Drug-coated balloons (DCB) represent an established technology aimed at combining the dilatation of obstructive atherosclerotic vascular lesions with the rapid transfer of an antiproliferative drug to avoid neointimal hyperplasia. In contrast to standard drug-eluting stent (DES) technology, DCBs are targeted to provide antirestenotic efficacy in the absence of a persisting vascular prosthesis, consequently avoiding chronic vascular irritation and resultant inflammation. With the mainstream introduction of DCBs in 2006, Bruno Scheller and colleagues were able to demonstrate their antirestenotic efficacy in the clinical setting of coronary in-stent restenosis relative to plain old balloon angioplasty (POBA) in a randomised controlled trial1. Subsequently, a wealth of studies resulted in European guidelines recommending their primary use in this specific clinical setting2. Even today, clinical adoption of their broader application in other coronary lesion-specific settings, such as de novo lesions, bifurcations and small vessel disease, is slow despite the existence of dedicated studies to support their use. In addition, reports about the biological consequences of downstream particulate shedding during the deployment of paclitaxel-coated balloons (PCB) instigated a careful review of the existing literature, and, despite concerns ultimately being resolved regarding the increased mortality associated with the use of PCBs, sustained hesitation fuelled the development of sirolimus-coated balloons (SCB), as the vascular toxicity profile of sirolimus is more benign.
In the current issue of EuroIntervention, Aihara and colleagues report the comparative preclinical outcome of 2 PCBs (AGENT, 2 μg/mm2 paclitaxel [PTX]; Boston Scientific and SeQuent Please NEO, 3 μg/mm2 PTX; B. Braun) and 1 SCB (MagicTouch, 1.25 μg/mm2 sirolimus [SRL]; Concept Medical Inc.)3. A total of 6 devices each were used for the measurement of drug concentrations and an additional 6 devices each for histological evaluation at 28 days. First and foremost, the authors must be congratulated for performing such important mechanistic studies which help us appraise the clinical utility and performance of different DCB technologies (Figure 1). Preclinical studies remain a cornerstone of medical device regulation, as they provide first-line evidence of biological safety in a living organism. Devices should be applied in their intended anatomical location; for DCBs, these may be used in the coronary arteries as well as in the peripheral circulation in animals. In addition, preclinical studies provide an opportunity to deliver a mechanistic understanding of the mode of action, as well as aiding in the assessment of comparative performance under consistent and predictable physiological conditions. Yet the predictive value of preclinical studies to support the clinical antirestenotic efficacy of DCBs remains speculative for now owing to their application in juvenile and healthy arteries, in the absence of human atherosclerotic disease conditions.
Against this background, Aihara et al used healthy Japanese white rabbits to investigate the comparative performance of the 2 PCBs and 1 SCB in the rabbits’ healthy, juvenile peripheral arteries. The results provided greater evidence to support the antirestenotic efficacy of both of the PCBs relative to the SCB, while vascular safety was confirmed in all comparators. The authors utilised established and sound preclinical methodology combining histopathological and pharmacological evaluations of the drugs’ effects. With regards to histology, the loss of medial smooth muscle cells (SMCs) was previously established as a proxy of PTX-induced vascular reactions, where viable SMCs undergo apoptosis and get replaced by an extracellular matrix (proteoglycan deposition) − a phenomenon that can be visualised under the microscope when using specific histopathological stains4. PTX is a highly lipophilic molecule, able to pass through the lipid bilayer of cell membranes, that interferes with cell division by binding to microtubules, stabilising them and subsequently leading to cell death. SRL (an analogue of rapamycin), on the other hand, requires binding to an intracellular protein (FKBP-12), which in complex, results in the inhibition of mammalian target or rapamycin (mTOR), leading to disruption of multiple signalling pathway reactions involved in cell growth and proliferation. As SRL does not directly induce apoptosis, loss of SMCs is expectedly less evident with its vascular application. While both of the PCBs showed greater SMC loss compared to the SCB in the current study, which may suggest more effective drug transfer to the vessel wall, the validity of such a comparison must be questioned, as this preclinical endpoint has never been validated for SCBs to date.
Furthermore, the authors report a higher ratio of non-target to target drug (muscle/artery ratio) persistence for the SCB relative to both the PCBs, which suggests that there is an increased downstream release of sirolimus during deployment and consequent accumulation in muscle tissue; this might result in less effective vascular transfer of the drug and greater loss into non-target organs. This particular ratio, however, has not previously been validated in dedicated preclinical studies and warrants further scrutiny. Along these lines, the authors failed to apply a preclinical model of in-stent restenosis, which would otherwise have allowed direct evaluation of the comparative antirestenotic efficacy of the DCBs tested.
Despite these important limitations, the authors have provided essential insights into the particularities and differences between various DCB-coating formulations. The clinician may ultimately remember these findings when interpreting results from major randomised controlled trials comparing these and other DCB brands in the near future. While the relative merits of SCBs over PCBs remain ambiguous to date, technical progress is evident and will eventually translate into improved patient outcome in clinical practice.
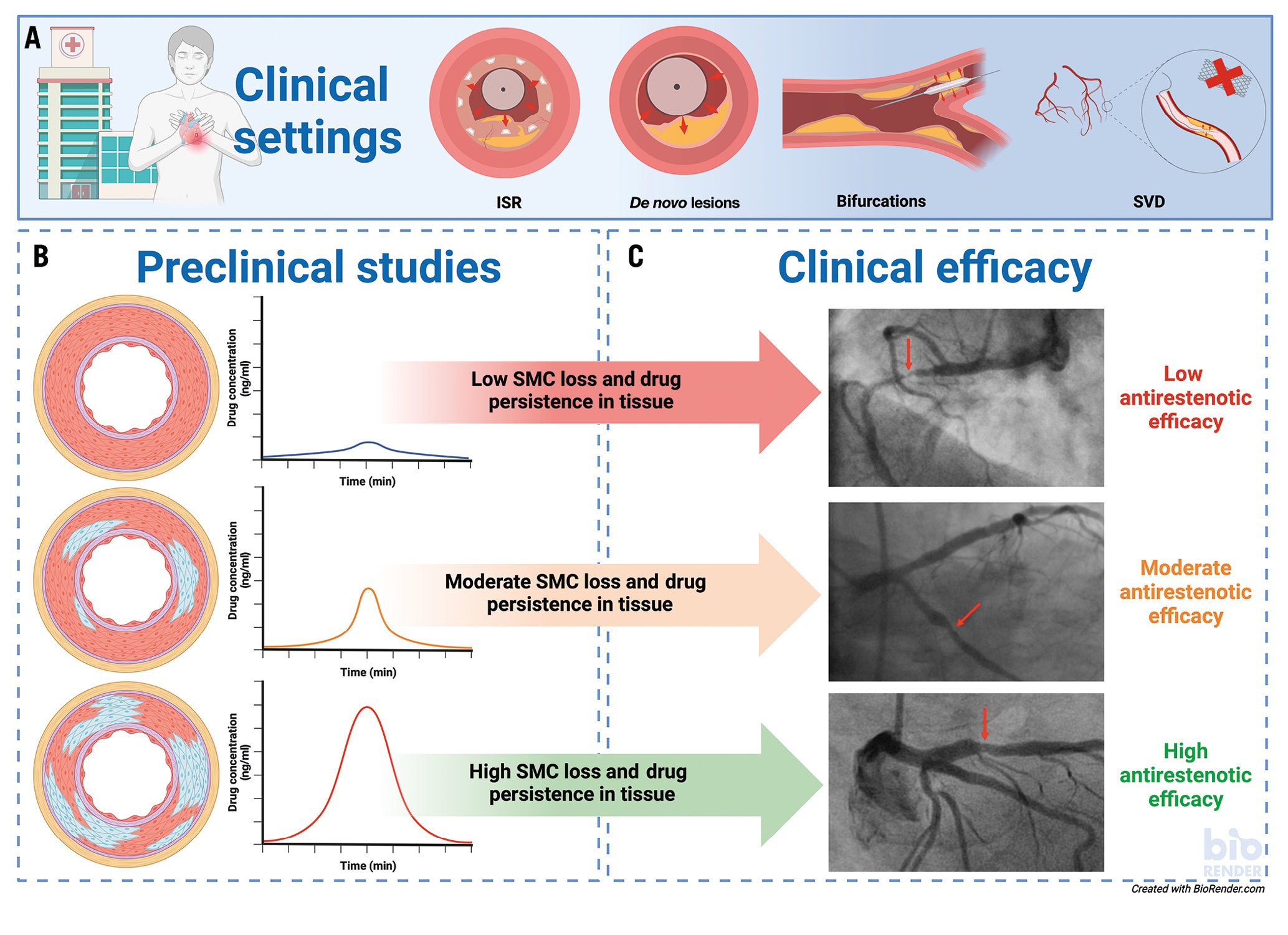
Figure 1. Transferability of preclinical study results into clinical outcome. A) Clinical indications are shown for the use of DCBs. B) Preclinical studies: a schematic overview of the correlation between histopathological findings in the arterial wall (blue areas represent SMC loss in arteries stained with Movat Pentachrome) and drug tissue concentration. C) Clinical efficacy: representative angiographic findings of patients with varying degrees of stenosis. Red arrows indicate the stenotic area. ISR: in-stent restenosis; SMC: medial smooth muscle cells; SVD: small vessel disease
Conflict of interest statement
M. Joner reports personal fees from OrbusNeich, AstraZeneca, and ReCor Medical; grants and personal fees from Biotronik, Boston Scientific, and Edwards Lifesciences; and grant support from Amgen outside the submitted work. He has also received funding from the German Center for Cardiovascular Research (DZHK; FKZ 81Z0600502; FKZ 81X2600526) and from the Leducq Foundation (grant agreement number 18CVD02). L. Wild has no conflicts of interest to declare.

