Abstract
Background: Drug-coated balloons (DCB) and drug-eluting stents (DES) represent the currently recommended treatments for in-stent restenosis (ISR). Optical coherence tomography (OCT) allows detailed neointimal characterisation which can guide treatment strategies.
Aims: The aims of this study were first, to assess the relation between neointimal pattern and clinical outcomes following in-stent restenosis (ISR) treatment, and second, to explore a potential interaction between neointimal pattern and treatment modality relative to clinical outcomes.
Methods: Patients undergoing OCT-guided treatment (DCB or DES) of ISR in three European centres were included. Based on the median of distribution of non-homogeneous neointima quadrants, patients were categorised into low and high inhomogeneity groups.
Results: A total of 197 patients (low inhomogeneity=100 and high inhomogeneity=97) were included. There were no significant differences in terms of major adverse cardiac events (MACE) (p=0.939) or target lesion revascularisation (TLR) (p=0.732) between the two groups. The exploratory analysis showed a significant interaction between neointimal pattern and treatment modality regarding MACE (pint=0.006) and TLR (pint=0.022). DES showed a significant advantage over DCB in the high (MACE: HR 0.26 [0.10-0.65], p=0.004; TLR: HR 0.28 [0.11-0.69], p=0.006), but not in the low inhomogeneity group (MACE: p=0.917; TLR: p=0.797).
Conclusions: In patients with ISR treated with DCB or DES, there were no significant differences in terms of MACE or TLR between the low and high inhomogeneity groups. A significant interaction was observed between treatment modality and neointimal pattern with an advantage of DES over DCB in the high and no difference in the low inhomogeneity group. This warrants confirmation from prospective dedicated studies.
Introduction
In-stent restenosis (ISR) represents the most frequent treatment failure modality following percutaneous coronary intervention (PCI)1. Although use of newer-generation drug-eluting stents (DES) has significantly reduced its occurrence, contemporary randomised clinical trials have shown cumulative rates of target lesion revascularisation (TLR) of 7-10% at five-year follow-up2, and real-world registries including surveillance angiography have shown even higher rates of angiographic restenosis3.
Although several treatment strategies for ISR have been tested4, drug-coated balloon (DCB) angioplasty and repeat DES implantation have emerged as the most effective therapeutic options5,6. However, one major limitation of clinical trials comparing treatment modalities for ISR is the isolated use of coronary angiography as a guide to treatment allocation; indeed, besides mere confirmation of ISR presence, such a coronary “luminogram” delivers little additional information to guide the treatment strategy.
In this regard, the use of high-resolution intravascular imaging techniques, such as optical coherence tomography (OCT), provides unique information regarding the mechanisms underlying ISR and the characteristics of neointimal tissue7. Based on its optical properties at OCT imaging, neointimal tissue has been subdivided into several patterns8 that correlate with different histological substrates9,10. Such different patterns might impact on the outcomes of patients with ISR undergoing PCI in a way dependent on the treatment approach (DES or DCB). However, the number of studies investigating the correlation between OCT-defined neointimal pattern and clinical outcomes following different treatment modalities is extremely scant11 and limited by either comparison of non-contemporary treatment options (such as plain old balloon angioplasty), short clinical follow-up, or isolated neointimal characterisation at one single frame.
This large multicentre European registry had two objectives: first, to assess whether the OCT neointimal pattern is related to the clinical outcomes of patients undergoing PCI for ISR; second, to explore whether there is an interaction between neointimal pattern and type of PCI –DCB or repeat DES– relative to clinical outcomes.
Methods
PATIENT POPULATION AND STUDY ENDPOINTS
Patients presenting with ischaemic symptoms and/or evidence of myocardial ischaemia in three European centres (Hospital Universitario de La Princesa and Hospital Universitario Clínico San Carlos, Madrid, Spain [from 2010 to 2011]; Deutsches Herzzentrum, Munich, Germany [from 2012 to 2017]) who underwent intravascular OCT and subsequent PCI (either DES or DCB) for ISR were considered eligible for the study. Informed consent was obtained prior to each procedure. Ethics approval was waived since all procedures were required on a clinical basis. Treatment modality was at the discretion of the operator. Clinical follow-up was performed by office visit, phone contact or structured follow-up letter.
The primary endpoint of the study was the cumulative incidence of major adverse cardiac events (MACE), defined as a composite of all-cause death, myocardial infarction (MI) or clinically driven target lesion revascularisation (TLR). The secondary endpoint was clinically driven TLR. Individual components of the primary endpoint were also assessed separately. Further details regarding study endpoint definitions are provided in Supplementary Appendix 1.
ANGIOGRAPHIC AND OCT DATA ACQUISITION AND ANALYSIS
Baseline and post-procedural angiograms as well as raw data of OCT image acquisitions were recorded and assessed off-line in a core laboratory (ISAResearch Center, Munich, Germany). The angiographic pattern of ISR was classified according to Mehran’s classification12. Quadrant-based neointimal characterisation was performed at the frame displaying the maximal % area stenosis as well as the five preceding and following analysed frames13,14,15 (Figure 1). Details and definitions regarding angiographic and OCT analysis are provided in Supplementary Appendix 1.
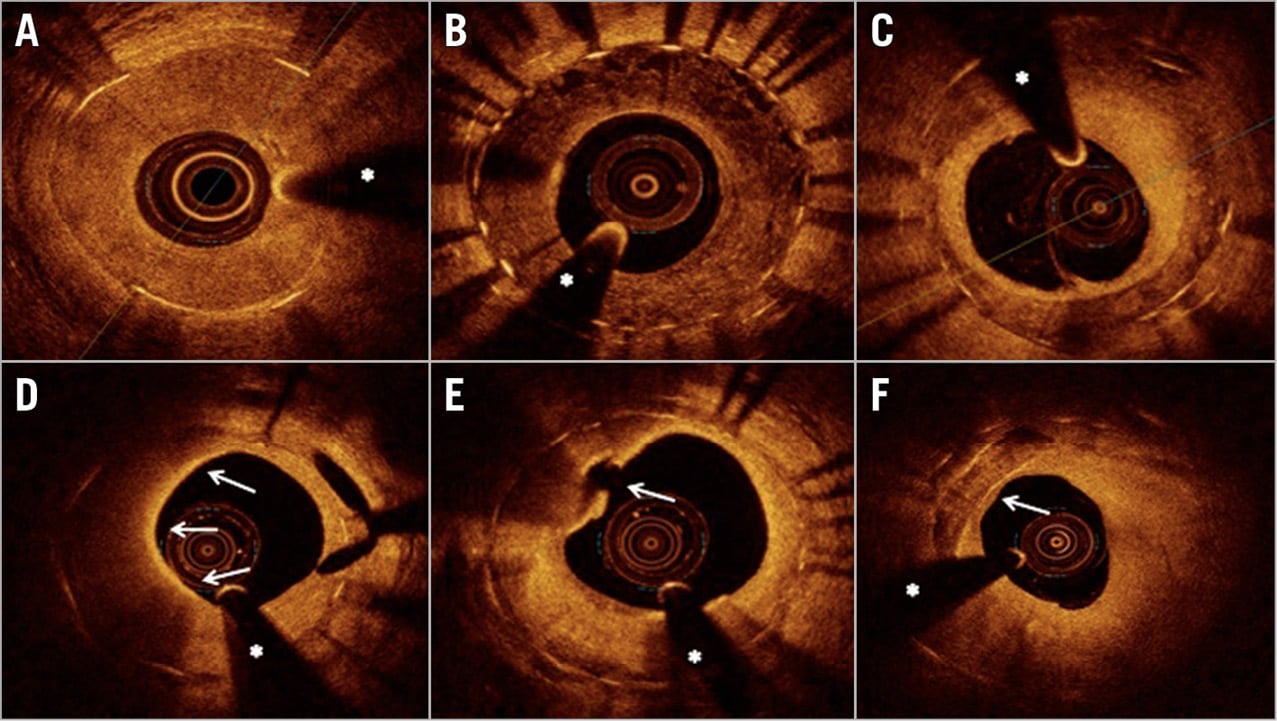
Figure 1. Representative images of optical coherence tomography findings in patients presenting with in-stent restenosis. A) Homogeneous neointimal pattern. B) Heterogeneous neointimal pattern. C) Layered neointimal pattern. D) Macrophage infiltration involving a 180° neointimal arc (arrows from 6 to 12 o’clock). E) Neoatherosclerosis and ruptured thin-cap fibroatheroma (arrow). F) Neointimal calcification (arrow). * guidewire artefact.
In order to investigate the relation between an increased expression of inhomogeneous quadrants and clinical outcomes, the study population was divided into low and high inhomogeneity groups, based on the median of distribution of non-homogeneous quadrants; analogously, the high inhomogeneity patient population was further classified into low and high neoatherosclerosis subgroups.
STATISTICAL ANALYSIS
Continuous data are presented as mean±SD or median (25th-75th percentiles) depending on the distribution pattern of the variable. Categorical data are presented as absolute and relative frequencies. Hypothesis testing of differences between the groups was performed using the Student’s t-test or the Wilcoxon rank-sum test for continuous variables and the Pearson χ2 test (or Fisher’s exact test where any expected cell count of the contingency table was <5) for categorical variables.
To account for the clustered nature of the data, a linear mixed model was used for the analysis of OCT data. The model contained a fixed-effects term (neointimal pattern) and a random intercept as random-effects term for patient in case of frame-level analysis and as nested random-effects term for patient and frame for strut-level analysis.
Event-free survival was estimated by the Kaplan-Meier method for each clinical outcome. Hazard ratios (HR) with two-sided 95% confidence intervals (95% CI) were calculated using Cox proportional hazards models. The two objectives of the study were addressed in a statistical two-step approach. First, we compared two patient groups defined by the OCT neointimal pattern (high and low inhomogeneity groups) regarding their clinical outcomes after PCI for ISR. The risk for the primary and secondary endpoints of the study was assessed using a) a univariable Cox proportional hazards model including only the OCT pattern of neointima as an independent vari-able, and b) a multivariable model including baseline clinical and angiographic characteristics in addition to the OCT pattern of neointima. Second, we assessed whether the relation between the OCT pattern of neointima and clinical outcomes is influenced by the type of PCI performed for treatment of ISR (DCB or DES). For this purpose, we entered the interaction between OCT pattern of neointima and PCI type into the multivariable model described above. In the case of a significant adjusted interaction between these two variables, we proceeded with an illustrative comparison of the outcomes for the two PCI types (DCB or DES) in each group of OCT pattern of neointima. All tests were two-sided and assessed at a significance level of 5%. Statistical analysis was performed using the R 3.6 Statistical Package (R Foundation for Statistical Computing, Vienna, Austria).
Results
BASELINE CLINICAL AND PROCEDURAL CHARACTERISTICS
A total of 197 patients undergoing PCI for ISR were included, with one lesion being imaged/treated per patient. Based on the median of the distribution of non-homogeneous quadrants, patients were categorised into low (n=100) and high (n=97) inhomogeneity groups. Treatment modality was DES implantation in 88 (44.7%) patients and DCB angioplasty in 109 (55.3%) patients. Baseline clinical, angiographic and procedural characteristics according to neointimal pattern are shown in Table 1 and Table 2. There were no relevant differences in the baseline characteristics between the two groups.
OPTICAL COHERENCE TOMOGRAPHY ANALYSIS
OCT morphometric data according to predominant neointimal type are shown in Table 3. Morphometric analysis included a total of 3,505 frames (33,298 struts) in the low inhomogeneity group and 2,647 frames (24,967 struts) in the high inhomogeneity group. There were no relevant between-group differences in terms of stent diameter/area, lumen diameter/area or neointimal thickness/area. Neointimal tissue characterisation was performed in a total of 7,675 quadrants; the proportion of inhomogeneous quadrants was 2.3% (0.0-6.4) in the low inhomogeneity group and 31.8% (18.2-60.7) in the high inhomogeneity group.
CLINICAL OUTCOMES
The median (25th-75th percentiles) follow-up was 701 (408-1,087) and 748 (361-1,083) days (p=0.962) in the low and high inhomogeneity groups, respectively. Clinical events and the primary and secondary endpoints are shown in Table 4. High neointimal inhomogeneity was not associated with a significantly higher risk of MACE (HR 1.02 [0.59-1.75], p=0.939) (Figure 2), clinically driven TLR (HR 1.10 [0.63-1.93], p=0.732) (Figure 3), or a composite of death or MI (HR 0.53 [0.14-2.08], p=0.372) (Figure 4). We performed a multivariable analysis using two separate Cox proportional hazards models for the primary and secondary endpoints. The OCT pattern of neointima and PCI type for ISR (DES or DCB) were entered into these models along with several baseline clinical and angiographic characteristics such as age, gender, smoking habit, body mass index, hypercholesterolaemia, arterial hypertension, diabetes mellitus, history of MI, history of coronary artery bypass grafting, multivessel disease, target vessel, ostial lesion, bifurcation lesion, complete occlusive ISR, reference diameter (vessel size) and diameter stenosis before PCI (restenosis severity). In the multivariable model for the primary endpoint of MACE at two years, the adjusted p-value was 0.567 for the OCT pattern of neointima and 0.022 for the PCI type. In the multivariable model for the secondary endpoint of TLR at two years, the adjusted p-value was 0.350 for the OCT pattern of neointima and 0.013 for the PCI type. Subsequently, we assessed the interaction between the OCT pattern of neointima and PCI type for ISR (DES or DCB) by adding the interaction term OCT pattern of neointima * PCI type to these two multivariable models. There were statistically significant interactions for both MACE (pint=0.006) and TLR (pint=0.022) at two years.
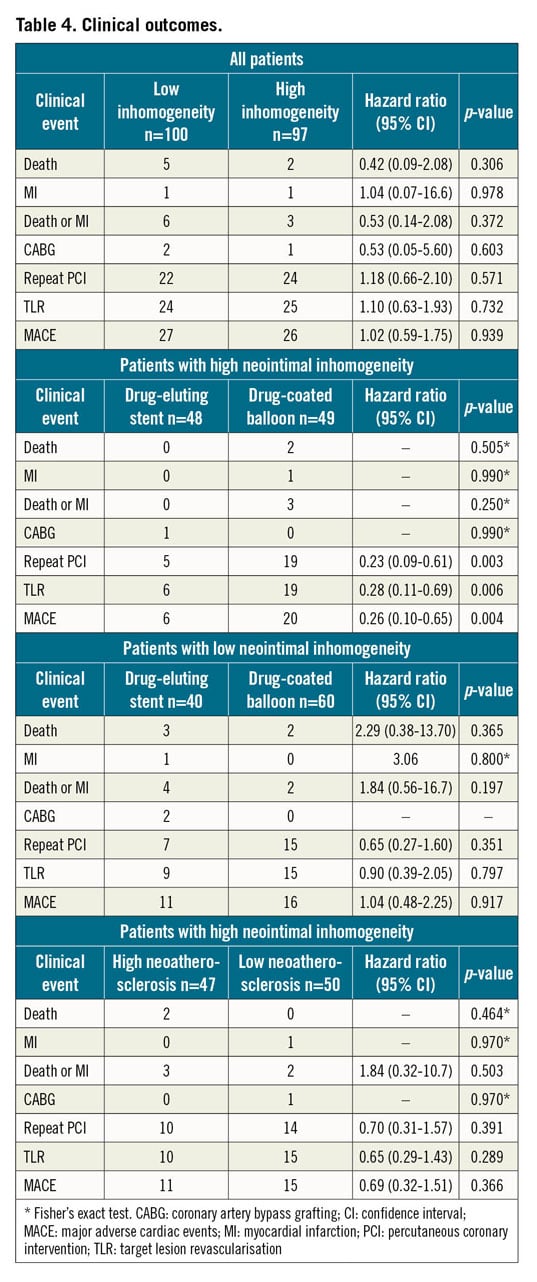
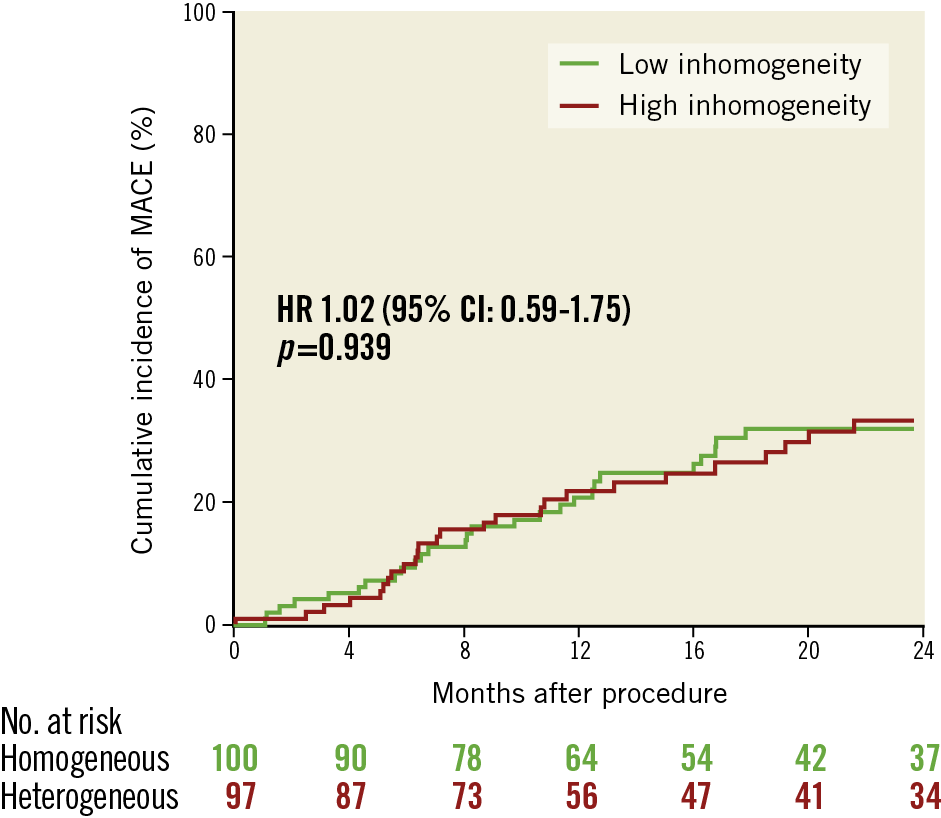
Figure 2. Cumulative incidence of major adverse cardiac events in the low and high inhomogeneity subgroups.
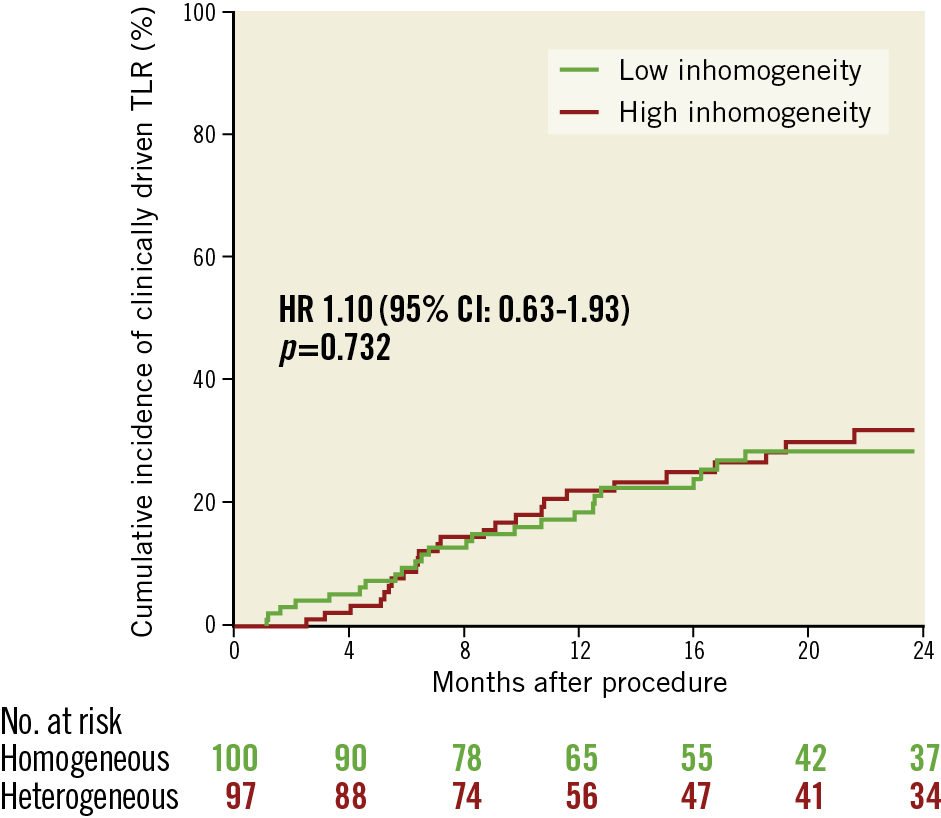
Figure 3. Cumulative incidence of clinically driven target lesion revascularisation in the low and high inhomogeneity subgroups.
Figure 4. Cumulative incidence of all-cause death or myocardial infarction in the low and high inhomogeneity subgroups.
INTERACTION BETWEEN NEOINTIMAL PATTERN, TREATMENT MODALITY AND CLINICAL OUTCOMES
The clinical, angiographic and procedural characteristics of the high and low inhomogeneity groups, respectively, according to treatment modality (DES or DCB) are shown in Supplementary Table 1-Supplementary Table 4. Table 4 shows the clinical outcomes for each neointimal group according to treatment modality.
Notably, DES was associated with a significant advantage over DCB in the high inhomogeneity group (MACE: HR 0.26 [0.10-0.65], p=0.004; TLR: HR 0.28 [0.11-0.69], p=0.006), but not in the low inhomogeneity group (MACE: HR 1.04 [0.48-2.25], p=0.917; TLR: HR 0.90 [0.39-2.05], p=0.797). The dependence of treatment effect of DES and DCB on the extent of inhomogeneity of the neointima is shown in Figure 5 for the primary endpoint.
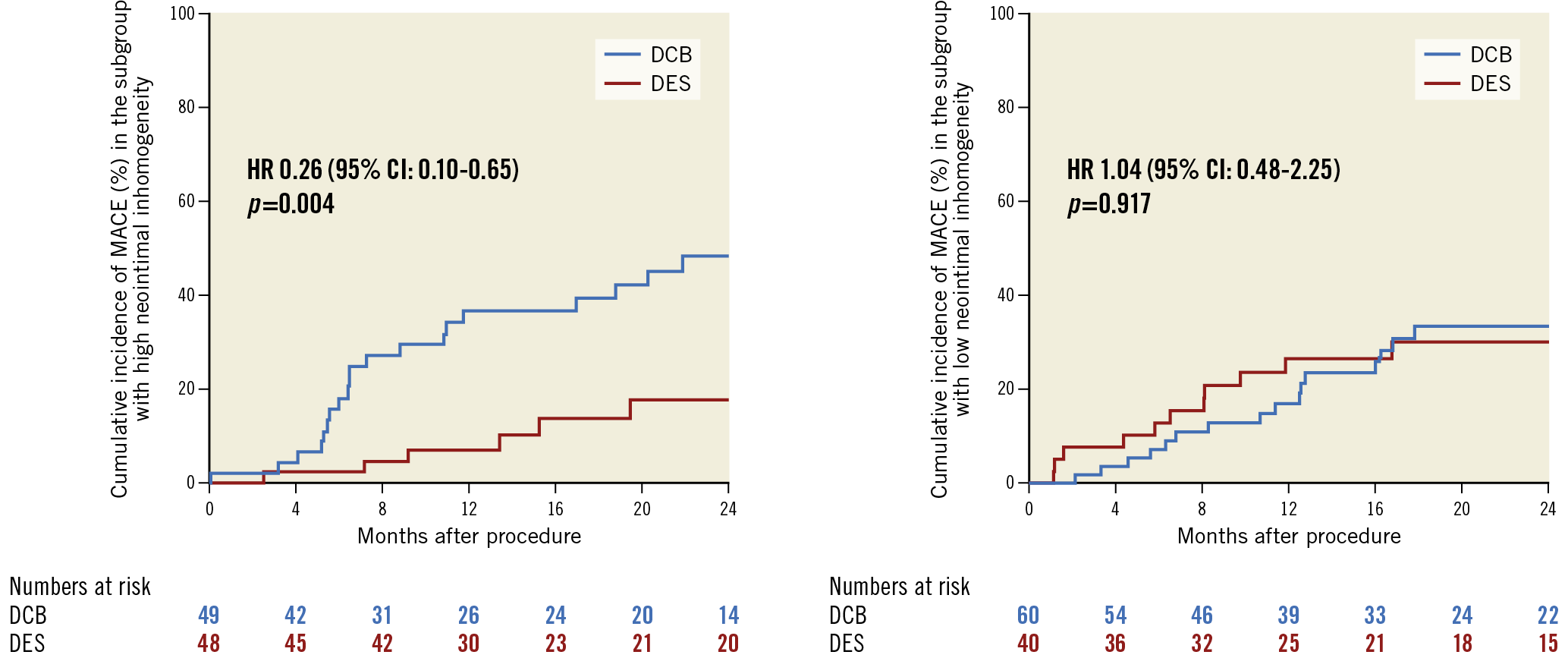
Figure 5. Cumulative incidence of major adverse cardiac events according to treatment type (DES vs DCB) in the subgroups of patients with high (left) and low (right) inhomogeneity of the neointima. There was a significant interaction between neointimal pattern and treatment modality regarding MACE (pint=0.006).
NEOATHEROSCLEROSIS
Clinical, angiographic and procedural characteristics according to the extent of neoatherosclerosis in the subgroup of patients with high inhomogeneity are shown in Supplementary Table 5 and Supplementary Table 6. Clinical outcomes of patients in the high inhomogeneity group according to the extent of neoatherosclerosis are shown in Table 4. There were no relevant differences in terms of clinical outcomes between the two groups.
Discussion
Based on the results of available randomised clinical trials5,6, current European guidelines recommend the use of either DES or DCB for the treatment of coronary ISR (class of recommendation I, level of evidence A)16. The main findings of the present study can be summarised as follows: i) in patients presenting with ISR and undergoing treatment with DCB or DES, there were no significant differences in terms of MACE or clinically driven TLR between the low and high inhomogeneity groups; ii) a significant interaction exists between treatment modality and neointimal pattern with an advantage of DES over DCB in the high inhomogeneity group and no difference in the low inhomogeneity group; iii) there were no relevant differences in terms of clinical outcomes between low and high neoatherosclerosis subgroups in the population of patients with high neointimal inhomogeneity.
The efficacy of DCB treatment relies on rapid initial transfer and subsequent tissue retention of the antiproliferative agent necessary for persistent suppression of cell proliferation17. DCB represents a particularly attractive treatment option due to its ability to provide favourable angiographic results without adding new stent layers. Such a mechanism of action suggests that the subset of smooth muscle cell-rich ISR lesions is particularly suitable for DCB treatment. OCT-histology correlation studies have shown an homogeneous neointimal pattern to correlate consistently with abundance of smooth muscle cells10.
On the other hand, clinical outcomes following repeat DES implantation might be less dependent on underlying neointimal patterns compared to DCB angioplasty. However, repeat DES implantation is associated with potential drawbacks, mainly due to additional stent layers and neoatherosclerosis development. Histological and clinical studies have confirmed an accelerated course of neoatherosclerosis following DES implantation14,18, which in turn triggers late adverse events, including repeat ISR and stent thrombosis13,19.
Our findings are in keeping with those of Tada et al11 who reported comparable TLR rates following DCB and repeat DES in patients with homogeneous neointima. From a practical standpoint, our results support the use of DES in ISR lesions with high neointimal inhomogeneity, while DCB angioplasty could be safely and effectively used in ISR lesions with low neointimal inhomogeneity. However, regardless of the treatment strategy adopted, the overall MACE rate remains considerable in patients presenting with ISR, highlighting the need for individualised treatment in this patient population.
To summarise, by tailoring treatment strategy to specific ISR lesion characteristics, incorporation of intravascular OCT in the treatment algorithm of ISR impacts positively on treatment outcomes in terms of efficacy and safety. Implementation of such an algorithm in clinical practice requires confirmation of our findings in specifically designed randomised clinical trials with relevant clinical and angiographic endpoints.
Our report has a number of strengths. First, it includes a detailed quadrant-based multi-frame neointimal characterisation for each ISR lesion. Indeed, categorisation of neointimal patterns based on the optical characteristics of a single frame11,20,21 is limited by significant intra-lesion neointimal heterogeneity13. Second, analysis of OCT pullbacks was performed in a central core laboratory according to a standardised protocol, thereby minimising between-centre variability. Third, extended clinical follow-up, with a median follow-up of two years, should have allowed capture of late-occurring events, such as those related to the development of neoatherosclerosis.
Study limitations
Some limitations should be considered when interpreting the results of the present report. First, due to the retrospective nature of the study, its results should be interpreted as exploratory, that is hypothesis-generating. Second, the possible bias of patient and lesion selection should be considered, since patients presenting with ISR were not included consecutively. Third, treatment strategy was at the discretion of the operator and could represent an additional bias. Fourth, the index stent interval, despite not being different between groups, showed considerable variability and could represent an additional confounding factor.
Conclusions
In a large multicentre European registry including patients undergoing intravascular OCT prior to percutaneous treatment of ISR lesions with the two currently recommended strategies, there was no significant difference in terms of MACE or clinically driven TLR between patients with low and high inhomogeneity of the neointima. The exploratory analysis showed that a significant interaction exists between neointimal pattern and treatment modality, with DES showing a significant advantage over DCB in the high, but not in the low inhomogeneity group. This warrants confirmation from prospective dedicated studies.
|
Impact on daily practice In patients presenting with ISR and undergoing treatment with either DCB or DES, there were no significant differences in clinical outcomes between the low and high neointimal inhomogeneity groups. The exploratory analysis showed a significant interaction between treatment modality and neointimal pattern, with a significant advantage of DES implantation as compared to DCB angioplasty in lesions with high neointimal inhomogeneity and comparable outcomes between the treatment strategies in lesions with low neointimal inhomogeneity. If confirmed by prospective dedicated studies, these results would support repeat DES implantation for lesions with high neointimal inhomogeneity, while DCB angioplasty could represent a particularly safe and effective treatment for lesions with low neointimal inhomogeneity. |
Conflict of interest statement
N. Gonzalo reports personal fees from Abbott Vascular and Boston Scientific, outside the submitted work. M. Joner reports personal fees from Biotronik, OrbusNeich, AstraZeneca and Recor, grants and personal fees from Boston Scientific and Edwards, and grants from Amgen, outside the submitted work. S. Kufner reports speaker fees from AstraZeneca and Bristol Myers Squibb, not related to the current work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

