Abstract
BACKGROUND: Drug-coated balloons (DCBs) are important treatment options for coronary artery disease; however, randomised controlled trials comparing various DCB technologies are sparse, and further investigations are needed.
AIMS: This preclinical study aimed to histologically and biologically compare the drug effects and safety of a low-dose paclitaxel-coated DCB (PCB; AGENT), a regular-dose PCB (SeQuent Please NEO) and a sirolimus-coated DCB (SCB; MagicTouch).
METHODS: The DCBs were inflated in the healthy iliac arteries of 18 rabbits, which were euthanised after 28 days. The treated iliac arteries and distal skeletal muscles were histopathologically evaluated, and drug concentrations were measured.
RESULTS: In the histopathological evaluation, the medial smooth muscle cell loss score regarding depth, an indicator of drug efficacy, was significantly higher with AGENT and SeQuent Please NEO than with MagicTouch (4.0 [3.6-4.0] vs 3.7 [3.7-4.0] vs 2.2 [2.0-2.4]), with significant differences in comparisons between AGENT and MagicTouch (p<0.01) and between SeQuent Please NEO and MagicTouch (p<0.01). AGENT and SeQuent Please NEO showed comparable drug concentrations in the treated artery (p=0.61). In contrast, the drug concentrations in distal skeletal muscles were the highest for MagicTouch, followed by SeQuent Please NEO and AGENT (28.07 [13.19-52.46] ng/mg vs 0.66 [0.22-3.76] ng/mg vs 0.25 [0.04-3.23] ng/mg, respectively).
CONCLUSIONS: This study demonstrated that PCBs might have higher efficacy and lower drug concentrations in distal skeletal muscles than the MagicTouch SCB. The efficacy of the AGENT low-dose PCB and the SeQuent Please NEO regular-dose PCB was comparable.
Drug-coated balloons (DCBs) have been one of the important treatment options for patients with coronary artery disease (CAD) since recent studies demonstrated the non-inferiority of the efficacy of DCBs to drug-eluting stents (DES) for in-stent restenosis (ISR), small vessel disease (SVD) and side branches of bifurcation lesions of coronary arteries123456. Specifically, the evidence for using DCBs in ISR of coronary arteries has been well established, and the latest guidelines of the European Society of Cardiology recommend DCBs for ISR as a Class I treatment7. A study of patients with SVD with a diameter of <3 mm demonstrated that the efficacy of DCBs was comparable to that of DES8910. Moreover, the third report of the International DCB Consensus group recently updated guidelines to include a DCB-only approach as a valid treatment alternative to DES for SVD11. Thus, DCBs have been shown to be effective for ISR and SVD, although their effectiveness for lesions with bifurcation, chronic total occlusion and calcified nodules has not been well evaluated in clinical studies.
Few randomised controlled trials (RCTs) have compared the efficacy of paclitaxel-coated coronary DCBs (PCBs) and sirolimus-coated DCBs (SCBs); these have shown similar efficacy in preventing target lesion revascularisation12131415. However, as the number of patients enrolled in these studies was limited (<100 cases in each group), the differences between the two types of drugs remain unclear. In addition, the endpoints of these studies were based on angiographic assessments, resulting in a lack of data on the biological effects of PCBs and DCBs. Thus, this preclinical study aimed to investigate the drug effects and safety of two CE (European conformity)-marked PCBs (a low-dose PCB [AGENT; Boston Scientific] and a regular-dose PCB [SeQuent Please NEO; B. Braun]) and a CE-marked SCB (MagicTouch; Concept Medical Inc.) histologically and biologically (Central illustration).
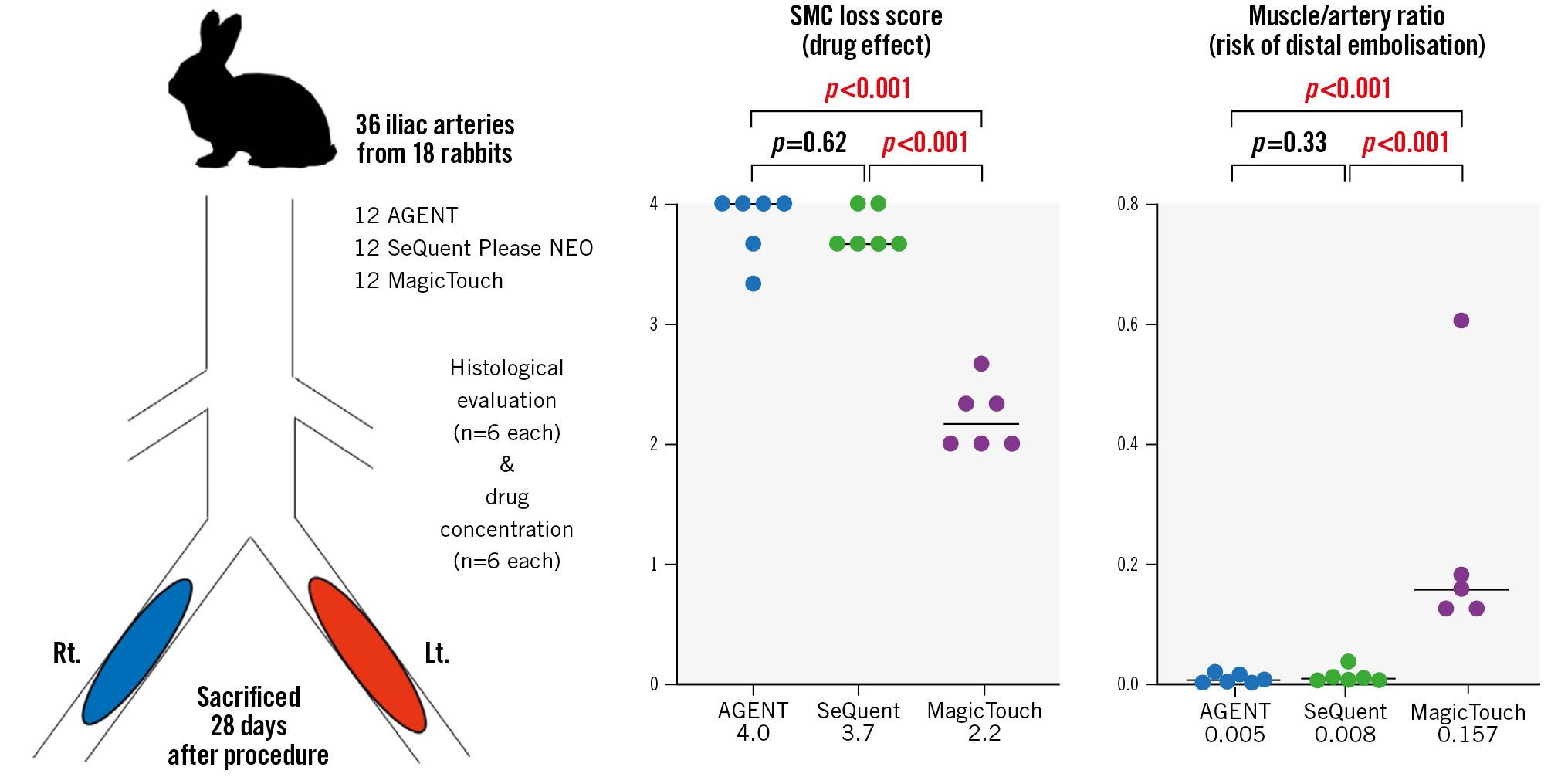
Central illustration. Drug effect and risk of distal embolisation of DCB. PCBs (AGENT and SeQuent Please NEO) have significantly higher drug efficacy and less risk of distal embolisation than SCBs (MagicTouch). DCB: drug-coated balloon; Lt.: left; PCB: paclitaxel-coated balloon; Rt.: right; SCB: sirolimus-coated balloon; SMC: smooth muscle cell
Methods
HEALTHY RABBIT ILIAC ARTERY MODEL
The study protocol was reviewed and approved by the Education and Research Support Center in the Department of Animal Care at Tokai University (Reference no. 222026).
The three DCBs were evaluated in this preclinical study with reference to past preclinical studies161718. Detailed characteristics of each DCB are demonstrated in Table 1. Briefly, AGENT is coated with a low-dose formulation of paclitaxel (2 μg/mm2) blended with an inactive excipient acetyl tri-n-butyl citrate (ATBC)19, and SeQuent Please NEO is coated with a regular-dose formulation of paclitaxel (3 μg/mm2) in an X-ray contrast agent called iopromide11. MagicTouch is an SCB with a specific phospholipid-based carrier and is coated with 1.25 μg/mm2 of sirolimus20.
Eighteen Japanese white rabbits (Tokyo Laboratory Animals Science Co., Tokyo, Japan) were fed with a normal diet and received dual antiplatelet therapy (aspirin and clopidogrel, starting 2 days before the procedure until the day of sacrifice) as previously described18. A total of six AGENT PCBs, six SeQuent Please NEO PCBs and six MagicTouch SCBs in nine rabbits were used for the measurement of drug concentrations. Likewise, six AGENT PCBs, six SeQuent Please NEO PCBs and six MagicTouch SCBs in nine rabbits were used for histological evaluation (Figure 1).
General anaesthesia was induced with isoflurane via an inhalation mask, and surgical access was obtained via the right carotid artery to insert a 5 Fr vascular sheath. After nitroglycerine and heparin injections, angiography of the bilateral iliac arteries and balloon injury was performed using a 3.0×15 mm non-compliant balloon catheter (NC Sprinter RX Balloon Dilatation Catheter; Medtronic) at a nominal pressure by slipping the inflated balloon from the distal femoropopliteal artery to induce endothelial denudation following neointimal proliferation. A 3.0×15 mm DCB was inflated at a target balloon-to-artery overstretch ratio of 1:1.1-1.2. Different DCBs were used for the right and left iliac artery of each rabbit. All DCBs were 3.0×15 mm in size. They were inflated for 30 s in the iliac arteries as previously described16. To document patency, angiography was performed after DCB inflation. After the procedure, the animals recovered in the recovery unit. For a month, the animals were carefully examined at least once a day by laboratory technicians. All animals received appropriate care in compliance with Animal Welfare Act and Public Health Services policies.
Table 1. Characteristics of DCB.
| Company | Drug | Drug dose (μg/mm²) | Additive | Substance class | Formulation | Excipient hydrophilic/hydrophobic | Guiding catheter compatibility | |
|---|---|---|---|---|---|---|---|---|
| AGENT | Boston Scientific | Paclitaxel | 2.0 | ATBC | Plasticiser | Crystalline | Hydrophobic | 5 Fr |
| SeQuent Please NEO | B. Braun | Paclitaxel | 3.0 | Iopromide | X-ray contrast medium | Crystalline | Hydrophilic | 5 Fr |
| MagicTouch | Concept Medical | Sirolimus | 1.25 | − | Phospholipids | Sirolimus encapsulated in phospholipids | Unknown | 6 Fr |
| ATBC: acetyl tri-n-butyl citrate; DCB: drug-coated balloon | ||||||||
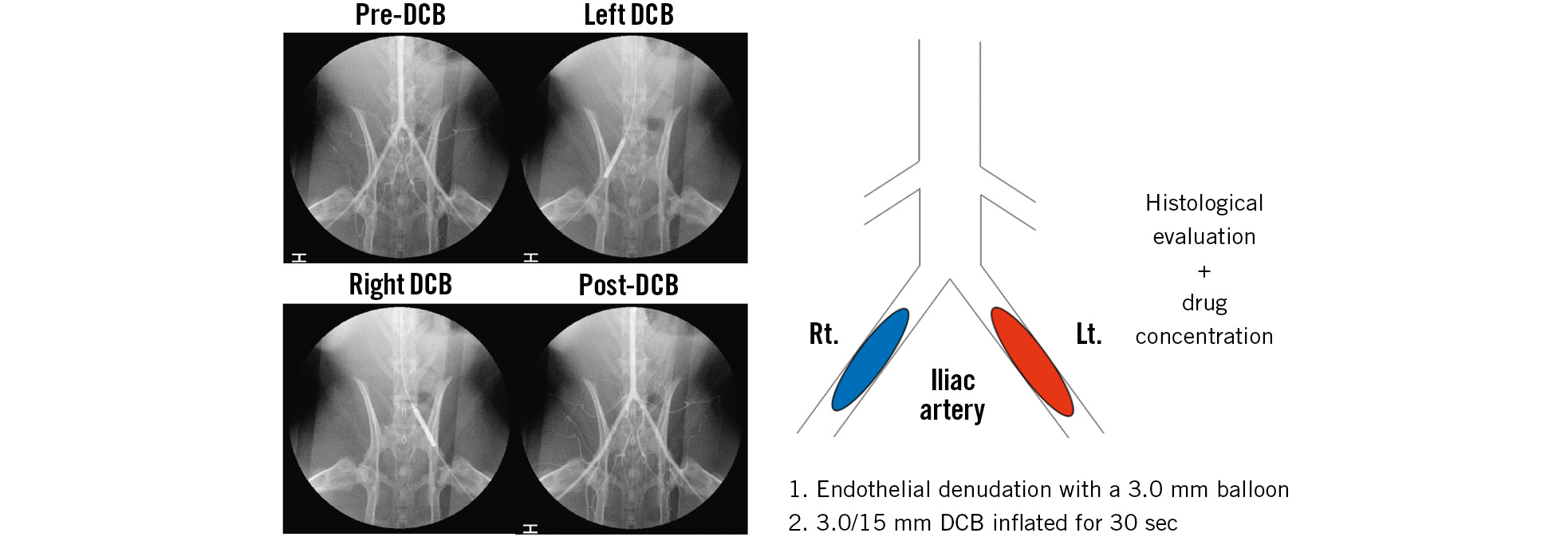
Figure 1. Study protocol schematic diagram and series of angiograms in rabbits. The treated iliac arteries were harvested 28 days after DCB use. Nine rabbits were used for the measurement of drug concentrations and the other nine rabbits for the histological evaluation. DCB: drug-coated balloon; Lt.: left; Rt.: right.
QUANTITATIVE VESSEL ANGIOGRAPHY ANALYSIS OF ILIAC ARTERIES
Angiography of the bilateral iliac arteries was performed at baseline, after DCB use and at harvest (28 days after DCB use). Quantitative vessel angiography analysis (Medis Suite XA, Medis Medical Imaging Systems) of the iliac arteries was also performed.
COLLECTION OF RABBIT ILIAC ARTERIES
Twenty-eight days after the procedure, the rabbits were again anaesthetised with isoflurane via an inhalation mask. A 5 Fr sheath was positioned in the left carotid artery and jugular vein. After nitroglycerine and heparin injections, angiography of the treated iliac arteries was performed to confirm patency. The treated iliac arteries were flushed with 1 L of lactated Ringer’s solution. We determined the exact location of the treated lesion by carefully reviewing angiographic images of the previous procedure, using anatomical landmarks such as the spinal vertebrae and aortoiliac bifurcation; from this, we were able to detect the precise position of the DCB-treated iliac arteries.
HISTOPATHOLOGICAL PREPARATION OF THE TREATED LESION
In the nine rabbits used for histopathological evaluation, the treated iliac arteries were removed, following fixation with 10% formalin perfusion, and divided into proximal, middle and distal segments. Each segment was subsequently subdivided into 4-6 subsegments, which were embedded in paraffin. Histological sections of 4-6 μm thickness were cut on a rotary microtome, mounted on charged slides and stained with haematoxylin and eosin and Movat pentachrome. From each treated iliac artery, the three most affected histological sections were used for histopathological analyses.
COLLECTION OF RABBIT DISTAL SKELETAL MUSCLES
Skeletal muscle samples distal to the treated iliac arteries were obtained from two skeletal muscles below the knee region from both legs, i.e., tibialis anterior and soleus muscles, as previously described1718. The skeletal muscles were fixed with 10% formalin, embedded in paraffin and stained with haematoxylin and eosin and Movat pentachrome.
HISTOLOGY AND HISTOMORPHOMETRY
The histomorphometric analysis of the treated iliac arteries was performed as previously described16182122232425. Briefly, the morphometric analysis was performed with computer-assisted software (ZEN2; Zeiss), and the external elastic lamina, internal elastic lamina and lumen areas were assessed. The percentage area stenosis was calculated as follows: (1–[lumen area/internal elastic lamina area])×100. The histological sections were examined for inflammation, fibrin deposition, calcification, haemorrhage and medial injury, as previously reported18. Biological drug effects were evaluated using the medial smooth muscle cell (SMC) loss score and medial proteoglycan/collagen score, as described in previous animal studies16182526. The SMC loss score is an indicator of DCB drug efficacy and has been used in DCB evaluation studies1626. In this study, two types of SMC loss scores were evaluated: depth of drug penetration and circumference of drug uniformity. The extent of medial SMC loss was semiquantified using a scoring system of 0-4, with 0 indicating none identified; 1, an SMC loss of <25% of the medial circumference; 2, 25%-50% loss; 3, 51%-75% loss; and 4, >75% loss. The medial proteoglycan/collagen score was also semiquantified using a scoring system of 0-4, with 0 indicating none identified; 1, proteoglycan/collagen of <25% of the medial area; 2, 25%-50%; 3, 51%-75%; and 4, >75%. A histological analysis of two downstream skeletal muscles per treated iliac artery was also performed to evaluate safety. One section per skeletal muscle was assessed, and the number of arterioles with DCB-induced changes was evaluated. All histomorphometric analyses were performed with the observer blinded to the treatment group.
BIOANALYSIS OF PACLITAXEL AND SIROLIMUS CONCENTRATIONS IN TREATED LESIONS AND DOWNSTREAM SKELETAL MUSCLES
Bioanalyses of paclitaxel and sirolimus concentrations in treated lesions and downstream skeletal muscles were performed by the technical assistants in the Medical Science College Office and the Support Center for Medical Research and Education of Tokai University, as previously reported18. In each batch analysis, 1 pmol of paclitaxel and sirolimus were analysed as controls. The data analysed in different batches were integrated by normalisation with the signal intensities of these standards. The drug concentrations in the distal skeletal muscles were measured using two sections from two skeletal muscles (tibialis anterior and soleus muscles) per lesion. In addition to drug concentrations in the distal skeletal muscles, the muscle/artery ratio was used to evaluate the safety of DCBs. Paclitaxel and sirolimus have different therapeutic ranges; therefore, comparing drug concentrations only was not sufficient. The muscle/artery ratio is a convenient alternative for comparing DCB safety. The harvested samples of nine rabbits from the same arteries and muscles as the histological samples were homogenised and finally prepared in 100 μL of 75% methanol in ultrapure water. Then, 2 μL of the 10-fold diluted samples (for paclitaxel quantitation) and 4 μL of undiluted samples (for sirolimus quantitation) were subjected to liquid chromatography-tandem mass spectrometry.
STATISTICAL ANALYSIS
Data are presented as mean with standard deviation and median with interquartile range (IQR). The normality of distributions was checked using graphic methods and the Shapiro-Wilk test. Variables with non-parametric distribution were compared by the Kruskal-Wallis test with Steel-Dwass post hoc analysis. The generalised estimating equation (GEE) method was used for histological and biological analyses. Continuous variables were tested using this method with log-linked gamma based on the data distribution. Moreover, categorical data were tested by using the GEE method with the ordinal logistic model. A p-value of <0.05 was considered statistically significant. JMP software (version 16.0; SAS Institute) and GraphPad Prism (GraphPad) were used for statistical analyses.
Results
ANGIOGRAPHIC ANALYSIS OF ILIAC ARTERIES
In this study, quantitative vessel angiography analysis of the iliac arteries was performed. There was no significant difference in the vessel diameter at baseline (AGENT vs SeQuent Please NEO vs MagicTouch: 2.52±0.22 mm vs 2.41±0.38 mm vs 2.38±0.38 mm; p=0.38), after DCB use (2.83±0.14 mm vs 2.79±0.24 mm vs 2.86±0.22 mm; p=0.69) or at harvest, after 28 days of DCB use (2.16±0.46 mm vs 2.22±0.43 mm vs 2.13±0.61 mm; p=0.74) among the three DCBs (Supplementary Table 1).
HISTOMORPHOMETRIC ANALYSES
All animals survived the scheduled 28 days without vessel dissections, ectasia or aneurysms on the angiographic examination before sacrifice. Morphometric analysis of the treated iliac arteries of the three DCBs was performed. The vessel dimensions, such as the external elastic lamina area (AGENT vs SeQuent Please NEO vs MagicTouch: 0.61±0.16 mm2 vs 0.56±0.14 mm2 vs 0.49±0.17 mm2), the internal elastic lamina area (0.48±0.13 mm2 vs 0.44±0.12 mm2 vs 0.37±0.15 mm2) and the neointimal area (0.04±0.03 mm2 vs 0.03±0.03 mm2 vs 0.06±0.03 mm2) were measured. The external and internal elastic lamina areas were significantly greater in AGENT than in MagicTouch (p=0.003 and p=0.003, respectively). The percentage stenosis tended to be higher in MagicTouch than in AGENT and SeQuent Please NEO (9.2% [2.4%-11.7%] vs 3.0% [1.7%-15.2%] vs 16.3% [11.5%-19.5%]; AGENT vs SeQuent Please NEO [p=0.70]; AGENT vs MagicTouch [p=0.13]; SeQuent Please NEO vs MagicTouch [p=0.05]) (Table 2).
Table 2. Morphometric analysis of the treated arteries.
| Parameters | AGENT | SeQuent Please NEO |
MagicTouch | A vs S | A vs M | S vs M |
|---|---|---|---|---|---|---|
| p-value | ||||||
| EEL area, mm2 | 0.61±0.16 | 0.56±0.14 | 0.49±0.17 | 0.45 | 0.03 | 0.37 |
| IEL area, mm2 | 0.48±0.13 | 0.44±0.12 | 0.37±0.15 | 0.54 | 0.03 | 0.24 |
| Lumen area, mm2 | 0.44±0.12 | 0.41±0.12 | 0.31±0.13 | 0.58 | 0.01 | 0.09 |
| Medial area, mm2 | 0.13±0.03 | 0.11±0.02 | 0.12±0.02 | 0.23 | 0.23 | 0.52 |
| Intimal area, mm2 | 0.04±0.03 | 0.03±0.03 | 0.06±0.03 | 0.56 | 0.08 | 0.07 |
| % stenosis, % | 9.2 (2.4-11.7) | 3.0 (1.7-15.2) | 16.3 (11.5-19.5) | 0.70 | 0.13 | 0.05 |
| Data are presented as mean±standard deviation or median (interquartile range). A: AGENT; EEL: external elastic lamina; IEL: internal elastic lamina; M: MagicTouch; S: SeQuent Please NEO | ||||||
BIOLOGICAL ANALYSES OF PACLITAXEL AND SIROLIMUS CONCENTRATIONS
In the DCB-treated iliac arteries, the drug concentrations of the treated lesions were comparable for the three DCBs (AGENT vs SeQuent Please NEO vs MagicTouch, median value [IQR]: 145 [9-517] ng/mg vs 84 [44-531] ng/mg vs 124 [58-365] ng/mg) (Figure 2A).
In the skeletal muscles distal to the treated vessel, the drug concentrations were as follows: AGENT vs SeQuent Please NEO vs MagicTouch: 0.25 [0.04-3.23] ng/mg vs 0.66 [0.22-3.76] ng/mg vs 28.07 [13.19-52.46] ng/mg (Figure 2B). The drug concentrations for AGENT and SeQuent Please NEO were comparable (p=0.53). The muscle/artery ratio of drug concentrations was the highest with MagicTouch, followed by SeQuent Please NEO and AGENT (AGENT vs SeQuent Please NEO vs MagicTouch: 0.005 [0.001-0.015] vs 0.008 [0.005-0.017] vs 0.157 [0.125-0.392], respectively) (Figure 2C). The differences were significant between AGENT and MagicTouch (p<0.001) and between SeQuent Please NEO and MagicTouch (p<0.001).
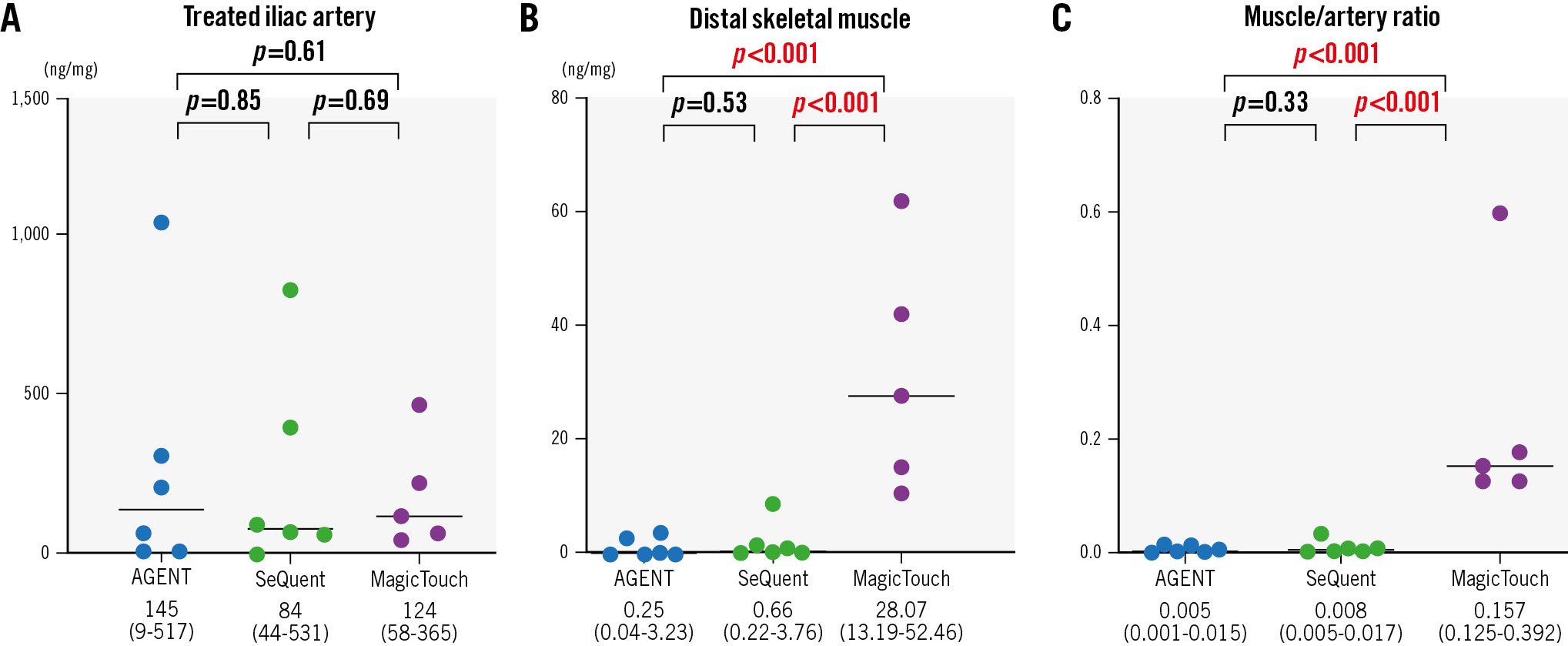
Figure 2. Drug concentrations of the treated lesions and distal skeletal muscles. The drug concentrations in treated lesions and downstream skeletal muscles were measured 28 days after drug-coated balloon use. A) In the treated lesion of the iliac arteries, drug concentrations were comparable among the three drug-coated balloons. B) In skeletal muscles distal to the treated vessel, drug concentrations were comparable in the two paclitaxel-coated balloons. C) The muscle/artery ratio of drug concentrations was the highest in the MagicTouch, followed by SeQuent Please NEO and AGENT.
HISTOPATHOLOGICAL EVALUATION OF THE TREATED ILIAC ARTERIES
In the histopathological evaluation of the treated lesions, the medial SMC loss score regarding depth was significantly higher with AGENT and SeQuent Please NEO than with MagicTouch (4.0 [3.6-4.0] vs 3.7 [3.7-4.0] vs 2.2 [2.0-2.4], respectively) (Figure 3, Figure 4A, Table 3). The difference between AGENT and SeQuent Please NEO was not significant (p=0.62). However, significant differences were found between AGENT and MagicTouch (p<0.001) and between SeQuent Please NEO and MagicTouch (p<0.001). The score regarding the circumferential medial SMC loss was the highest with AGENT and SeQuent Please NEO, followed by MagicTouch (3.0 [2.7-3.0] vs 3.0 [2.9-3.4] vs 1.7 [1.7-2.3], respectively) (Figure 3, Figure 4B, Table 3) with significant differences in the comparisons between AGENT and MagicTouch (p<0.001) and between SeQuent Please NEO and MagicTouch (p<0.001). The medial proteoglycan/collagen score was comparable among the three DCBs (1.5 [0.3-2.2] vs 1.0 [0.3-2.4] vs 0.3 [0.0-0.7]) (Table 3); however, MagicTouch showed a trend towards a lower proteoglycan/collagen score (AGENT vs SeQuent Please NEO [p=0.77]; AGENT vs MagicTouch [p=0.05]; SeQuent Please NEO vs MagicTouch [p=0.08]).
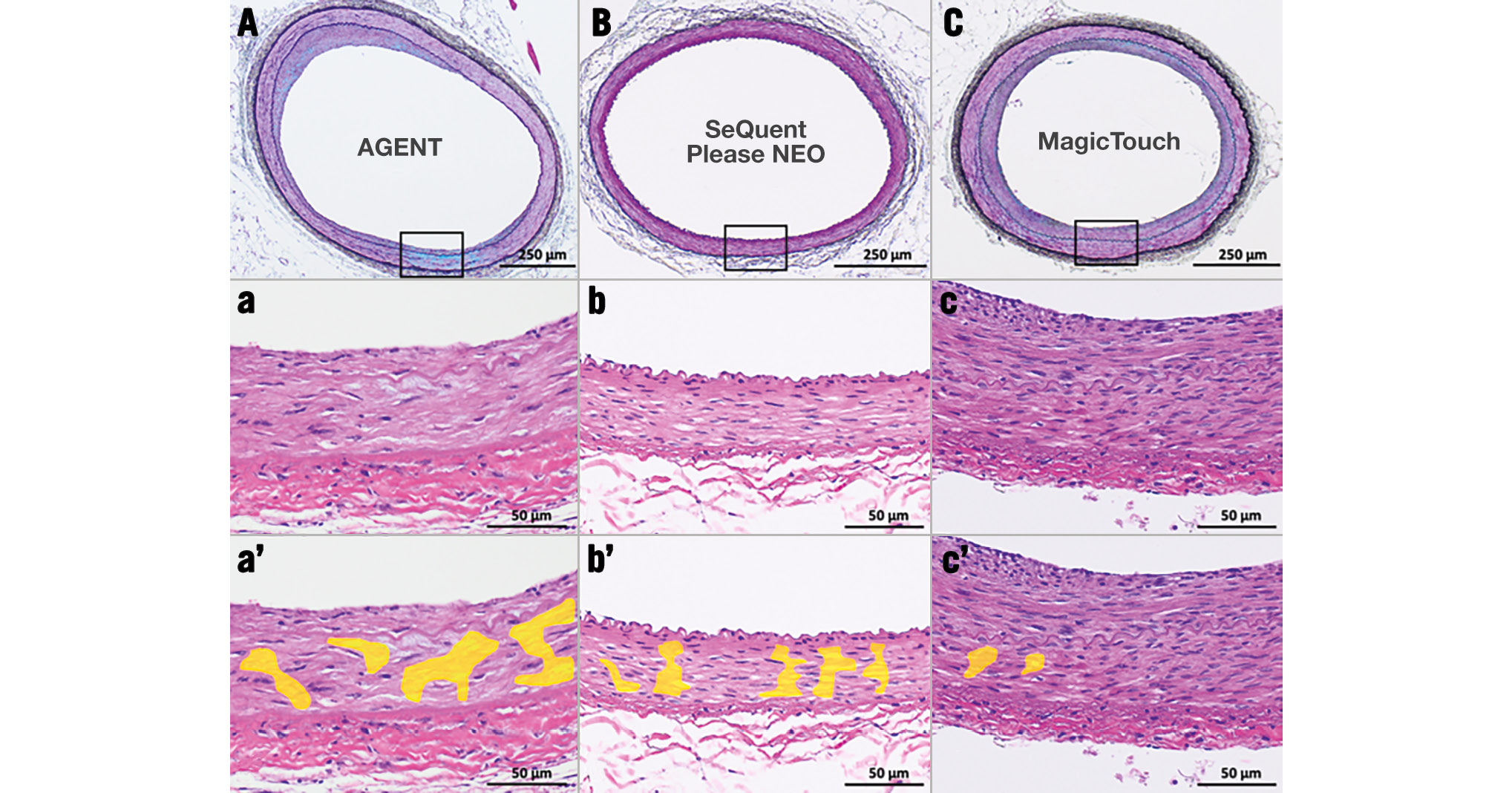
Figure 3. Representative pictures of the treated lesions after drug-coated balloon use. A, B, C) Low-power (Movat pentachrome stain) and a, b, c) high-power (haematoxylin and eosin stain) images of the iliac arteries 28 days after drug-coated balloon use. a’, b’, c’) Areas with smooth muscle cell loss are coloured yellow (haematoxylin and eosin stain).
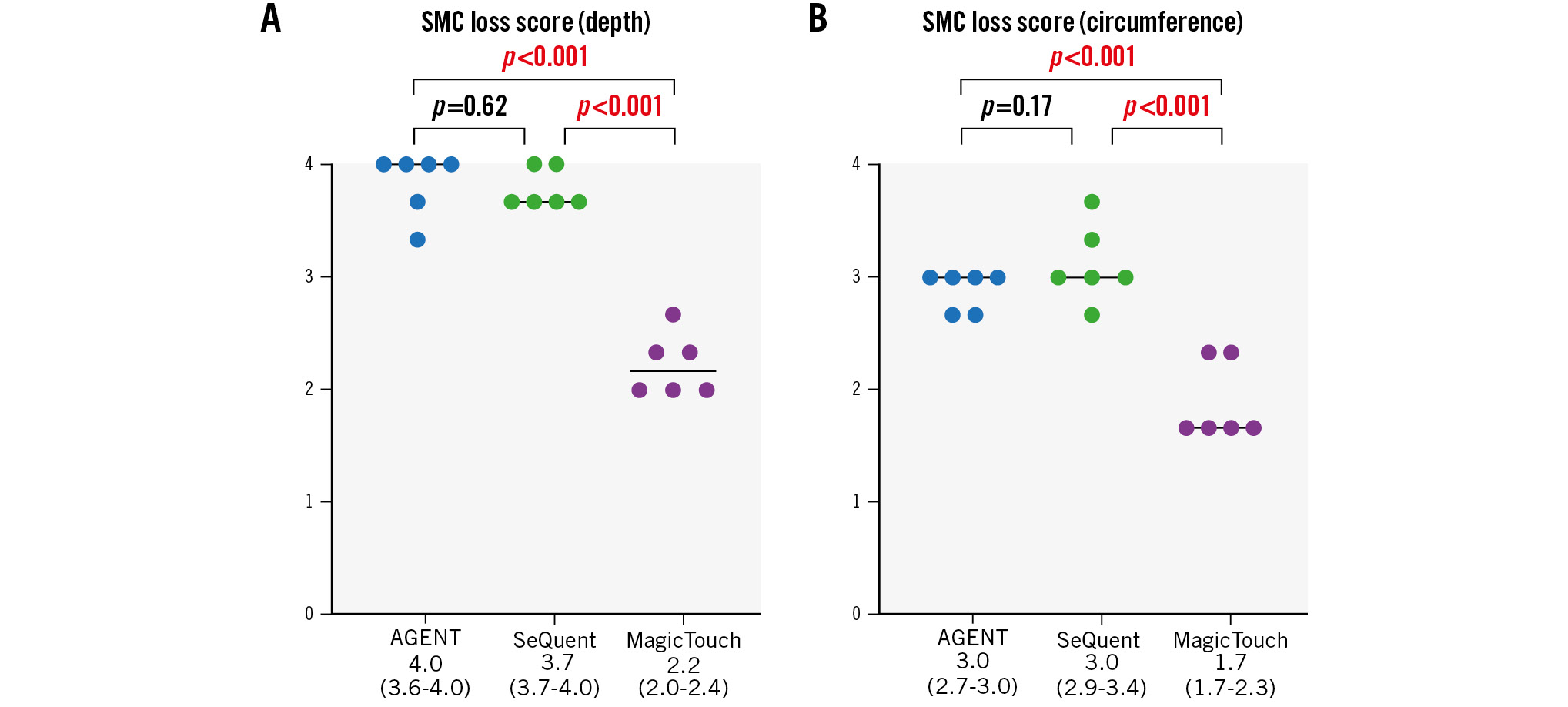
Figure 4. Histological scores of SMC loss (depth and circumference). A) AGENT and SeQuent Please NEO had higher scores for the depth of medial SMC loss than MagicTouch. B) AGENT and SeQuent Please NEO had higher scores for the circumference of medial SMC loss than MagicTouch. SMC: smooth muscle cell
Table 3. Pathological analysis of the treated arteries and distal skeletal muscles.
| Agent |
SeQuent Please NEO |
MagicTouch | A vs S | A vs M | S vs M | |
|---|---|---|---|---|---|---|
| p-value | ||||||
| SMC loss score (depth) | 4.0 (3.6-4.0) | 3.7 (3.7-4.0) | 2.2 (2.0-2.4) | 0.62 | <0.001 | <0.001 |
| SMC loss score (circumference) | 3.0 (2.7-3.0) | 3.0 (2.9-3.4) | 1.7 (1.7-2.3) | 0.17 | <0.001 | <0.001 |
| Fibrin/platelet thrombus score | 0.0 (0.0-0.1) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | NA | NA | NA |
| Neointimal fibrin score | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | NA | NA | NA |
| Medial proteoglycan score | 1.5 (0.3-2.2) | 1.0 (0.3-2.4) | 0.3 (0.0-0.7) | 0.77 | 0.05 | 0.08 |
| Calcification score | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | NA | NA | NA |
| Inflammation score | 1.0 (1.0-1.5) | 1.0 (1.0-1.1) | 1.3 (1.3-1.8) | 0.26 | 0.06 | 0.003 |
| Haemorrhage score | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | NA | NA | NA |
| Medial injury score | 0.0 (0.0-0.0) | 0.0 (0.0-0.3) | 0.0 (0.0-0.0) | NA | NA | NA |
| No. of distal arterioles with changes per section | 0.0 (0.0-0.0) | 0.0 (0.0-1.0) | 0.0 (0.0-1.0) | 0.16 | 0.003 | 0.54 |
| Data are presented as median (interquartile range). A: AGENT; M: MagicTouch; NA: not applicable; S: SeQuent Please NEO; SMC: smooth muscle cell | ||||||
HISTOPATHOLOGICAL EVALUATION OF DISTAL SKELETAL MUSCLES
The histological analysis of the downstream skeletal muscles demonstrated vascular changes in the evaluated arterioles (Figure 5). There was no arterial necrosis or granular change resulting from drug particulate embolisation. The number of arterioles with DCB-induced downstream effects was as follows: AGENT vs SeQuent Please NEO vs MagicTouch: 0.0 (0.0-0.0) vs 0.0 (0.0-1.0) vs 0.0 (0.0-1.0) (Table 3), showing significantly greater values in MagicTouch than in AGENT (p=0.003).
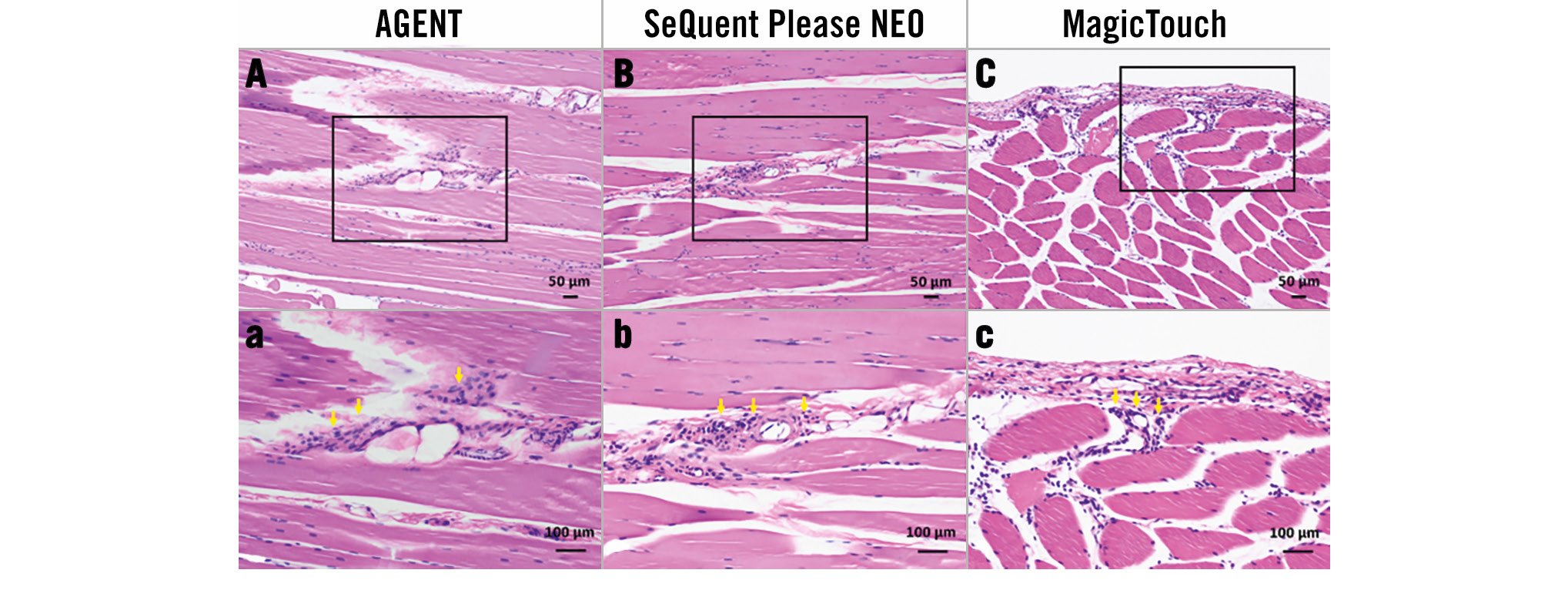
Figure 5. Vasculitis due to distal particulate emboli after drug-coated balloon use. A, B, C) Low-power (haematoxylin and eosin stain) and a, b, c) high-power (haematoxylin and eosin stain) images of distal skeletal muscle arterioles 28 days after drug-coated balloon use. Note the moderate inflammatory cell reaction around arterioles of the distal skeletal muscles (yellow arrows).
Discussion
In this study, the efficacy and safety of two CE-marked PCBs and one CE-marked SCB were evaluated and compared histologically and biologically. This study has some notable findings: 1) The drug concentration and pathologically evaluated drug effect of the treated lesion were comparable in the two PCBs; 2) Histopathologically, the drug effect of the PCBs was distributed more circumferentially and deeply than that of the MagicTouch SCB; 3) The drug concentrations in the distal skeletal muscles were comparable between AGENT and SeQuent Please NEO, whereas the muscle/artery ratio of the drug concentration was significantly higher in the MagicTouch SCB than in the two PCBs, suggesting a higher risk of distal emboli with the MagicTouch SCB than with the PCBs.
Several animal studies have evaluated DCBs in peripheral arteries using healthy swine and rabbit models; however, to the best of our knowledge, this is the first animal study to compare the drug efficacy of the currently available DCBs for coronary arteries. The results from these studies were consistent, and there were significant differences in drug efficacy between the DCBs16171825. Interestingly, the results of several clinical trials to compare the 1-year patency of femoropopliteal arteries after DCB use, which showed that there were significantly different clinical outcomes between the DCBs, have been shown to be in line with the findings of these animal studies2728. Therefore, we believe that animal studies can predict clinical outcomes and help to understand the differences between DCBs.
This study demonstrated the comparable drug effect of the AGENT low-dose (2 μg/mm2) PCB and the regular-dose SeQuent Please NEO (3 μg/mm2) PCB. In a previous RCT, Hamm et al demonstrated the non-inferiority of AGENT to SeQuent Please for the treatment of ISR29. In another RCT, Nakamura et al recently demonstrated that AGENT showed non-inferiority to SeQuent Please and SeQuent Please NEO for patients with SVD measuring <3 mm30. The present study provides histological and biological support for the results of these previous studies. Another preclinical study also demonstrated comparable paclitaxel tissue concentrations at the treatment site with the AGENT low-dose PCB (2 μg/mm2) and the Pantera Lux (BIOTRONIK) regular-dose PCB (3 μg/mm2)31. These two preclinical studies demonstrated that the AGENT low-dose PCB has a comparable drug effect despite a lower paclitaxel loading dose than other regular-dose paclitaxel PCBs. Preclinical studies in peripheral artery disease have suggested the importance of the drug coating integrity rather than the drug dose of DCBs16171825, and the unique ATBC coating of AGENT may contribute to efficient drug transfer.
In the present preclinical study, the PCBs had a deeper and more circumferential distribution than the MagicTouch SCB. Two RCTs have demonstrated that there was no significant difference between the efficacy of the SeQuent Please NEO and the SeQuent SCB (4 μg/mm2) for ISR and de novo coronary disease1415. However, these two previous studies compared two SeQuent Please DCBs made by the same company, and no clinical trials have examined comparisons between other PCBs and SCBs. In the present study, the histopathological evaluation demonstrated that the PCBs had more uniform and deeper drug transfer than the MagicTouch SCB. In addition, the percentage stenosis tended to be higher in the MagicTouch SCB than in the PCBs, and the proteoglycan score, another indicator of drug efficacy, also tended to be higher in the PCBs than in the MagicTouch SCB. These results suggested a higher drug efficacy of the PCBs as compared to that of the MagicTouch SCB. The result might be attributed to paclitaxel being liposoluble, whereas sirolimus is hydrosoluble. This could allow paclitaxel to remain in the target lesion, whereas sirolimus would be more likely to disperse to distal skeletal muscles.
This preclinical study also demonstrated that the muscle/artery ratio of drug concentrations was higher with the MagicTouch SCB than with the PCBs, suggesting a higher risk of distal emboli with the MagicTouch SCB than with the PCBs. On the other hand, there were fewer distal arteriole changes for the PCBs and MagicTouch SCB than those in previous animal studies on peripheral DCBs1618, suggesting that the risk of flow-limiting emboli was low in all three of the DCBs evaluated in the current study. However, the muscle/artery ratio of drug concentrations was the highest with MagicTouch SCB, followed by SeQuent Please NEO and AGENT, suggesting the presence of histologically invisible emboli in the distal skeletal muscles. A previous meta-analysis suggested that DCB use for femoropopliteal lesions in patients with chronic limb-threatening ischaemia resulted in a higher risk of major amputation probably due to distal emboli of the coating drug32. No studies to date have reported adverse events, such as increased periprocedural myocardial infarction in coronary arteries after DCB use, due to the lower drug dose coated on the coronary DCBs than that of the peripheral DCBs. However, in previous clinical studies, myocardial injury markers such as troponin I were not measured after DCB use. Therefore, the effect of distal embolisation on the microvessels of the heart after DCB use in coronary arteries has never been thoroughly analysed. Further clinical studies with a larger number of patients are needed to evaluate the effect of “histologically invisible” distal emboli after DCB use in coronary arteries.
Limitations
This preclinical study has several limitations. First, this study was performed using the iliac arteries of healthy rabbits not including ISR and de novo SVD lesions. As with all other preclinical studies using animals, the rabbit iliac artery model may not fully represent the histological and biological responses of atherosclerotic coronary arteries in humans. However, in daily clinical practice, it is almost impossible to evaluate histological and biological changes after DCB use. In addition, although we have previously performed several animal studies with atherosclerotic rabbit iliac arteries and pig coronary arteries1624, a healthy rabbit iliac artery model was considered to be best for aligning the baseline vessels. Therefore, this model should be an effective alternative for comparing the drug effects of several DCBs. Second, only three DCBs were assessed, and other available DCBs were not evaluated in this study. Further studies are needed to evaluate biological differences among other currently available DCBs. Third, the results of this study might have varied depending on the procedural and individual differences. One of the main causes of the high drug concentration variability might be the time from treatment to sacrifice (28 days) after DCB use. Nevertheless, similar variability has been shown in previous studies evaluating drug concentrations1718. Fourth, blood drug concentrations and the amount of drug lost during DCB usage were not measured in this study. Fifth, we used the SMC loss score of the “media”, not the changes in intimal tissue, to compare the efficacy of DCBs, as described in other animal studies16182526. Moreover, SMC loss might not be adequate to evaluate the drug efficacy of SCB, as sirolimus is not cytotoxic to SMCs, whereas paclitaxel is. Finally, there was a limited number of evaluated samples (6 each). However, as several animal studies performed with a similar number of samples were able to predict clinical outcomes17182124, the number of lesions in this study was assumed to be sufficient to show the characteristics of each DCB.
Conclusions
This study demonstrated that PCBs might have higher efficacy and lower drug concentrations in the distal skeletal muscles than the MagicTouch SCB. The efficacy of the AGENT low-dose PCB and the SeQuent Please NEO regular-dose PCB was comparable.
Impact on daily practice
Drug-coated balloons (DCBs) are important treatment options for coronary artery disease; however, randomised controlled trials comparing various DCB technologies are sparse, and further investigations are needed. This first animal study comparing the histological and biological drug efficacy of the currently available DCBs for coronary arteries demonstrated that paclitaxel-coated DCBs might have higher efficacy and lower drug concentrations in the distal skeletal muscles than the MagicTouch sirolimus-coated DCB. The efficacy of the AGENT low-dose paclitaxel-coated DCB and the SeQuent Please NEO regular-dose paclitaxel-coated DCB was comparable despite the difference in paclitaxel dose, suggesting the importance of the integrity of the drug coating of the DCB rather than the drug dose itself.
Acknowledgements
The authors thank the Medical Science College office and the Support Center for Medical Research and Education, Tokai University, for their technical assistance.
Funding
This study is sponsored by Boston Scientific Japan (Tokyo, Japan).
Conflict of interest statement
S. Torii received research grants from Abbott, Boston Scientific Japan, Medtronic, Asahi Intecc, OrbusNeich, and Japan Medical Device Technology Co. Ltd; and received honoraria from Boston Scientific Japan. G. Nakazawa is a consultant for Boston Scientific, Abbott, Terumo Corp., Asahi Intecc, and Japan Medical Device Technology Co. Ltd; and received research grants from Boston Scientific, Abbott, Terumo Corp., Japan Medical Device Technology Co. Ltd. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

