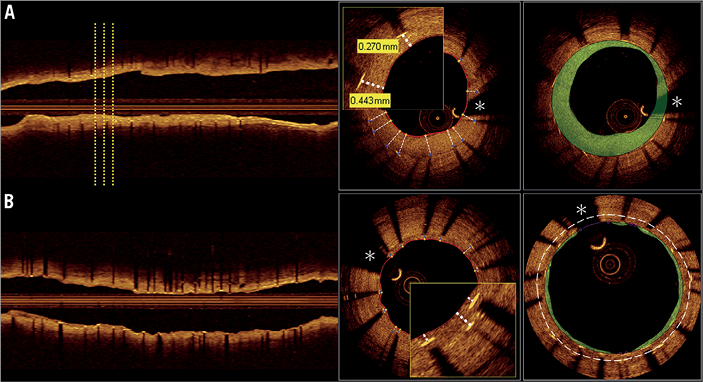
Figure 1. OCT analysis. OCT core lab assessment for drug-eluting balloon (A) and everolimus-eluting stent (B) treated patients. On the left side, longitudinal view illustrating assessment every third frame (0.6 mm interval). In the drug-eluting stent group, where a double layer of stent struts is seen, all detectable stent struts were analysed with respect to coverage. For the assessment of stent area and neointimal hyperplasia area, separate measurements taking into account the inner (lining the green area) and the outer (white dashed line) layer of struts were performed. *coronary wire artefact; OCT: optical coherence tomography
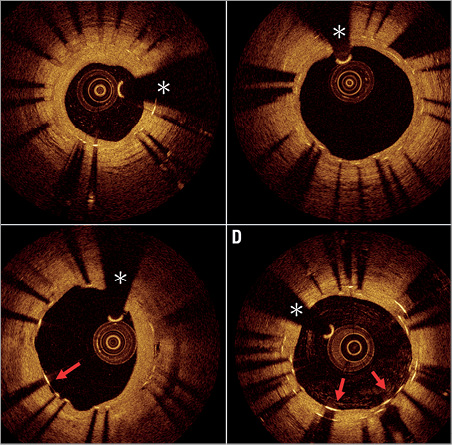
Figure 2. Healing patterns at nine months in ISR lesions treated with DEB or EES. A) Favourable nine-month healing in ISR treated with DEB. All struts are well covered. There is a homogeneous layer of neointimal tissue with a well preserved lumen area. B) Unfavourable nine-month healing in ISR treated with DEB. There is an asymmetrical healing pattern with some intimal hyperplasia in the right half of the frame and very discrete neointima growth with some uncovered struts in the left half of the frame. The red arrow indicates a non-covered strut. C) Favourable nine-month healing in ISR treated with EES. There is a double layer of stent struts, which all appear well covered. There is a thin but healthy appearing layer of neointimal tissue. D) Unfavourable nine-month healing in ISR treated with EES. Some struts of the inner stent layer are uncovered (red arrows). *coronary wire artefact; DEB: drug-eluting balloon; EES: everolimus-eluting stent; ISR: in-stent restenosis
For every analysed pullback, the presence of neoatherosclerosis and the presence of the black hole phenomenon were assessed in the nine-month OCT pullback. Neoatherosclerosis, the transformation of neointima into a lipid-laden atherosclerotic plaque, is considered an important mechanism in late stent failure18. The black hole phenomenon is described as an accumulation of larger signal-poor areas inside restenotic neointimal tissue19.
STATISTICAL METHODOLOGY
All randomised patients were included in the analysis according to the intention-to-treat (ITT) principle. Summary statistics are given per randomised treatment group. For continuous measurements, the number of observations with non-missing data, means and standard deviations (SD) or medians and interquartile ranges (IQR) are presented, as appropriate. For categorical variables, the observed frequencies and percentages are reported.
For baseline lesion and angiographic characteristics, treatment groups are compared using a t-test for continuous variables and a chi-square test for categorical variables. All tests are two-sided and assessed at a significance level of 5%.
The primary endpoint and all secondary OCT and QCA endpoints were analysed by means of a Wilcoxon rank-sum test on all patients in the ITT set who had data. In order to account for all patients included in the ITT analysis set, multiple imputation techniques were used, whereby patients with missing OCT data were imputed 100 times. These analyses yielded similar results to the complete cases analyses, and therefore only the latter are presented in this manuscript.
No formal sample size calculation based on a primary endpoint hypothesis could be performed, given the absence of any data on the magnitude of the effect in either group at the time the trial was designed. Compatible with the assumptions made in other studies with OCT-based endpoints (e.g., the OCTAMI trial)20, we estimated requiring a minimum of 20 patients per group to provide meaningful results. To accommodate for loss of follow-up and loss of assessable OCTs, we therefore planned to randomise 50 patients.
The statistical analysis for this paper was generated using SAS/STAT software, version 9.2 (TS2M3), of the SAS System for Windows (copyright © 2002-2008 SAS Institute Inc.). SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
Results
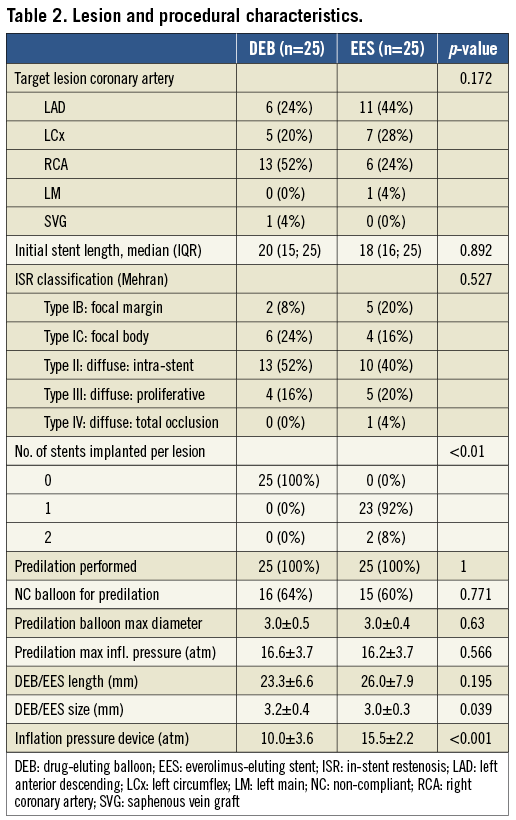
Between June 2009 and October 2011, fifty patients with ISR were enrolled in the trial. Only a single patient screened for the study failed our inclusion criteria, because of an unsatisfactory result after predilation. All other patients were treated according to the randomly assigned treatment modality, without any exclusion or crossover of patients. The study flow is presented in Figure 3. Baseline patient characteristics were well balanced between groups, except for more patients with diabetes mellitus and fewer males in the DEB group (Table 1). Clinical presentation at the time of inclusion was comparable to earlier series, with 3/4 of patients presenting with stable angina or silent ischaemia, and 1/4 with an acute coronary syndrome. The median time interval between initial stent implantation and the diagnosis of ISR was 20.3 (IQR 8.9-57.8) and 7.8 (IQR 5.0-47.9) months in the DEB and EES groups, respectively. Baseline lesion characteristics were comparable between groups and are presented in Table 2. Most stents presented diffuse intra-stent neointimal hyperplasia (Mehran classification type II), while the remainder had focal (type I) or diffuse proliferative (type III) disease21. Although per protocol only BMS ISR in native coronary arteries qualified for participation in the trial, four cases of DES ISR (three in the DEB and one in the EES arm) and one restenosis in a saphenous venous graft were included. The mean lengths of DEB and EES were comparable. The stented segment was completely treated with DEB or EES, except for five cases in the DEB and four in the EES arm, where a more focal treatment was applied, both in focal and more proliferative restenosis. Procedural characteristics are also presented in Table 2.
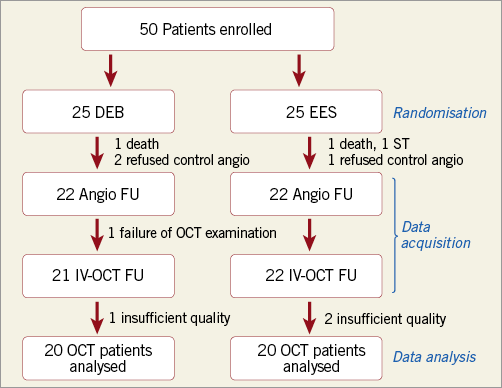
Figure 3. SEDUCE study flow chart. Of 25 patients treated with drug-eluting balloon (DEB), 22 underwent follow-up angiography (two refused, one patient died before the planned follow-up). One additional patient could not undergo optical coherence tomography (OCT) for technical reasons (impossible to advance the catheter distal to the lesion). One OCT pullback could not be analysed due to insufficient image quality. Of 25 patients treated with everolimus-eluting stents (EES), one patient refused FU angiography and one patient died before the planned follow-up angiography. Two additional OCT examinations were of insufficient quality to be included in the analysis. DEB: drug-eluting balloon; EES: everolimus-eluting stent; OCT: optical coherence tomography
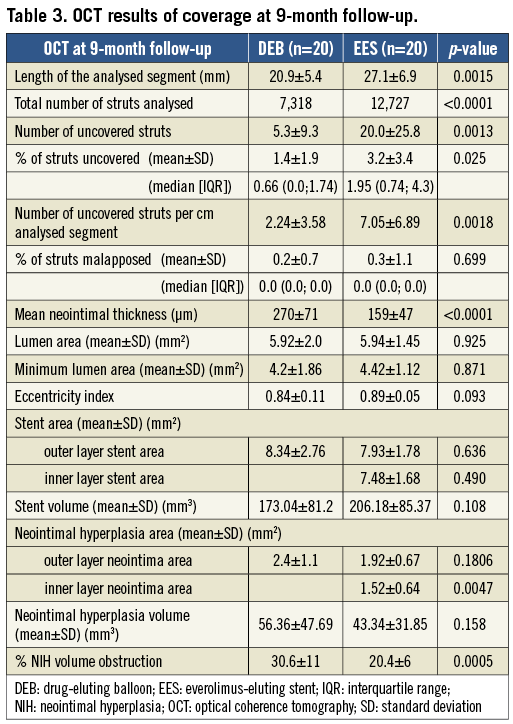
Forty-three patients underwent OCT at nine months. The quality of three OCT examinations was insufficient for quantitative analysis. Two patients died before the nine-month follow-up visit, and another suffered stent thrombosis within one month of inclusion and was excluded from the OCT analysis. An additional three patients refused nine-month control angiography and OCT, while in one the OCT catheter could not be advanced distally to the target lesion. The OCT analysis group thus consisted of 40 patients, 20 in each group, and 20,045 stent struts were analysed in total. Quantitative data are presented in Table 3. The study flow of patients is described in Figure 3.
A mean of 366±135 and 636±184 struts were analysed per patient in the DEB and EES groups, respectively. The percentage of uncovered struts per patient was significantly lower in patients treated with DEB as compared to those treated with EES (1.4% vs. 3.1%, p=0.025). Mean neointimal thickness was 270±71 µm in DEB vs. 159±47 µm in EES (p<0.0001). Mean neointimal hyperplasia area was 2.4±1.08 mm² in DEB, vs. 1.92±0.67 mm² in EES (p=0.1806). The percentage of malapposed struts per patient was very low in both groups (0.2% vs. 0.3%, p=0.699). Representative illustrations of covered and uncovered struts are shown in Figure 2. A graphical representation of strut coverage in both treatment groups is presented in Figure 4.
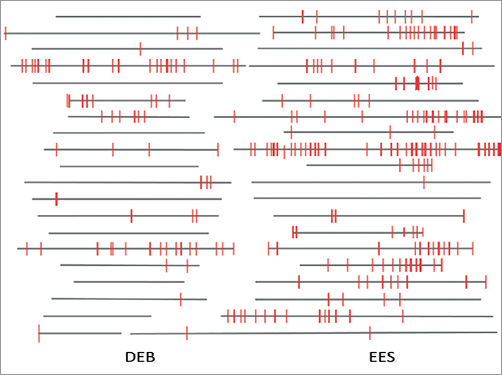
Figure 4. Graphical representation of stent strut coverage in lesions in DEB versus EES. Grey horizontal bars represent stented segments. Uncovered struts are represented by red lines. DEB: drug-eluting balloon; EES: everolimus-eluting stent
Results of lumen area, stent area and neointimal area are presented in Table 3. The eccentricity index (calculated as minimal/maximal diameter at the level of minimal lumen area in the OCT images) was 0.84±0.11 vs. 0.89±0.05 (p=0.093) in the DEB vs. EES arm. With respect to the qualitative analysis, three vs. two cases of black hole phenomenon and two vs. one case of neoatherosclerosis were observed in the DEB vs. EES group, respectively.
QCA data are presented in Table 4. Before treatment, MLD in the DEB group was significantly larger (0.98 mm vs. 0.57 mm in EES, p=0.005). Acute gain in these patients was smaller (1.43 vs. 2.03 mm, p=0.001), resulting in a similar in-stent MLD after treatment in both groups (2.41 vs. 2.6 mm, p=0.1). LLL at nine months was small in both groups (0.28 vs. 0.07 mm, p=0.1), but ultimately resulted in a significantly smaller MLD in DEB-treated patients (2.13 mm vs. 2.54 in EES, p=0.006) and a higher percentage of diameter stenosis (26.4% vs. 11.4%, p=0.002).
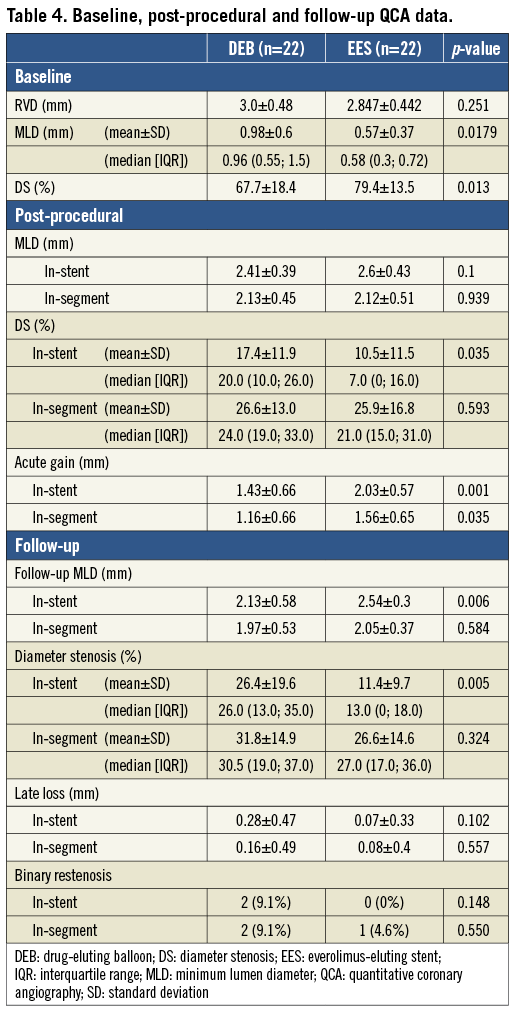
We did not observe significant differences in clinical outcome at one-year follow-up (Table 5). There was one death in each group (one cardiac death in the DEB arm, not related to the target lesion and one non-cardiac death in the EES arm), one TLR in the DEB arm, and two TLR (including one case of angiographically proven early stent thrombosis at three days after stent implantation) in the EES arm.
Discussion
The present study is the first one to compare healing characteristics, as assessed with OCT and QCA, in patients treated for BMS ISR with a paclitaxel-eluting balloon vs. an EES. Our observations resulted in the following main findings. 1) The percentage of uncovered struts at nine months, as assessed with OCT, is significantly lower in patients treated with a DEB. Nevertheless, EES also show a favourable degree of stent coverage, comparable to EES implanted in de novo coronary lesions11. 2) Despite comparable angiographic MLD in both groups after treatment, and moderate differences in LLL at nine months, MLD at follow-up was significantly lower and diameter stenosis significantly larger in patients treated with DEB. 3) Although underpowered for clinical outcome, the lower MLD and higher percentage of diameter stenosis at follow-up did not translate into clinically significant differences in rates of TLR. The findings of the current study support the use of either DEB or EES as safe and efficacious treatment options for ISR.
The current study represents the first prospectively randomised comparison between DEB and EES in the setting of ISR. Former studies compared DEB with first-generation DES, especially paclitaxel-eluting stents, which have been associated with a less favourable efficacy and safety profile as compared to EES1,6,12. In the absence of large randomised trials comparing safety of DEB and DES in the setting of BMS ISR, human pathology studies suggest that the extent of stent strut coverage and apposition at midterm is a valid proxy of long-term stent healing22-24. In our study, stent strut coverage in patients treated with DEB appeared to be excellent, with almost 99% of struts already covered at nine months, as assessed with OCT. Several hypotheses can be formulated to explain why we did not observe complete strut coverage in these restenotic lesions only treated with the DEB in the absence of an additional layer of stent struts. First, adjacent to the in-stent restenotic segment, there are also areas of normal (non-excessive) healing. These areas usually have between 1 and 2% of struts left uncovered, as demonstrated by previous OCT studies with a BMS comparator group20,25. Second, restenotic lesions were extensively predilated before application of the DEB, damaging the existing neointimal layer of a significant number of struts. Finally, the known cytotoxic effects of paclitaxel might also have contributed to the incomplete strut coverage after DEB. Furthermore, the percentage of uncovered struts after treatment with DEB for ISR in our study is very similar to that in a recently published registry10.
The percentage of uncovered struts of between 3 and 4% after EES in our study is also comparable to that described in de novo lesions11. Despite the distinct composition and architecture of the tissues underlying the implanted stent in ISR, a similarly favourable healing pattern was observed with an EES in more challenging circumstances. Because EES implantation after ISR results in a double layer of struts, it remains unclear, however, whether these favourable OCT results also translate into an equally favourable clinical advantage. In the analysis of stent strut coverage, which was performed blinded using automated software, almost twice as many struts were counted in this group. As a consequence, the absolute number of uncovered struts is four times higher in the EES compared to the DEB arm.
Differences in stent strut coverage between DEB and DES can at least in part be explained by the absence of an additional layer of struts after DEB treatment as well as a more uniform drug distribution and shortened drug elution. Indeed, with DES, the newly implanted stent results in a de novo healing process before full strut coverage can be expected. Another factor is that drug elution with DES occurs progressively over weeks, while the residual drug concentration in the vessel wall with DEB is usually very low after 12-24 hrs, although its antiproliferative effect has been shown to be sustained over time26,27. These differences could ultimately lead to delayed stent endothelialisation in patients undergoing DES implantation, and could potentially increase their risk for later events4,28,29. Indeed, rates of stent thrombosis of up to 5.9% at five years after DES implantation for ISR reported previously suggest that delayed re-endothelialisation does result in a clinically less favourable outcome4. In contrast, no instances of stent thrombosis were seen up to five years in the PACCOCATH-ISR trial, in which coated balloons were compared with uncoated balloons for the treatment of ISR5,30. While awaiting long-term clinical outcome results in large trials, the favourable healing characteristics after DEB observed in our study support the hypothesis of improved safety of DEB.
The late luminal loss in the DEB arm of our study was slightly higher as compared to the late loss previously reported in the PEPCAD II trial, but slightly lower compared to PEB used in DES restenosis patients (presumed to be more challenging than BMS restenosis) in the ISAR-DESIRE 3 trial6,31. This difference can in part be explained by the more severe pattern of initial restenosis in our experience (predominantly type II and III ISR lesions)6. Vice versa, in our study, the extent of late luminal loss after EES (0.07 mm) was similar to that observed in de novo coronary lesions32. This compares favourably with trials using other DES for ISR, such as the SISR and ISAR-DESIRE trials3,6,28.
Due to the relatively small number of patients, the current study is limited in terms of angiographic assessment of the efficacy of DEB vs. DES in in-stent restenotic lesions. However, efficacy with respect to angiographic endpoints of DEB in ISR was demonstrated earlier in the PEPCAD II and ISAR-DESIRE 3 trials6,31.
As mentioned, the MLD in our study was significantly lower at follow-up in the DEB versus the DES arm. However, these findings did not translate into differences in the rate of binary restenosis, need for target lesion revascularisation or clinical outcome. Another reassuring observation is the performance of the DEB at the stent edges, reflected in favourable in-segment results of MLD and diameter stenosis in the angiographic follow-up examinations.
The small and insignificant difference in the minimal luminal area on OCT contrasts to some extent with the statistically significant difference in minimal luminal diameter on QCA. The difference in the QCA assessment of a group with a single layer vs. a double layer of struts might have played a role, as well as the fact that lesions in the DEB group tended to be a little bit more eccentric, influencing QCA but not OCT analysis.
Finally, our study also offered a unique opportunity to assess specific patterns associated with impaired healing that cannot be adequately addressed by angiography, including the black hole phenomenon and in-stent neoatherosclerosis (Figure 5). Careful analysis revealed that there was no excess observation of these phenomena in either of the treatment groups.
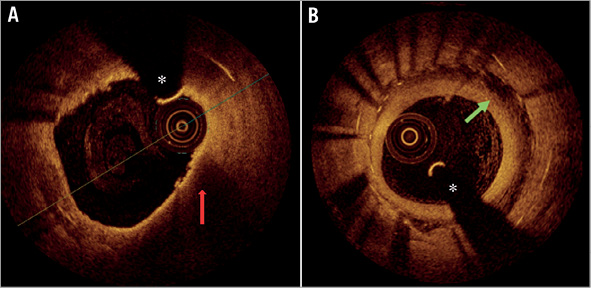
Figure 5. Neoatherosclerosis and black hole phenomenon at nine-month OCT follow-up. A) In-stent neoatherosclerosis with a thin-capped fibroatheroma (TCFA) (red arrow) containing neointima surrounded by a signal-poor, lipid area. B) “Black hole phenomenon” (green arrow) extending over 270° in a patient with ISR treated with EES. *coronary wire artefact
Limitations
Our study has several limitations. First, the sample size was relatively small, and the primary endpoint could only be assessed in 40 patients (20 in each group); the study was not powered to detect differences with respect to clinical outcome.
A second inevitable limitation is the fact that a group with a single layer of struts is compared to a group with a double layer of struts. This has led to a much higher number of stent struts analysed in the EES group, possibly underestimating the burden of uncovered struts when expressed as a percentage of total struts.
Randomisation to one of the two treatment arms was performed only after an acceptable angiographic result after extensive predilation had been obtained. An additional bias might have been introduced by the fact that patients not suitable for treatment with the DEB (e.g., very extensive restenosis extending far beyond the edges of the stent) or the EES (e.g., very tortuous or calcified lesions predicting difficulties in advancing the DES to the target lesion site) were not considered good candidates for the study. The reasons behind both inclusion and exclusion criteria were mainly aimed at avoiding crossover after failed lesion preparation. Therefore, the results of the current study are only generalisable to in-stent restenotic lesions in which an acceptable angiographic result is obtained after careful predilation.
In a quarter of the patients in both treatment arms, the time interval between initial stent implantation and diagnosis or treatment for restenosis exceeded three years. It is not unlikely that in-stent neoatherosclerosis was responsible for ISR in this subgroup of patients, rather than neointima formation33. We performed an additional analysis of the main endpoints of the study only taking into account the patients with ISR <3 years, which did not affect the conclusions of our study.
Despite their high resolution, OCT images do not provide the context to determine exact composition of the material covering stent struts. In addition, it remains unclear whether the observed neointimal tissue is covered by a functional endothelium34. In addition, OCT does not provide information about vasomotor function in the treated segment. A final limitation with regard to the OCT analysis concerns the assessment of late malapposition. Arbitrarily, a cut-off distance of 110 µm was used to quantify apposition of all struts, since it is impossible to discriminate completely the struts from the EES from those from the underlying BMS. Therefore, these results should be interpreted with caution.
Conclusions
Treatment of BMS ISR with DEB leaves significantly fewer stent struts uncovered at nine-month follow-up, as compared with an EES. However, the healing course of EES in this challenging lesion subset should also be considered favourable, as it appears to be comparable to that of EES in de novo coronary lesions. Patients treated with EES had a significantly lower percentage of diameter stenosis at nine-month angiography. However, this did not translate into differences in clinical outcome. The findings of the our study give support to the use of both DEB and EES as valuable treatment options for ISR, with DEB probably offering a slightly better safety profile at the price of a lower efficacy compared to EES. Since the current study is exploratory in nature, further research is needed to preclude large differences in the primary endpoint between the groups.
| Impact on daily practice The favourable healing characteristics observed with both DEB and EES in this study add support for the use of both technologies in BMS ISR. While healing might be slightly superior with DEB, there seems a small price to pay with respect to antirestenotic efficacy as compared with EES. Therefore, a patient- and lesion-tailored approach might be preferable, with a preference for DEB in those patients at increased bleeding risk or where a treatment with anticoagulant therapy is needed, given the benefit of a shorter course of dual antiplatelet therapy. In patients deemed at high risk for stent thrombosis, or in a tortuous or calcified anatomy where optimal expansion of the stent is not achievable, a DEB would be equally preferred. Conversely, drug-eluting stent implantation could be considered as the first treatment modality for ISR on critical locations, such as proximal LAD lesions. |
Funding
The work was supported by a grant from the Research Foundation Flanders (FWO) (G.0690.09N) and by an Industrial Research Grant of the Catholic University Leuven (KU Leuven) IOF grant (ZKC2992). T. Adriaenssens is supported by a clinical doctoral grant from the Research Foundation Flanders.
Conflict of interest statement
The investigators received unrestricted research grants from Abbott Vascular (Santa Clara, CA, USA) and B. Braun Melsungen AG (Berlin, Germany). T. Adriaenssens, P. Sinnaeve, C. Dubois and W. Desmet are consultants for Abbott Vascular. The other authors have no conflicts of interest to declare.

