
Abstract
Aims: The study aimed to assess drug adherence, transfer to the vessel wall, tolerance and efficacy of a constrained angioplasty balloon coated with an excipient-enhanced paclitaxel coating (Chocolate coated balloon [CCB]) in the porcine model.
Methods and results: Drug adherence was investigated in vitro. Drug transfer was evaluated in porcine arteries. A stent overstretch model was chosen to provoke intimal thickening in the efficacy and tolerance study. Conventional uncoated balloons were used as controls. CCB were coated with a nominal (3 µg/mm2) and high dose (two completely overlapping inflations each at 6 µg/mm2) of paclitaxel. Efficacy was assessed by histomorphometry and quantitative coronary angiography (QCA). Tolerance, including potential downstream effects, was assessed by myocardial function and histopathology. The CCB lost 6±12% of dose during in vitro simulated delivery to the lesion; drug transfer to the vessel wall was 14±4%. QCA and histomorphometry revealed no baseline differences between treatment groups. Thirty days after treatment, both doses of the CCB resulted in a 50% reduction in neointimal thickening of arteries relative to the uncoated balloon group. Maximum neointimal thickness was 1.12±0.36 mm for uncoated control specimens and 0.46±0.06 mm and 0.44±0.30 mm for the two CCB doses (3 and 2×6 µg/mm2), respectively. There were no device-related animal deaths or changes in left ventricular ejection fraction or device-specific myocardial histopathologies. There were no statistically significant differences between inflammatory scores among treatment groups.
Conclusions: The results demonstrate efficacy and tolerance of a mechanically unique constrained angioplasty balloon within the tested dose range of the selected paclitaxel coating in the chosen porcine preclinical model.
Introduction
Balloon angioplasty as a revascularisation treatment modality has gained renewed interest with the advent of paclitaxel coatings. Numerous clinical trials have examined the usefulness of treating both de novo lesions, as well as in-stent restenosis, in the coronary and peripheral vasculature1-9. The ability of paclitaxel to persist in the tissue bed and exert its inhibitory influence on smooth muscle cell replication is implicated in its efficacy as a one-time delivery therapy10-13. It is well known that drug-eluting stents drastically reduce restenosis rates compared to bare metal stents and it has been shown that paclitaxel-coated balloons can be used with bare metal stents14-17 if certain limitations are observed. The efficacy of one-time paclitaxel delivery by an angioplasty balloon suggests that long-term elution may not be necessary18,19 and may obviate undesired complications from permanent implants such as late stent thrombosis. However, drug coating was only available over the smooth cylindrical surface of regular balloons.
The Chocolate® balloon (TriReme Medical Inc., Pleasanton, CA, USA) is the first and only drug-coated, CE-marked, percutaneous transluminal angioplasty (PTA) balloon catheter that differs from standard-shaped balloon catheters. It exhibits an outer nitinol constraining structure (Figure 1). This constraining structure is designed to distribute the balloon forces more uniformly than standard angioplasty balloons, thereby offering potential benefits in reducing therapy-associated injury. Interim results from CHOCOLATE BAR, a prospective core lab-adjudicated registry conducted in 33 clinical centres within the USA, report high rates of success and limb preservation in patients with peripheral arterial disease when uncoated Chocolate PTA balloon angioplasty was employed. The acute benefits of the Chocolate platform may be maintained in the drug-coated version. A single-arm, core lab-adjudicated, multicentre clinical trial of the coated Chocolate balloon had no flow-limiting dissections and less than 1.5% bail-out stenting in 70 lesions. The constraining structure on the device may protect the coated balloon during catheter delivery and minimise premature unfolding of the balloon.
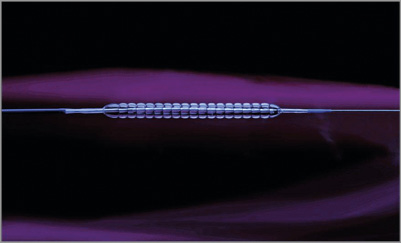
Figure 1. The Chocolate angioplasty balloon, demonstrating the surface topography formed by the outer nitinol-constraining structure with an inflated balloon. The constraining structure results in the formation of multiple balloon “pillows”.
The present study was undertaken to evaluate the tolerance and efficacy of a nitinol constraining structure covered balloon with a novel paclitaxel coating (Chocolate coated balloon [CCB]) in porcine coronary arteries at 30 days. In order to provoke intimal thickening, an overstretch stent model was employed. The anticipated clinical dose (3 µg/mm2) was evaluated as well as a fourfold dose (2×6 µg/mm2).
Materials and methods
BALLOON CATHETERS AND COATING OF BALLOONS
For the coronary studies, Chocolate balloons (3.5×20 mm) were coated with 3 µg/mm2 and 6 µg/mm2 of a paclitaxel coating containing a proprietary excipient. Uncoated standard PTCA catheters (3.5×20 mm, Maverick™ 2; Boston Scientific, Marlborough, MA, USA), were used as negative controls.
For the peripheral studies, Chocolate balloons (6.0×40 mm) were coated with 3 µg/mm2 of paclitaxel coating containing a proprietary excipient. Commercially available paclitaxel urea-coated balloons (IN.PACT Pacific; Medtronic, Dublin, Ireland) of the same size (6.0×40 mm) were used as positive control.
CCB were sterilised with ethylene oxide and commercial products were obtained in their original sterile packaging.
ADHERENCE TO THE BALLOON DURING PASSAGE THROUGH A HAEMOSTATIC VALVE AND GUIDING CATHETER
Balloons were introduced through a haemostatic valve with on/off closure (Medex Medical, Klein-Winternheim, Germany) and a blood-filled guiding catheter (Launcher® JL 3.5, 6 Fr; Medtronic) into a vial filled with blood, at 37°C with constant stirring. After the passage of the guiding catheter, the balloons remained in the blood for one minute, were removed from the blood without retraction into the guiding catheter, and placed in an empty vial, inflated and cut off the shaft. The samples were prepared and analysed as described below.
IN VIVO EXPERIMENTS
All animal experiments and housing were conducted in accordance with the guidelines for animal experiments set forth by the animal protection committee of the Sachsen Anhalt government, Germany. Anaesthesia of the animals and the interventional procedure, except for the sequence of balloon dilatation and stenting, have been described previously20,21. Briefly, conventional farm pigs (castrated males) approximately three months old and weighing 23-32 kg were used in the coronary and peripheral studies.
Oxygen, heart rate, and electrocardiography were continuously monitored during the interventional procedures and assessed at follow-up. Two days prior to angioplasty and every day following the interventional procedure, 75 mg of clopidogrel (Plavix™; Sanofi Winthrop, Frankfurt, Germany) and 100 mg acetylsalicylic acid (ASS; Ratiopharm, Ulm, Germany) was administered orally. Long-acting verapamil hydrochloride (VeraHexal™ 120 mg; Hexal AG, Holzkirchen, Germany) was given 24 hours in advance of the procedure to prevent vascular spasm during the intervention.
The pigs underwent baseline angiography via carotid arterial access. All devices were delivered via the carotid artery through 6 Fr or 8 Fr guiding catheters. A 0.014” guidewire was positioned in the left or right coronary system, as well in an external or internal femoral artery of appropriate size. The test CCB and control balloons were advanced over the guidewire and positioned at the desired locations.
DRUG TRANSFER STUDIES – CORONARY AND PERIPHERAL ARTERIES
To identify unequivocally the treated vessel segments in the drug-transfer studies, short stainless steel marker stents mounted on uncoated balloons were implanted distal to the selected vessel segment prior to angioplasty. With the drug-coated balloons a vessel overstretch of 1.1-1.2 was obtained. Inflations in coronary arteries were performed for 60 sec, and in peripheral arteries for 120 sec. After deflation, catheters were closely inspected upon removal from the animal and transferred to vials for evaluation of the paclitaxel remaining on the catheters.
EFFICACY AND TOLERANCE STUDIES – CORONARY ARTERIES
In the coronary efficacy and tolerance studies, the left ramus circumflex (CX) and the left anterior descending artery (LAD) of each pig were treated according to the group assignment applying 6-14 atm inflation pressure resulting in an overstretch of 1.1-1.2 versus the reference diameter of the vessel. The duration of each inflation was 60 seconds. To enhance neointimal proliferation, stainless steel stents (3.5×16 mm) pre-mounted on uncoated standard balloon catheters (OMEGA™; Boston Scientific) were implanted in all treatment groups immediately after angioplasty in the location identical to the balloon inflations using anatomical landmarks. The length of the stents was chosen to be less than the total treated length to avoid injury where no drug had been deposited.
POST PROCEDURE
At predetermined time points (20 min, 24 hours, 30 days), follow-up measurements including blood sampling were completed. The animals were euthanised under anaesthesia. Necropsy with gross inspection of the major organs was performed. All deployment locations identified and excised for analytical chemistry evaluation or the arteries were irrigated with saline and arteries fixed with 10% neutral buffered formalin at 100 mmHg.
QUANTIFICATION OF PACLITAXEL
SAMPLE PREPARATION AND QUANTIFICATION OF PACLITAXEL
A defined volume of ethanol (>96%) was added to both the balloon and tissue samples. The vials with balloons were closed and intensely shaken, followed by treatment in an ultrasound bath for 30 minutes and centrifugation. The tissue samples were homogenised (Precellys® 24-Dual Homogenizer; PEQLAB Biotechnologie GmbH, Erlangen, Germany), thereafter treated for 30 min with ultrasound at room temperature and then centrifuged for 10 minutes. Blood samples for pharmacokinetic analysis were collected in ethylenediamine tetraacetic acid (EDTA) containing tubes prior to the treatment in all animals and in all surviving animals at 20 min, 24 hours, and at 30 days post treatment, centrifuged, and frozen until analysis.
MEASUREMENT OF PACLITAXEL
Paclitaxel was determined by HPLC using UV detection. Column: Waters Symmetry, C18, 5-μm, 25 cm×4.6 mm. Mobile phase: 45% phosphate buffer 0.005 M (pH 3.5) and 55% acetonitrile, 1 ml/min. Detection: 230 nm. For tissue samples, the same column and detection method was used but the mobile phase was changed to 55% phosphate buffer 0.005 M (pH 3.5) and 45% acetonitrile. Paclitaxel in the plasma was quantified by extraction and LC-MS-MS using a validated method by a third-party laboratory (BASi, West Lafayette, IN, USA).
QUANTITATIVE ANGIOGRAPHIC ANALYSIS
Quantitative coronary angiography (QCA) was performed using AXIOM Artis zee fluoroscopy (Siemens, Erlangen, Germany): pre-treatment (native vessel), study treatment images with inflated balloons, stent implantation images with inflated balloons, and final results after treatment of both arteries and at angiographic follow-up. Global left ventricular ejection fraction was assessed prior to the vascular treatments and at the one-month follow-up. Clinical observation of all animals was conducted daily, and animal weight was measured at baseline and follow-up.
The CAAS II System (Pie Medical, Maastricht, the Netherlands) was used for quantitative coronary and peripheral artery analysis by experienced observers blinded to the treatment groups. Left ventricular ejection fraction (LVEF) was quantified using the Siemens module (AXIOM Artis dBC; Siemens).
HISTOMORPHOMETRY AND PATHOLOGY
The target segments were embedded in methyl-methacrylate (Heraeus Kulzer, Wehrheim, Germany). Two in-stent sections and segments proximal and distal to the stent were stained by haematoxylin and eosin (H&E), and one in-stent section was stained by Masson Goldner. Histomorphometric measurements were taken using the “NIS BR 3.0” image programme (Nikon Corporation, Tokyo, Japan). Luminal diameter, external elastic lamina (EEL) diameter (vessel diameter), maximal neointimal thickness, EEL area (vessel area), luminal area, and neointimal area were evaluated. Semi-quantitative histopathology assessments for injury and inflammation were conducted according to Schwartz22 and Kornowski23.
The hearts were sectioned in the left ventricle close to the septum, at the apex and in the right ventricle close to the right coronary artery. The slices were examined for gross and microscopic abnormalities.
STATISTICAL ANALYSIS
Histomorphometric variables of the three cross-sectional planes within the stent were averaged to obtain a mean value per treated segment. Angiographic and histomorphometric continuous variables were compared by ANOVA analysis or Student’s t-test using the software package SPSS for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA). All data are presented as mean values±standard deviation.
Results
DRUG ADHERENCE
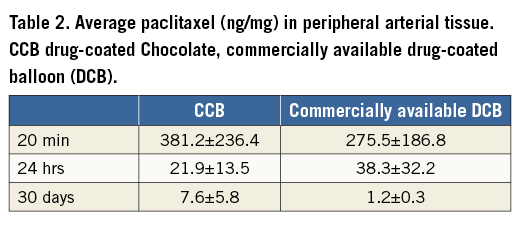
The adherence of the drug on the way to the treatment site was simulated in vitro. Drug loss was measured as the percent difference between the drug content of the original CCB-coated devices and the devices after passing through a blood-filled haemostatic valve into a guiding catheter and floating in blood (Table 1). The mean drug loss of eight CCB catheters evaluated in two experiments was 6±12%.
In the coronary arteries, drug transfer to the vessel wall in vivo was 14±4% (n=10) of the dose and 17±3% (n=10) remaining on the balloons after deflation and retraction (Table 1). In the peripheral arteries, an average of 23±6% of paclitaxel remained on the CCB catheters (n=19).
PERIPHERAL TISSUE KINETICS STUDY
Twelve animals allowed a total of thirty-one deployments in the peripheral arteries, twelve control and nineteen test articles. All devices were successfully deployed with no device malfunction.
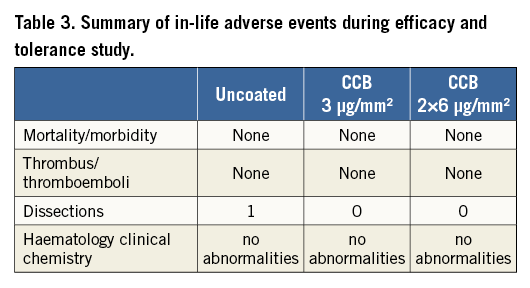
The kinetics of the CCB demonstrated a biphasic mechanism, as reported in the literature for commercially available drug-coated balloon products. The mean therapeutic dose tissue concentration at 20 min post final inflation was Cmax=381±236 ng/mg for the CCB and Cmax=276±187 ng/mg for the positive control DCB. Initial clearance of paclitaxel seemed to be faster for the CCB product, with slower clearance in the second phase up to 30 days. The results of the arterial tissue pharmacokinetics are displayed in Table 2 for the CCB and the positive control DCB.
The clinical dose plasma concentration for the CCB was Cmax=1.93 ng/mL at 20 min after deployment of two to three balloons in swine of 23-32 kg, dropping to below the limit of quantitation (0.3 ng/ml) at the next time point (24 hours).
CORONARY EFFICACY AND TOLERANCE STUDY
GENERAL OBSERVATIONS
There were no device failures or device-related animal morbidity or mortality in the study. All animals exhibited normal weight gain and there were no device-related findings discovered at necropsy. No target-site thrombi or thromboemboli were observed in any of the paclitaxel treatment groups (Table 3). There was a total absence of vasospasm observed in the high dose CCB treatment group. One small arterial dissection was observed in the uncoated balloon cohort. There were no significant differences in the haematological or clinical chemistry profiles from pre-treatment to follow-up (30 days) for any of the groups. The change in left ventricular ejection fraction was small between baseline and follow-up and consistent across all treatment groups (Table 4). The electrocardiograms (obtained before treatment, immediately post treatment, and at 30-day follow-up) remained stable in the majority of animals. The few animals that did show mild abnormalities, beyond the expected short-lasting reaction to ischaemia during balloon inflation, were equally spread across all treatment groups. Blood pressure (taken before treatment, post treatment and at 30-day follow-up) dropped during the procedure by approximately 40 mmHg due to anaesthesia and the injection of intracoronary nitroglycerine but did not change from before treatment to follow-up in all treatment groups.
QUANTITATIVE CORONARY ANGIOGRAPHY
In-stent diameter stenosis at 30 days was 56% in the uncoated balloon treatment group compared to only 6% to 7% for both paclitaxel balloon-treated groups (p=0.001) (Table 5). There were no statistically significant differences between the paclitaxel-treated groups (3 µg/mm2 CCB, and 2×6 µg/mm2 CCB). The results demonstrate that paclitaxel delivered by CCB suppressed neointimal thickening compared to uncoated balloons.
HISTOMORPHOMETRY
Histomorphometric measurements confirmed and supported the findings of QCA. The paclitaxel-treated groups exhibited approximately half the neointimal area observed in the uncoated balloon group (Table 6). Stent diameters were statistically equivalent across all groups (coated and uncoated treatments) and all histomorphometric parameters reflected the inhibition of neointimal proliferation in the paclitaxel-treated groups, i.e., lumen diameter, maximal neointimal thickness, lumen area, neointimal area, diameter stenosis, and area stenosis. There were no statistically significant differences in neointimal proliferation between the paclitaxel-treated groups. Visual inspection of low power magnification histology images demonstrated obvious neointimal suppression in the paclitaxel-treated groups relative to uncoated balloon treatments (Figure 2).
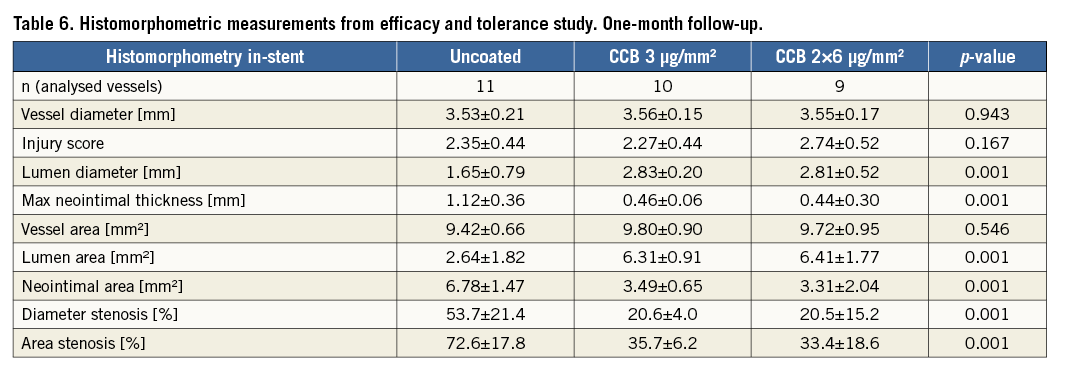
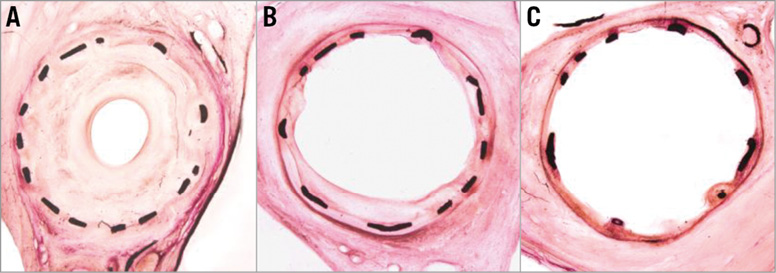
Figure 2. Low magnification images of uncoated (A), CCB 3 µg/mm2 (B), and CCB 2×6 µg/mm2 (C) balloon treatments of stented arteries demonstrating neointimal suppression at 30 days. All specimens were left anterior descending coronary arteries and stained with H&E.
HISTOPATHOLOGY
Treated arteries were scored for injury and inflammation across three sections (proximal, mid and distal stent) and averaged. The injury score reflects mechanical damage due to the intended overstretch of the arteries. The multi-inflation, high-dose paclitaxel CCB group exhibited a higher injury score than the 3 µg/mm2 CCB group (p=0.046), which was expected due to multiple balloon inflations at the same site. No statistically significant differences were observed among any of the groups for inflammation.
DOWNSTREAM HISTOPATHOLOGY: MYOCARDIUM
Myocardial specimens were evaluated for haemorrhage, degeneration, necrosis, inflammation, fibrosis, vasculitis, thrombosis, endocarditis, and epicarditis. Histopathological findings were observed in seven of 11 animals of the uncoated group, three of five animals in the 3 µg/mm2 CCB group, and five of five animals in the (2x) 6 µg/mm2 CCB group. Findings in the CCB drug-coated groups were limited to inflammation, vasculitis and fibrosis ranging from mild to moderate in severity. The incidence of findings tended to increase at the high dose level. Non-occluding thrombi were observed in two hearts downstream of the treatment site in the uncoated balloon group, and one heart from the uncoated group exhibited extensive fibrosis. No thrombi or signs of ischaemia or necrosis were observed in any hearts of the paclitaxel-treated animals. Overall, the incidence and quality of findings in the uncoated balloon group was similar to that in the paclitaxel-treated groups, including the high dose.
Discussion
Paclitaxel balloon angioplasty is proving to be a viable therapeutic approach for revascularisation of stenosed arteries1-9. It can also be used as an adjunct to bare metal stenting to provide inhibition of restenosis. Whether the drug-coated balloon was used first or last in the procedure had no impact in one published report24. So far, only positive randomised clinical trials have been published for paclitaxel-coated balloons. It remains unclear if drugs other than paclitaxel (e.g., zotarolimus25 or sirolimus21) will be clinically efficacious when applied locally by coated balloon catheters.
In the present study, a paclitaxel coating on an angioplasty system utilising a nitinol constraining structure over the balloon was investigated. Bench top studies indicate that the superficial constraining structure protects the drug coating during balloon transit through the catheter and prevents premature loosening of balloon folds, thereby minimising drug loss prior to delivery. Furthermore, the balloon surface forms pillows upon inflation with a three-dimensional geometry that is different from a conventional balloon. This results in a larger surface area, being approximately 20% greater than a conventional balloon without a constraining nitinol structure.
Clinical results for the non-drug-coated and drug-coated Chocolate balloon also demonstrate that the nitinol-constraining structure results in fewer flow-limiting arterial dissections and resultant bail-out stents, clinical events that remain with standard balloon use and drug-coated balloon use. Computer simulations indicate that the constraining structure distributes the forces more uniformly across the target site, perhaps explaining the reduction in dissections. Although not at all conclusive, the only dissection observed in these studies occurred in the standard balloon cohort.
Drug transfer in these studies was high and reliable when compared to studies in the same animal model20,26 for the pillow and groove balloon membrane configuration of the CCB. In the coronary porcine model, our results compare well to published data on paclitaxel urea-coated balloons13. The variation in the peripheral drug transfer profile compared to the positive control PCB (Table 2) may be attributed to multiple factors; however, given the small number of vessels and large standard deviations, a difference cannot be established. Additional data would be required to investigate differences due to balloon shape, mechanical inflation mechanism, impact to vessel wall, contact area, lipophilic additives, etc. No difference in the clearance of paclitaxel is anticipated after being taken up by the artery.
The primary aim of the present study was to assess the efficacy and tolerance of the CCB in porcine arteries. In coronary arteries, a stent overstretch model was used to provoke intimal thickening so that the antiproliferative effects of paclitaxel could be detected. A marked (~50%) reduction in neointimal thickness compared to uncoated balloons as assessed by QCA and histomorphometry was documented for the CCB at two different doses. The inability of the high-dose CCB to reduce intimal thickening beyond the anticipated clinical dose suggests that the clinical dose balloons have already reached the maximum therapeutic effect. Kinetics of peripheral deployment of the CCB were comparable to the positive control DCB and as expected for paclitaxel in porcine arteries.
A number of parameters support a positive safety profile for the CCB. In this study, there were no device-related animal deaths, no in-life clinical symptoms, no treatment-associated thrombi or thromboemboli, no alterations in ECGs beyond signs of ischaemia during balloon inflation and no diminution of left ventricular ejection fraction when comparing CCB to uncoated balloon treatments. Likewise, myocardial histopathologic examination failed to reveal abnormalities associated with the drug-coated devices at the dose intended for clinical use. Plasma levels of paclitaxel after deployment of up to four balloons remained far below the limit of approximately 90 ng/ml known to cause systemic adverse events in patients according to Huizing et al27 and were below the limit of detection within 24 hours.
Conclusions
The CCB is effective in suppressing neointimal thickening. In this respect, it is comparable to commercial products20,26. It appears to be well tolerated up to at least the intended clinical paclitaxel dose of 3 µg/mm² balloon surface as assessed in this preclinical model. Furthermore, the nitinol constraining structure of the Chocolate balloon may offer advantages over standard drug-coated balloons in reducing undesired drug loss during balloon transit in the introductory sheath or guiding catheter and vessel wall injury during dilatation.
| Impact on daily practice Balloon catheters with a nitinol constraining structure are designed to distribute the balloon forces more uniformly than standard angioplasty balloons, thereby offering potential benefits in reducing therapy-associated injury. Drug coating may combine the benefits of DCB technology and a constrained angioplasty balloon to facilitate the concept of leaving nothing behind in the treatment of de novo coronary and peripheral artery disease. The results of the preclinical studies demonstrate efficacy and tolerance within the tested dose range of the selected paclitaxel coating in the chosen porcine model. |
Conflict of interest statement
G. Binyamin and P. Seifert are employees of TriReme. E. Konstantino is an employee and shareholder of TriReme. S. Bienek is an employee of InnoRa. U. Speck and B. Scheller are shareholders of InnoRa. The other authors have no conflicts of interest to declare.

