Abstract
BACKGROUND: The treatment of in-stent restenosis (ISR) after drug-eluting stent (DES) implantation remains challenging in current clinical practice.
AIMS: The study was conducted to investigate a novel biolimus-coated balloon (BCB) for the treatment of coronary DES-ISR compared with the best-investigated paclitaxel-coated balloon (PCB).
METHODS: This was a prospective, multicentre, randomised, non-inferiority trial comparing a novel BCB with a clinically proven PCB for coronary DES-ISR. The primary endpoint was in-segment late lumen loss (LLL) at 9 months assessed by an independent core laboratory. Baseline and follow-up optical coherence tomography were performed in a prespecified subgroup of patients.
RESULTS: A total of 280 patients at 17 centres were randomised to treatment with a BCB (n=140) versus a PCB (n=140). At 9 months, LLL in the BCB group was 0.23±0.37 mm compared to 0.25±0.35 mm in the PCB group; the mean difference between the groups was −0.02 (95% confidence interval [CI]: −0.12 to 0.07) mm; p-value for non-inferiority<0.0001. Similar clinical outcomes were also observed for both groups at 12 months. In the optical coherence tomography substudy, the neointimal area at 9 months was 2.32±1.04 mm2 in the BCB group compared to 2.37±0.93 mm2 in the PCB group; the mean difference between the groups was −0.09 (95% CI: −0.94 to 0.76) mm2; p=non-significant.
CONCLUSIONS: This head-to-head comparison of a novel BCB shows similar angiographic outcomes in the treatment of coronary DES-ISR compared with a clinically proven PCB. (ClinicalTrials.gov: NCT04733443)
In-stent restenosis (ISR), particularly in patients with drug-eluting stents (DES), poses a significant clinical challenge, frequently necessitating repeat revascularisation interventions123. A meta-analysis published in 2020 indicates that, for DES-ISR, paclitaxel-coated balloons (PCB) are marginally less effective when compared to contemporary DES4. The distinct advantage of drug-coated balloons (DCBs) is their ability to deliver medication without requiring a new stent implantation. This underscores the crucial significance of the advancing DCB technology5. Different drug-coating formulations and coating-process technologies will result in different vascular responses due to variations in drug formulation, dosage, pharmacokinetics, and interactions with lesions6. Although previous research on DES has illuminated a range of benefits of -limus derivatives over paclitaxel3, it remains unclear whether these benefits are transferable to DCB treatment.
Biolimus A9 (BA9 [Biosensor International]), a sirolimus derivative, has been modified to increase its lipophilicity 10-fold in comparison to sirolimus and other -limus compounds, while retaining its rapamycin inhibition properties. This enhancement makes biolimus particularly well suited for targeted, short-term delivery to vascular tissues. The biolimus-coated balloon (BCB), a semicompliant angioplasty balloon, is coated with 3 μg/mm2 of biolimus, employing polyethylene oxide as the delivery matrix. Preclinical testing using a standard porcine coronary model (n=15) showed that, 1 hour after deployment, the maximum systemic blood concentration of biolimus was approximately 2.0 ng/ml per balloon. Of note, this concentration is 40 times less than the established safety threshold for biolimus7. Moreover, tissue analysis from the treated coronary arteries demonstrated that biolimus levels remained above 1 ng/mg 28 days after the procedure, exceeding the accepted therapeutic threshold for -limus-based drugs.
Recent studies have shed light on the efficacy of BCBs, yet they have not converged on a consensus. The BIO-RISE CHINA study confirmed the superior efficacy of a novel BCB over plain old balloon angioplasty in patients with small-vessel coronary disease undergoing percutaneous coronary intervention (PCI)8. On the other hand, the REFORM study portrayed a different picture, suggesting that a DCB coated with BA9 was less effective when compared to one coated with paclitaxel9. In contrast to the REFORM study, the crystallisation coating process employed in the BIO-RISE CHINA study for BA9 crystals yields a more consistent range of crystal size, which may have had a favourable impact on clinical outcomes. Given the ongoing uncertainty regarding the efficacy of BCBs for coronary artery disease, including ISR, there is an imperative need for additional research to elucidate their role in modern cardiovascular interventions.
Therefore, a prospective, multicentre, non-inferiority, randomised controlled clinical trial was initiated to compare the safety and efficacy of biolimus- versus paclitaxel-coated coronary balloon catheters in the treatment of DES-ISR.
Methods
STUDY DESIGN AND PATIENT POPULATION
The BIO ASCEND ISR study is a multicentre, randomised controlled (1:1), single-blinded, non-inferiority study conducted at 17 hospitals in China. The study was conducted in accordance with the Declaration of Helsinki and was registered on 29 January 2020 (ClinicalTrials.gov: NCT04733443). The main inclusion criteria were patients with Mehran type I, II, and III DES-ISR10 with stable angina, acute coronary syndrome, or asymptomatic myocardial ischaemia. Main exclusion criteria included acute myocardial infarction within 1 week prior to intervention or without recovery of cardiac enzymes, previously treated ISR, left main lesions, and patients with total occlusion. The full list of inclusion and exclusion criteria is provided in Supplementary Appendix 1.
The study protocol received approval from independent ethics committees at each participating centre. All patients provided written informed consent before enrolment. All clinical events were adjudicated by an independent clinical events committee. Analysis of all angiograms was conducted by trained and blinded personnel at the central core laboratory, utilising standard methodologies (Supplementary Appendix 2 and Supplementary Appendix 3).
Patients were randomly allocated (1:1) to treatment with either BCB or PCB. After successful lesion preparation, central randomisation was completed with a computed-generated allocation sequence, stratified by site. Patients and treating physicians were aware of the group allocations, whereas outcome and core laboratory assessors were masked to this allocation.
STUDY DEVICES AND PROCEDURES
The control device is a commercially available PCB (SeQuent Please NEO [B. Braun Melsungen AG]) made in Germany. The PCB was coated with 3 μg of paclitaxel/mm² of balloon surface. The tested device, a BCB (BioAscend JWMS China), was coated with 3 μg biolimus per mm² using polyethylene oxide as an excipient8; this was the same BCB used in the BIO-RISE CHINA study. More detail is shown in Supplementary Appendix 1.
Based on clinical recommendations, all patients were administered aspirin, either in a daily dose of 100 mg for at least 3 days prior to PCI or a one-time loading dose of 300 mg before the procedure, along with clopidogrel (given as a loading dose of 300 or 600 mg, followed by 75 mg per day) or ticagrelor (administered as a loading dose of 180 mg, then continued with 90 mg twice daily). Following PCI, patients were advised to continue dual antiplatelet therapy for a minimum of 1 month, followed by lifelong aspirin use. In all cases, predilation with plain, scoring, or cutting balloons was required to reduce stenosis to less than 30%. Once adequate predilation of the lesion was achieved, patients were randomly assigned in a 1:1 ratio to be treated with either the PCB or the BCB, according to their group, while cases without proper predilation were not included in the randomisation. The selection of balloon sizes was left to the discretion of the interventional cardiologists. Inflation of the DCB lasted between 45 to 60 seconds at normal pressure, tailored to the specific morphological features of the lesion. Successful treatment was defined as achieving a postprocedural residual stenosis of less than 30% based on visual assessment. In a prespecified subgroup of 60 patients, optical coherence tomography (OCT) was performed at baseline, after the procedure, and at the 9-month follow-up to assess the outcomes.
ENDPOINTS
Patients were scheduled for follow-up visits at 1, 6, 9 and 12 months, with an angiographic assessment planned within a period of 9±1 months. Data collection was conducted using electronic clinical report forms throughout the treatment process at all participating centres. The collection of data was prospectively finalised during the hospital stay and continued during subsequent follow-up visits.
The primary endpoint was in-segment late lumen loss (LLL) at 9 months after the procedure (defined as the postprocedural minimal lumen diameter minus the minimal lumen diameter at 9 months).
The major secondary endpoint was neointima area at 9 months (for the OCT subgroup only). Secondary endpoints included the following: (1) device success (defined as successful delivery, expansion and withdrawal), lesion success (defined as residual stenosis ≤30% and Thrombolysis in Myocardial Infarction [TIMI] flow 3 without type C [or above] dissection11) and clinical success (defined as lesion success with absence of death, myocardial infarction and target lesion revascularisation prior to discharge); (2) binary restenosis (≥50% diameter stenosis); (3) target lesion failure (TLF) as a device-oriented composite of cardiac death, target vessel myocardial infarction, and clinically driven target lesion revascularisation; and (4) a patient-oriented composite of all-cause mortality, myocardial infarction and any revascularisation, and definite or probable stent thrombosis12. All clinical events were evaluated by an independent clinical events committee unaware of the group assignment of the subjects.
QUANTITATIVE CORONARY ANGIOGRAPHY AND OCT ASSESSMENT
Trained and blinded personnel at the central core laboratory used standard methodologies to analyse all the angiograms. Quantitative coronary angiographic analysis was conducted using QAngio XA software, version 7.3 (Medis Medical Imaging Systems), in a blinded manner. The neointimal area was analysed using OCT 9 months after the procedure (OCT subgroup). Accordingly, OCT measurement indices contain lesion length, lumen diameter, lumen area, minimal lumen area, stent diameter, stent area, neointimal hyperplasia thickness, average intimal thickness, uncovered struts and their proportions, lipid-rich neointima, calcified neointima, mixed neointima, thin-cap fibroatheroma (TCFA), and thrombus. The neointima area was then calculated based on the above parameters.
STATISTICAL ANALYSIS
The study’s sample size was determined to evaluate the non-inferiority of the investigational device concerning the primary endpoint, based on the following assumptions: a comparable LLL at 9 months of 0.46 mm in both groups, a common LLL standard error of 0.5, and a non-inferiority margin of 0.195 mm1314. To ensure 80% power at a 2.5% alpha level for detecting non-inferiority, 105 patients per group were initially deemed necessary. Considering a potential 25% dropout rate for angiographic follow-up, the sample size was increased to 140 patients per group. This adjustment led to a total sample size of 280 patients. The sample size of the OCT substudy was based on neointimal area at 9 months. Assuming the comparable difference between groups as 0.5 mm2, the standard error as 1.18 mm2, and the alpha level as 5%, 204 OCT sections would be enough to guarantee 80% power15. Considering the cluster effect of multiple cross-sections of a patient, the intragroup correlation coefficient was conservatively estimated to be 0.05, and the corresponding design effect was approximately equal to 2, which translates into 408 OCT sections in 21 patients. With a maximum of a 25% rate of loss to angiographic follow-up, the OCT subgroup randomised 60 patients equally into 2 groups.
The full analysis set (FAS) and per-protocol set (PPS) were used to evaluate the endpoint. The FAS population comprised patients meeting the inclusion criteria, without fulfilling any exclusion criteria, who could provide informed consent. They were randomly allocated to either the BCB or PCB group, and their assessment data were collected after the procedure. The FAS is the main population in clinical events reporting. Patients were excluded from the PPS if they violated the protocol, were lost to follow-up, or had missing primary endpoint data. The PPS was specifically designated for reporting angiographic data.
For continuous variables, the mean±standard deviation was calculated in each group. For in-segment LLL as the primary outcome measure, a covariance analysis of adjusted centre and baseline (the minimal lumen diameter within the lesion segment immediately after intervention) effects was used to compare groups at the beginning of the study. The paired t-test was used to compare the secondary efficacy indices with normal distribution. The measurement data of non-normal distribution were tested by the Wilcoxon signed-rank test. McNemar’s paired χ2 test was used for intragroup comparison of qualitative indicators. Binary variables were presented as counts and percentages. Differences between the two groups were evaluated using the χ2 or Fisher’s exact tests, as deemed appropriate. The two 1-sided tests were employed to test non-inferiority. For lesion-level analysis, generalised estimating equations were used to account for the cluster effect. The Kaplan-Meier method was applied for the estimation of cumulative event rates, with the log-rank test used to assess differences between the groups. Analyses were conducted according to the intention-to-treat principle. A p-value of <0.05 was considered statistically significant. Additionally, SAS 9.4 (SAS Institute) was used for the analysis.
Results
PATIENTS AND PROCEDURAL RESULTS
From December 2020 to January 2022, we screened 290 patients with coronary DES-ISR, and finally, 280 of them were included, leaving 10 cases excluded before randomisation, as no significant in-stent stenosis was observed. There were no patients excluded on account of predilation failure. Five patients withdrew consent before receiving any study treatment (Figure 1). The average age of the patients was 64 years, with 204 (74.2%) males. The FAS population involved 138 patients with 152 lesions in the BCB group and 137 patients with 153 lesions in the PCB group. No significant differences were observed between the BCB and PCB groups regarding demographic, clinical, or lesion characteristics (all p-values>0.05) (Table 1).
Different balloon types were comparably utilised across the groups, with uniform measurements of DCB diameter, length, and inflation parameters, presenting no significant differences (all p-values>0.05). One DCB per lesion was utilised in all cases. Bailout procedures (stent or non-compliant balloon) performed because of edge dissection and obvious residual stenosis were similar in both groups (2.6% vs 1.3%, p=0.448). Following the intervention, TIMI flow grade 3 was observed in all vessels. Device and lesion success were achieved in all cases. Clinical success was observed in all patients in the BCB group and in 99.3% of patients in the PCB group, with only one case of periprocedural myocardial infarction noted (Table 2).
The comparison between the BCB group and the PCB group showed no significant differences in most preprocedural and postprocedural angiographic outcomes. Both groups had similar reference vessel diameters (2.79±0.40 mm vs 2.80±0.39 mm; p=0.779) and minimal lumen diameters (0.84±0.35 mm vs 0.87±0.36 mm; p=0.544) before the procedure. No significant differences in diameter stenosis (69.91±11.05% vs 69.11±11.56%; p=0.540) or lesion length (16.26±7.21 mm vs 15.97±6.85 mm; p=0.718) were observed. The BCB group showed greater in-device diameter stenosis after the procedure than the PCB group (21.94±7.52% vs 20.15±7.06%; p=0.034). The minimal lumen diameter and acute lumen gain for both the in-device and in-segment measurements were also comparable after the procedure (all p-values>0.05) (Table 3, Figure 2).
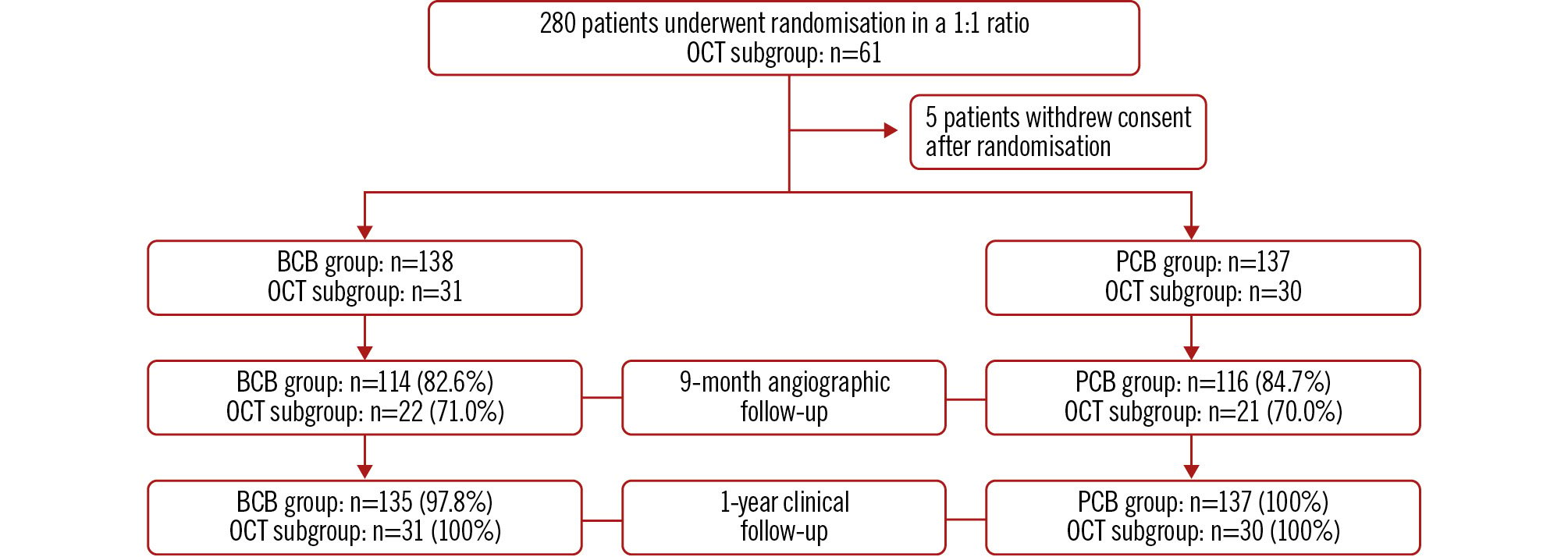
Figure 1. Patient flow and follow-up. BCB: biolimus-coated balloon; OCT: optical coherence tomography; PCB: paclitaxel-coated balloon
Table 1. Baseline patient and lesion characteristics (full analysis set population).
| BCB group (n=138 patients; n=152 lesions) | PCB group (n=137 patients; n=153 lesions) | p-value | |
|---|---|---|---|
| Age, years | 63.64±8.90 | 64.24±8.84 | 0.576 |
| Male | 103 (74.6) | 101 (73.7) | 0.862 |
| Body mass index, kg/m2 | 25.96±3.59 | 25.91±3.31 | 0.887 |
| Diabetes mellitus | 51 (37.0) | 59 (43.1) | 0.301 |
| Insulin-treated diabetes | 23 (46.0) | 29 (50.9) | 0.614 |
| Hypertension | 86 (62.3) | 98 (71.5) | 0.104 |
| Hyperlipidaemia | 61 (44.2) | 53 (38.7) | 0.353 |
| Previous MI | 22 (15.9) | 33 (24.1) | 0.091 |
| Previous CABG | 0 (0) | 0 (0) | - |
| Unstable angina | 111 (94.1) | 108 (93.1) | 0.763 |
| Left ventricular ejection fraction, % | 61.02±8.01 | 60.87±8.96 | 0.883 |
| Multivessel disease | 0.30±0.49 | 0.29±0.50 | 0.837 |
| Target vessel location | 152 (100) | 153 (100) | 0.346 |
| Left anterior descending artery | 71 (46.7) | 78 (51.0) | |
| Left circumflex artery | 23 (15.1) | 13 (8.5) | |
| Right coronary artery | 54 (35.5) | 58 (37.9) | |
| Other | 4 (2.6) | 4 (2.6) | |
| Number of non-target lesions | 138 (100) | 137 (100) | 0.799 |
| 0 | 98 (71.0) | 100 (73.0) | |
| 1 | 38 (27.5) | 34 (24.8) | |
| 2 | 2 (1.4) | 3 (2.2) | |
| Mehran type | 152 (100) | 153 (100) | 0.617 |
| I | 25 (16.4) | 31 (20.3) | |
| II | 88 (57.9) | 81 (52.9) | |
| III | 39 (25.7) | 41 (26.8) | |
| IV | 0 (0) | 0 (0) | |
| Values are mean±SD or n (%). The p-value is the difference in the biolimus DCB group compared with the SeQuent Please NEO DCB group. BCB: biolimus-coated balloon; CABG: coronary artery bypass grafting; DCB: drug-coated balloon; MI: myocardial infarction; PCB: paclitaxel-coated balloon; SD: standard deviation | |||
Table 2. Procedural characteristics and results (full analysis set population).
| BCB group (n=138 patients; n=152 lesions) | PCB group (n=137 patients; n=153 lesions) | p-value | |
|---|---|---|---|
| Transradial approach | 146 (96.1) | 141 (92.2) | 0.372 |
| Predilation | 152 (100) | 153 (100) | 0.728 |
| Plain old balloon | 217 (63.6) | 218 (66.7) | |
| Scoring balloon | 54 (15.8) | 49 (14.9) | |
| Cutting balloon | 52 (15.3) | 42 (12.8) | |
| Number of DCBs | 152 (100) | 153 (100) | - |
| 1 | 152 (100) | 153 (100) | |
| 2 | 0 (0) | 0 (0) | |
| Mean diameter of DCB, mm | 3.04±0.41 | 3.01±0.35 | 0.525 |
| Total length of DCB, mm | 25.13±6.63 | 24.38±6.82 | 0.328 |
| Maximum inflation pressure with DCB, atm | 9.20±2.33 | 9.27±2.52 | 0.782 |
| Duration of inflation with DCB, sec | 59.38±8.16 | 60.56±11.49 | 0.302 |
| Bailout strategy | 4 (2.6) | 2 (1.3) | 0.448 |
| Postprocedural TIMI flow | 152 (100) | 153 (100) | - |
| 1 | 0 (0) | 0 (0) | |
| 2 | 0 (0) | 0 (0) | |
| 3 | 152 (100) | 153 (100) | |
| Successful outcomes* | |||
| Device success | 152 (100) | 153 (100) | - |
| Lesion success | 152 (100) | 153 (100) | - |
| Procedural success | 138 (100) | 136 (99.3) | 0.498 |
| Values are n (%) or mean±SD. *Definitions for device, lesion, and procedural success are provided for the prespecified endpoints in the definitions section of Supplementary Appendix 1. BCB: biolimus-coated balloon; DCB: drug-coated balloon; PCB: paclitaxel-coated balloon; SD: standard deviation; TIMI: Thrombolysis in Myocardial Infarction | |||
Table 3. Quantitative coronary angiography results (full analysis set population).
| BCB group | PCB group | p-value | |
|---|---|---|---|
| Preprocedure | n=150 | n=151 | |
| Reference vessel diameter, mm | 2.79±0.40 | 2.80±0.39 | 0.779 |
| Minimal lumen diameter, mm | 0.84±0.35 | 0.87±0.36 | 0.544 |
| Diameter stenosis, % | 69.91±11.05 | 69.11±11.56 | 0.540 |
| Lesion length, mm | 16.26±7.21 | 15.97±6.85 | 0.718 |
| Post-procedure | n=150 | n=151 | |
| Minimal lumen diameter, mm | |||
| In-device | 2.24±0.37 | 2.27±0.35 | 0.375 |
| In-segment | 2.14±0.37 | 2.17±0.34 | 0.557 |
| Diameter stenosis, % | |||
| In-device | 21.94±7.52 | 20.15±7.06 | 0.034 |
| In-segment | 23.24±7.34 | 21.69±6.84 | 0.059 |
| Acute lumen gain, mm | |||
| In-device | 1.39±0.41 | 1.40±0.39 | 0.803 |
| In-segment | 1.30±0.40 | 1.30±0.40 | 0.988 |
| 9-month follow-up | n=125 | n=130 | |
| Minimal lumen diameter, mm | |||
| In-device | 1.96±0.62 | 1.98±0.56 | 0.832 |
| In-segment | 1.87±0.60 | 1.88±0.54 | 0.868 |
| Diameter stenosis, % | |||
| In-device | 31.25±19.31 | 29.54±17.30 | 0.456 |
| In-segment | 32.59±18.78 | 31.61±16.99 | 0.662 |
| Late lumen loss, mm | |||
| In-device | 0.26±0.42 | 0.29±0.40 | 0.579 |
| In-segment | 0.25±0.40 | 0.27±0.39 | 0.670 |
| Net lumen gain†, mm | |||
| In-device | 1.13±0.62 | 1.11±0.55 | 0.772 |
| In-segment | 1.04±0.59 | 1.02±0.54 | 0.720 |
| Binary restenosis, % | |||
| In-device | 17 (11.3) | 20 (13.1) | 0.806 |
| In-segment | 17 (11.3) | 20 (13.1) | 0.806 |
| Values are n (%) or mean±SD. †Net lumen gain was defined as the difference between the minimal lumen diameter at follow-up and baseline. BCB: biolimus-coated balloon; PCB: paclitaxel-coated balloon; SD: standard deviation | |||
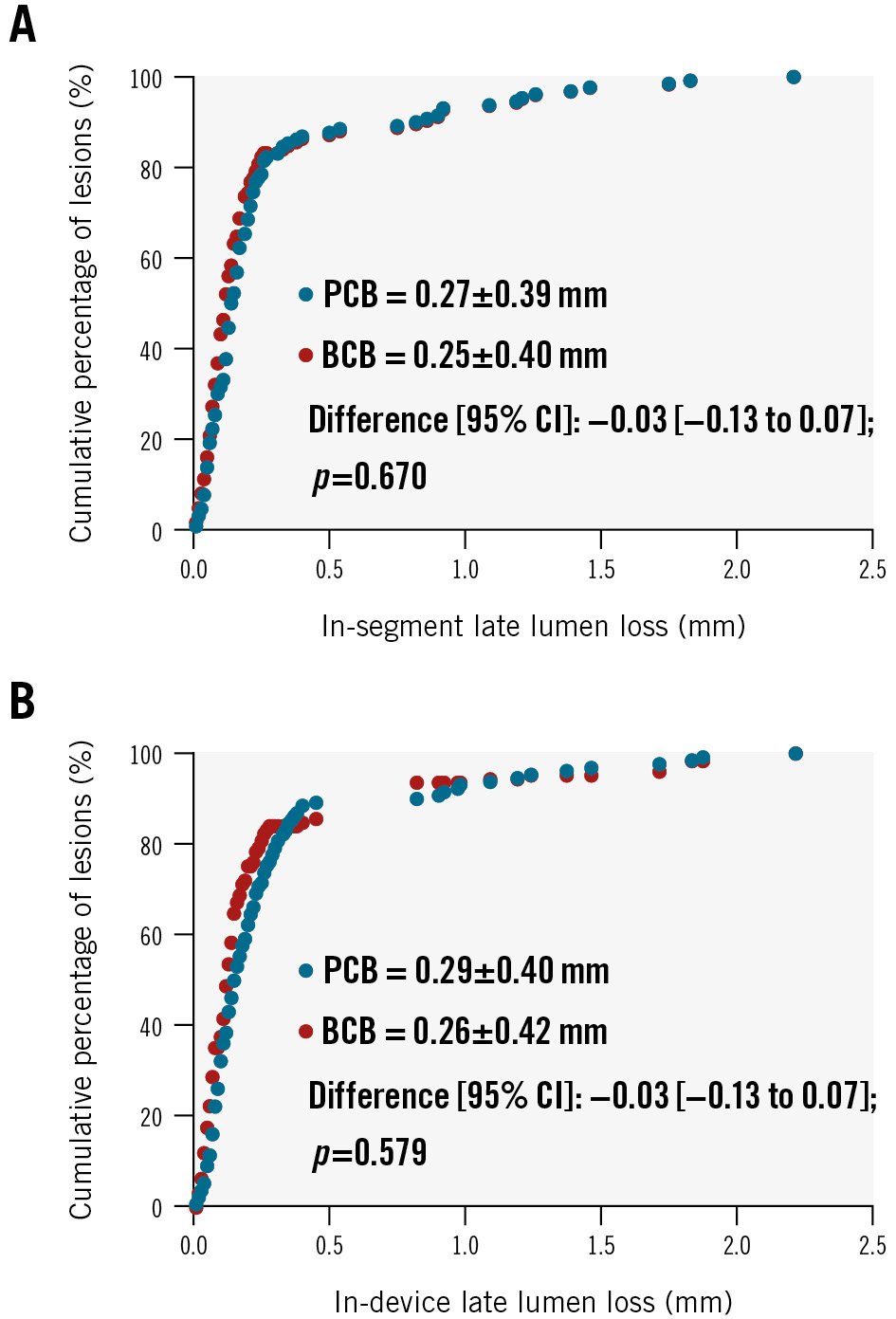
Figure 2. Nine-month in-segment and in-device late lumen loss distribution. Cumulative frequency distribution curves of in-segment (A) and in-device (B) late lumen loss at 9-month angiographic follow-up. BCB: biolimus-coated balloon; CI: confidence interval; PCB: paclitaxel-coated balloon
ANGIOGRAPHIC OUTCOMES
A total of 230 patients (83.6%) underwent the 9-month angiographic follow-up: 114 (82.6%) in the BCB group and 116 (84.7%) in the PCB group. In the per-patient analysis, the in-segment LLL at 9 months was 0.23±0.37 mm in the BCB group compared to 0.25±0.35 mm in the PCB group (p=0.632). The mean difference between the groups was −0.02 (95% CI: −0.12 to 0.07) mm; p<0.0001 for non-inferiority (Central illustration, Supplementary Table 1). The per-lesion analysis revealed an in-segment LLL of 0.25±0.40 mm and 0.27±0.39 mm in the two groups, respectively, with a mean difference of −0.03 (95% CI: −0.13 to 0.07); p<0.001 for non-inferiority. This aligns with the comparison of in-segment LLL per patient. Furthermore, follow-up showed no significant distinctions between the BCB and PCB groups concerning other critical metrics, such as maintenance of lumen diameter, stenosis rates, and the occurrence of binary restenosis (Table 3).
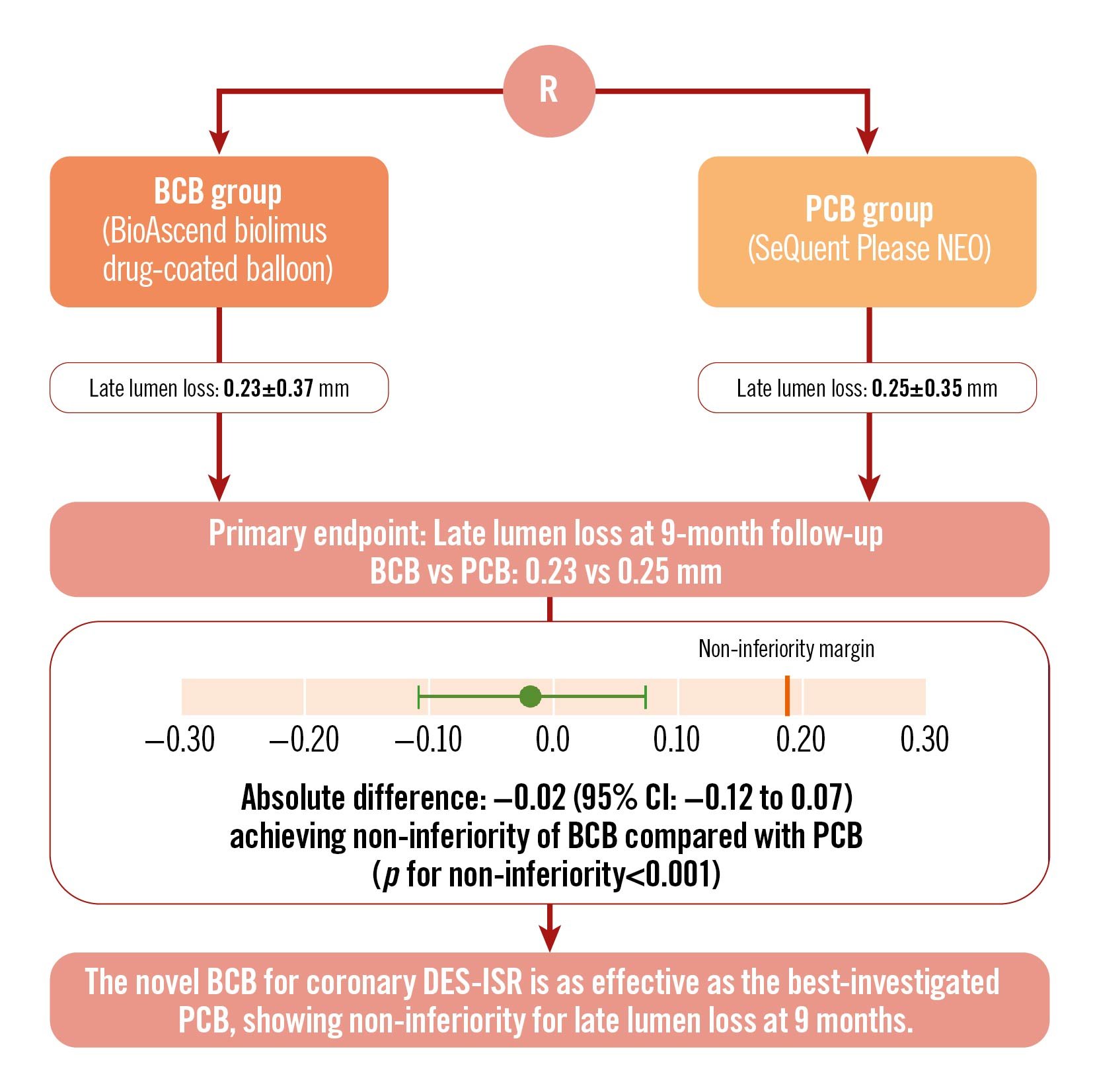
Central illustration. BIO ASCEND ISR study: a prospective, multicentre, non-inferiority trial in patients with coronary in-stent restenosis*. BCB: biolimus-coated balloon; CI: confidence interval; DES-ISR: drug-eluting stent in-stent restenosis; PCB: paclitaxel-coated balloon; R: randomisation
*This is a corrected version of the original illustration that was published ahead of print in May 2024.
CLINICAL OUTCOMES
At 1-month follow-up, the analysis between the BCB and PCB groups showed no significant differences in major clinical outcomes. The incidence of the patient-oriented composite endpoint was equivalent for both groups at 0.7% (p=1.000). Target lesion failure rates were similarly low, with 0% in the BCB group and 0.7% in the PCB group (p=0.498). Rates of myocardial infarction, all-cause death, cardiac death, stent thrombosis, target vessel revascularisation (TVR), and target lesion revascularisation (TLR) were comparable in both groups, suggesting similar safety profiles in the early stage.
At 1-year follow-up, there were no significant differences in the incidence of target lesion failure between the BCB and PCB groups, with rates of 13.3% and 9.5%, respectively (p=0.318). The patient-oriented composite endpoint, with a rate of 23.4% in the BCB group and 14.6% in the PCB group, also indicated no statistically significant difference (p=0.064). Revascularisation rates, including any revascularisation (22.2% for the BCB group vs 13.2% for the PCB group) and TVR (17.0% for the BCB group vs 9.6% for the PCB group), showed no significant differences (p=0.052 for any revascularisation; p=0.068 for TVR). Similarly, there were no statistically significant differences in the rates of all-cause death, cardiac death, or myocardial infarction between the groups, confirming the comparative safety of the treatments during the first year (Table 4, Figure 3).
Table 4. Clinical outcomes in the full analysis set.
| BCB group | PCB group | p-value | |
|---|---|---|---|
| At 1 month* | n=138 | n=137 | |
| Target lesion failure† | 0 (0) | 1 (0.7) | 0.498 |
| Patient-oriented composite endpoint‡ | 1 (0.7) | 1 (0.7) | 1.000 |
| All-cause death | 0 (0) | 0 (0) | - |
| Cardiac death | 0 (0) | 0 (0) | - |
| Myocardial infarction | 0 (0) | 1 (0.7) | 0.498 |
| Target vessel MI | 0 (0) | 1 (0.7) | 0.498 |
| Periprocedural MI | 0 (0) | 1 (0.7) | 0.498 |
| Any revascularisation | 1 (0.7) | 0 (0) | 1.000 |
| TVR | 0 (0) | 0 (0) | - |
| TLR | 0 (0) | 0 (0) | - |
| Stent thrombosis | 0 (0) | 0 (0) | - |
| At 1 year** | n=135 | n=137 | |
| Target lesion failure† | 18 (13.3) | 13 (9.5) | 0.318 |
| Patient-oriented composite endpoint‡ | 32 (23.4) | 20 (14.6) | 0.064 |
| All-cause death | 2 (1.5) | 1 (0.7) | 1.000 |
| Cardiac death | 0 (0) | 1 (0.7) | 1.000 |
| Myocardial infarction | 0 (0) | 1 (0.7) | 1.000 |
| Target vessel MI | 0 (0) | 1 (0.7) | 1.000 |
| Periprocedural MI | 0 (0) | 1 (0.7) | 1.000 |
| Any revascularisation | 30 (22.2) | 18 (13.2) | 0.052 |
| TVR | 23 (17.0) | 13 (9.6) | 0.068 |
| TLR | 18 (13.3) | 11 (8.1) | 0.161 |
| Stent thrombosis | 0 (0) | 1 (0.7) | 1.000 |
| Definite | 0 (0) | 0 (0) | |
| Probable | 0 (0) | 0 (0) | |
| Acute (0-24 h) | 0 (0) | 0 (0) | |
| Subacute (>24 h to 30 days) | 0 (0) | 0 (0) | |
| Late (>30 days to 1 year) | 0 (0) | 1 (0.7) | |
| Values are n (%). *1-month follow-up includes a window of ±7 days; **1-year follow-up includes a window of ±30 days. †Target lesion failure was defined as a composite of cardiac death, target vessel MI, or TLR. ‡Patient-oriented composite endpoint was defined as a composite of all-cause death, all MI, or any revascularisation. BCB: biolimus-coated balloon; MI: myocardial infarction; PCB: paclitaxel-coated balloon; TLR: target lesion revascularisation; TVR: target vessel revascularisation | |||
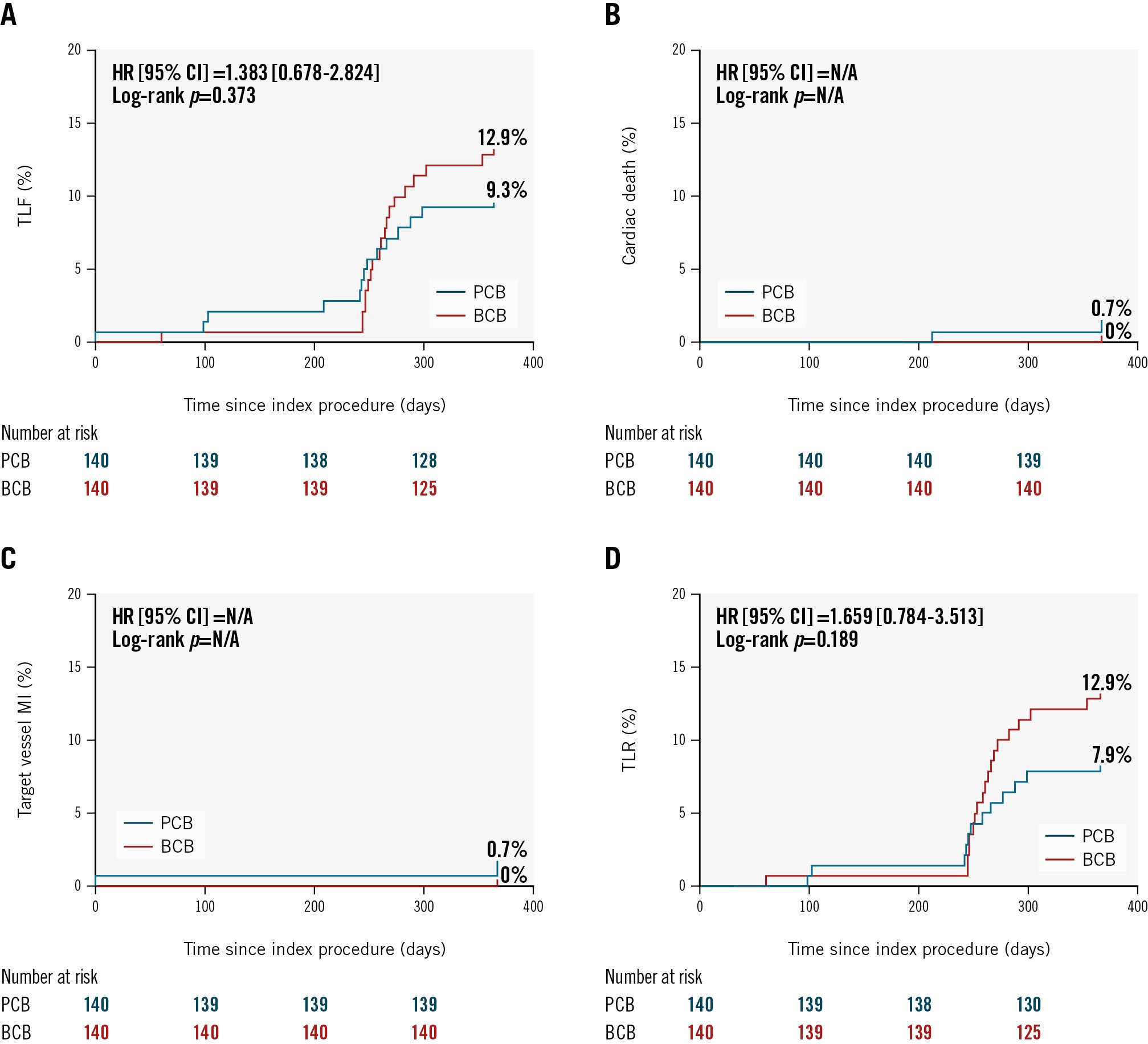
Figure 3. Time-to-event curves for selected clinical endpoints up to 1 year. Kaplan-Meier curves show the cumulative incidence of (A) target lesion failure; (B) cardiac death; (C) target vessel MI; and (D) TLR. BCB: biolimus-coated balloon; CI: confidence interval; HR: hazard ratio; MI: myocardial infarction; N/A: not applicable; PCB: paclitaxel-coated balloon; TLF: target lesion failure; TLR: target lesion revascularisation
OCT SUBSTUDY RESULTS
In the 9-month OCT follow-up of patients treated with either BCB or PCB, data revealed no significant difference in the average neointimal cross-sectional area between the two groups, with values of 2.32±1.04 mm² for the BCB group and 2.37±0.93 mm² for the PCB group (p=0.882). Other parameters, including mean and minimal luminal areas, stent areas, neointimal volume, the number of analysed struts, and uncovered struts, also showed no significant differences between the groups, indicating comparable outcomes for both treatments (Supplementary Table 2).
Discussion
This is a head-to-head randomised controlled trial directly comparing a novel Chinese BCB, utilising optimised biolimus drug crystallisation technology, against a commercially accessible PCB. In patients with coronary DES-ISR, the study demonstrated that (1) a novel BCB (BioAscend biolimus drug-coated balloon) was non-inferior to the PCB (SeQuent Please NEO paclitaxel-coated balloon) in terms of in-segment LLL when treating coronary DES-ISR; (2) the rates of adverse clinical events were similar between both treatment groups with 1-year clinical follow-up, except the rates of TLF and any revascularisation, which were numerically increased in the BCB group; (3) no significant difference in the major secondary endpoint of neointima area at 9 months was observed between the two devices.
DCBs integrate angioplasty with drug-coating techniques to affix antiproliferative drugs onto the surface of the balloon. As a lipophilic drug, paclitaxel rapidly traverses the cell membrane, irreversibly binds to microtubules, and continuously inhibits cell division and proliferative inflammation. It stands as the main clinical DCB coating drug16. In recent years, drugs with a better antiproliferation effect and higher safety (such as rapamycin) have not been applied to DCBs, as they cannot quickly pass through the cell membrane to achieve an effective residence time. Apart from the drug itself, excipients, pharmacokinetics, and interactions with the lesion can also cause different vascular responses6. With advancements in drug formulation and coating technology, rapamycin and its derivatives have emerged as potential candidates for DCBs17. BA9, a modified sirolimus analogue with increased lipophilicity, aims to optimise local drug delivery from stents and balloons. Unlike sirolimus, BA9 is a crystallised drug with less drug loss during delivery and has 10 times more lipophilic solubility than sirolimus. This allows rapid absorption by tissues while minimising exposure loss, resulting in more efficiency in inhibiting endovascular hyperplasia and reduction of late lumen loss.
A prospective trial conducted at 10 centres in China, known as the BIO-RISE CHINA study8, demonstrated that a novel biolimus-coated balloon exhibited superior efficacy to plain old balloon angioplasty in patients with small-vessel coronary disease in terms of LLL. The present study demonstrated no significant difference at 9 months in neointimal formation after treatment of DES-ISR between the BCB investigated here and the PCB counterpart. The specific BCB (biolimus in a dose of 3 μg/mm2 using polyethylene oxide as an excipient) analysed in this study was equivalent to the best-investigated PCB with regard to the angiographic endpoint of DES-ISR.
The in-segment LLL of 0.25±0.40 mm observed at 9 months with the BCB in this trial is consistent with findings from other -limus-coated balloon trials in DES-ISR. The first clinical experiment was reported on 50 patients with ISR treated with sirolimus in a liquid formulation delivered by a porous balloon (SABRE [Sirolimus-eluting Angioplasty Balloon for In-Stent REstenosis] Trial)18. In this patient population, in-segment LLL at 6 months was 0.31±0.52 mm. Scheller et al conducted a joint analysis of two parallel randomised trials comparing sirolimus-coated (SCB) and paclitaxel-coated balloons in coronary in-stent restenosis lesions19. After 6 months, in-segment LLL was 0.25±0.57 mm in the PCB group versus 0.26±0.60 mm in the SCB group. Clinical events up to 12 months did not differ between the groups. It is worth mentioning that the preliminary findings of the randomised REFORM trial (A Prospective, Randomized, Non-Inferiority Trial to Determine the Safety and Efficacy of the BA9TM Drug Coated Balloon for the Treatment of In-Stent Restenosis: First-in-Man Trial), involving 201 patients, were showcased at EuroPCR 202320. This trial revealed that the biolimus A9-coated balloon did not exhibit non-inferiority compared to the paclitaxel-iopromide device. Both the REFORM study and the current study used biolimus DCBs to treat coronary ISR lesions, but their results varied because of several factors. Firstly, the REFORM study included both bare metal stent ISR and DES-ISR patients, while our study only included DES-ISR patients. The varying pathophysiological processes between the two types of lesions may impact the efficacy of DCB treatment. Secondly, the REFORM study had a smaller sample size and a shorter angiographic follow-up period of 6 months, significantly limiting its statistical power. Furthermore, the BA9 drug-coated balloons in the two studies were from different manufacturers, which resulted in differences between the systems’ production quality and manufacturing processes. The excipient on the balloon of both DCBs was exactly the same, but the crystallisation coating process of the two pellets was different. The crystallisation process of BA9 used in this study can obtain more uniform BA9 crystals, which may considerably influence the outcomes.
In the subgroup analysis using OCT, no notable disparity was found in the 9-month neointimal area between the two groups. This finding further supports the conclusion that there was no inferiority in in-segment late lumen loss between the two DCBs. Nevertheless, quantitative coronary angiography analysis revealed that the BCB group exhibited a higher level of percentage stenosis after the procedure. This could potentially be attributed to the higher compliance of the PCB group compared to the BCB group. Consequently, under the same dilation pressure, it is plausible that the PCB group would experience a larger lumen diameter, resulting in a lower degree of postprocedural stenosis.
Although there were no significant differences in the rates of all clinical events, the BCB group exhibited numerically higher rates of the patient-oriented composite endpoint and any revascularisation compared to the PCB group. The observed differences may stem from various factors. The limited sample size undermines robust statistical comparisons of clinical event rates, and the possibility of random occurrences cannot be entirely excluded. Moreover, the potential shorter duration of biological activity associated with -limus compared to paclitaxel may contribute to a late catch-up phenomenon in clinical event rates at the 12-month follow-up21. Therefore, due to the moderate sample size of this study, further research with larger participant cohorts and extended follow-up durations should be conducted to provide a more comprehensive understanding of this matter.
Limitations
Firstly, the study had an insufficient number of patients to detect differences in clinical endpoints, and the completion rate for the 9-month angiographic follow-up fell slightly below expectations (83%). Secondly, there lacked the necessary scale for conducting subgroup analyses, and a significant number of high-risk patients and complex lesions were excluded from participation. Additional research should be carried out to ascertain the efficacy of BCBs in these specific patient populations and lesion types. Thirdly, due to the limited availability of fully mature conditions for conducting OCT examinations across all participating centres, only a small subset of patients completed the OCT assessments, limiting the statistical power of the OCT subgroup data analysis. Finally, the current follow-up period only spanned one year. As per the study protocol, a 3-year clinical follow-up should be conducted to evaluate the long-term prognosis of BCBs in treating DES-ISR. Moreover, it would have been intriguing to consider the emerging paradigm that ISR treatment should be tailored based on its underlying causative mechanism. It should be noted that there was no class effect among DCBs. Therefore, our findings cannot be widely generalised to other -limus-coated DCBs.
Conclusions
This randomised trial has confirmed that the novel BCB for coronary DES-ISR is as effective and safe as the established PCB, showing non-inferiority for 9-month LLL and neointimal area, with no stent thrombosis or myocardial infarction up to 12 months. These results suggest the potential of BCBs to improve clinical outcomes in coronary ISR treatment.
Impact on daily practice
This clinical trial reveals that, for patients dealing with coronary drug-eluting stent in-stent restenosis, the use of a biolimus-coated balloon (BCB) was non-inferior in terms of angiographic outcomes at 9 months compared to a well-established paclitaxel-coated balloon. However, a higher number of revascularisation events were seen in the BCB group at 1 year. Due to the unique pharmacokinetic profile of -limus analogues, long-term follow-up is needed to establish the clinical efficacy of these technologies.
Acknowledgements
The authors thank the patients who participated in the BIO ASCEND ISR study and appreciate the dedicated efforts of the clinical research collaborators in the BIO ASCEND ISR study organisation and the contributions of the participating centres listed in Supplementary Appendix 3.
Funding
Funding for the study was provided by Shandong JW Medical Systems Ltd, China.
Conflict of interest statement
The authors have no conflicts of interest to declare in regard to this manuscript.
Supplementary data
To read the full content of this article, please download the PDF.

