
Abstract
Aims: The aim of this study was to evaluate the biological efficacy of a novel lower-dose (2.5 µg/mm2) encapsulated paclitaxel nanocrystal-coated balloon (Nano-PCB) in the familial hypercholesterolaemic swine (FHS) model of iliofemoral in-stent restenosis.
Methods and results: Nano-PCB pharmacokinetics were assessed in 20 femoral arteries (domestic swine). Biological efficacy was evaluated in ten FHS: 14 days following bare metal stent implantation each stent segment was randomised to a clinically available PCB (IN.PACT, n=14), the Nano-PCB (n=14) or an uncoated balloon (n=12). Angiographic, optical coherence tomography and histological evaluation was performed at 28 days after treatment. Arterial paclitaxel concentration was 120.7 ng/mg at one hour and 7.65 ng/mg of tissue at 28 days with the Nano-PCB. Compared to the control uncoated group, both PCBs significantly reduced percent area stenosis (Nano-PCB: 36.0±14.2%, IN.PACT: 29.3±9.2% vs. control: 67.9±15.1%, p<0.001). Neointimal distribution in the entire stent length was more homogenous in the Nano-PCB. Histological evaluation showed comparable degrees of neointimal proliferation in both PCBs; however, the Nano-PCB showed slightly higher levels of neointimal maturity and endothelialisation.
Conclusions: Lower-dose encapsulated paclitaxel nanocrystals delivered via a coated balloon displayed comparable biological efficacy with superior healing features compared to a clinically validated PCB technology.
Abbreviations
%AS: percent area stenosis
CSA: cross-section area
DCB: drug-coated balloon
EEL: external elastic lamina
FHS: familial hypercholesterolaemic swine
IEL: internal elastic lamina
ISR: in-stent restenosis
MLD: minimum lumen diameter
NIA: neointimal area
NIT: neointimal thickness
OCT: optical coherence tomography
PCB: paclitaxel-coated balloon
RVD: reference vessel diameter
QVA: quantitative vascular angiography
SEM: scanning electron microscopy
Introduction
Randomised control trials have demonstrated the clinical efficacy of paclitaxel-coated balloons (PCB) in the prevention of restenosis amongst patients undergoing revascularisation of the femoropopliteal arterial territory1-4. Experimental studies have shown that the biological efficacy of these technologies relies on maintaining long-term tissue levels of paclitaxel following single-time balloon delivery5. In addition, several studies suggest that paclitaxel crystallinity plays an important role in achieving such levels of drug retention by depositing crystals on the vessel surface6-8. In recent years, concerns have been raised about the safety profile of PCB technologies, mainly their potential to result in unpredictable paclitaxel tissue levels and produce distal particle embolisation following balloon inflation9.
Paclitaxel-coated technologies are evolving towards the use of lower drug concentrations or non-crystalline forms of paclitaxel aiming at improving the clinical safety of PCB technologies. In particular, the encapsulation of paclitaxel nanocrystals offers a novel opportunity to achieve homogeneous long-term drug distribution and lower particulate formation compared to standard crystalline PCB technologies. In this study, we aimed to evaluate the pharmacokinetic profile and the biological effect on vascular healing of a novel nanoparticle-coated PCB (Nano-PCB) in the familial hypercholesterolaemic swine model of iliofemoral in-stent restenosis (ISR).
Methods
DEVICE DESCRIPTION
The PCB used in this study comprises a paclitaxel nanocrystal encapsulation coating technology developed for local drug delivery (Vascular Nanotransfer Technologies, Auburndale, MA, USA; and Concept Medical Research Private Limited, Surat, India). The basic concept relies on the development of paclitaxel nanocarriers by using biocompatible phospholipids to encapsulate nanocrystallised paclitaxel. The drug is encapsulated in a phospholipid bilayer that has amphiphilic properties in which the head is hydrophilic and the tail is lipophilic. Once in the solution, the paclitaxel nanocarriers result in a nanometer size range varying from 50 to 300 nanometers. The nanocrystals were synthesised using an ultrasonic homogenisation process. The nanocarrier formulation was coated to a stand-alone balloon catheter (2.5 µg/mm2 on a 4×40 mm balloon) using an inert gas-assisted spray process. The coated balloon was analysed by scanning electron microscopy (SEM) to evaluate surface smoothness and coating features (Figure 1). This coating process produces a thinner and uniform coating resulting in a concentration variability along the balloon of less than 10 percent. In vitro drug release analysis revealed that there was a paclitaxel loss during transit of ~2%. Approximately 68% of the paclitaxel coating was released upon the 60-second balloon inflation with only less than 11% of the drug remaining on the balloon surface. In the current study, a lower-dose (2.5 µg/mm2) nanocrystalline paclitaxel-coated balloon was used and compared to a commercially available paclitaxel-eluting balloon (IN.PACT™; Medtronic, Minneapolis, MN, USA) (3 µg/mm2).
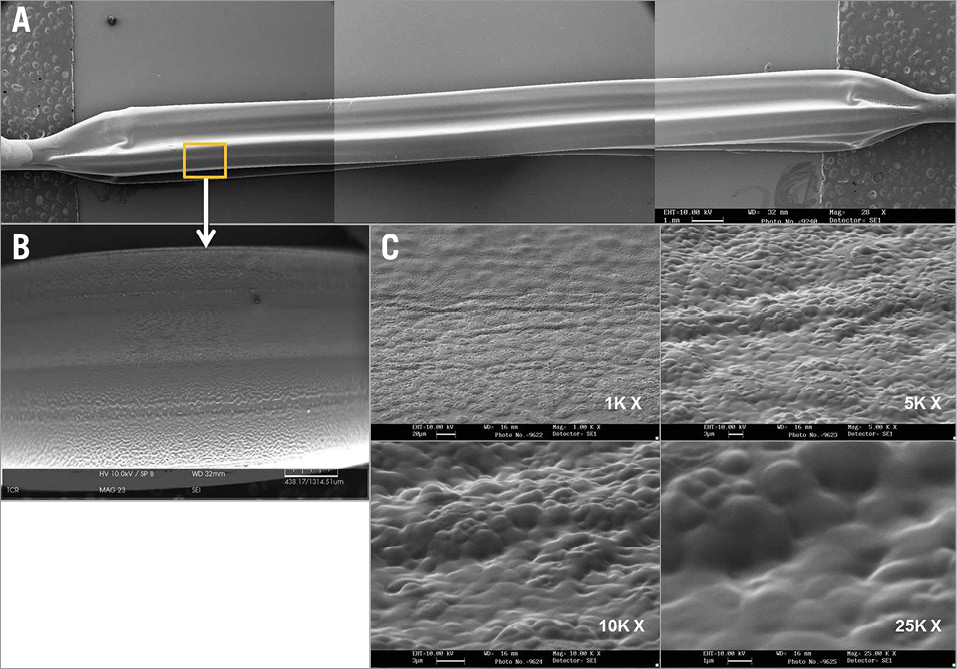
Figure 1. Nanocrystalline paclitaxel coating features by scanning electron microscopy (SEM). A) SEM of the nanocrystalline paclitaxel-coated balloon catheter. B) Coating process results in uniform drug connection with low magnification (28x). C) SEM with high magnification (1~25K x) showing that the surfaces of the coated balloon are smooth, without irregularities or cracks.
STUDY DESIGN
The study design is shown in Figure 2. The study was approved by the Institutional Animal Care and Use Committee. All animals received the standard care outlined by the study protocol and in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals formulated by the Institute of Laboratory Animal Resources (National Research Council, NIH Publication No. 85-23, revised 1996). The drug release profile of the Nano-PCB was assessed in 20 peripheral arteries of five domestic swine. In the efficacy study, a total of 10 familial hypercholesterolaemic swine (FHS) were included in the study. Forty peripheral arteries were injured at day zero with an uncoated balloon followed by self-expanding stent implantation. At day 14 (baseline), peripheral arteries (superficial femoral artery 20, profunda 20) were randomly treated with either a clinically proven PCB (IN.PACT; Medtronic, n=14), an experimental Nano-PCB (n=14), or a control uncoated balloon (n=12). At day 42 (four weeks post treatment), angiographic, optical coherence tomography (OCT) and histological evaluation was performed in all treated segments.
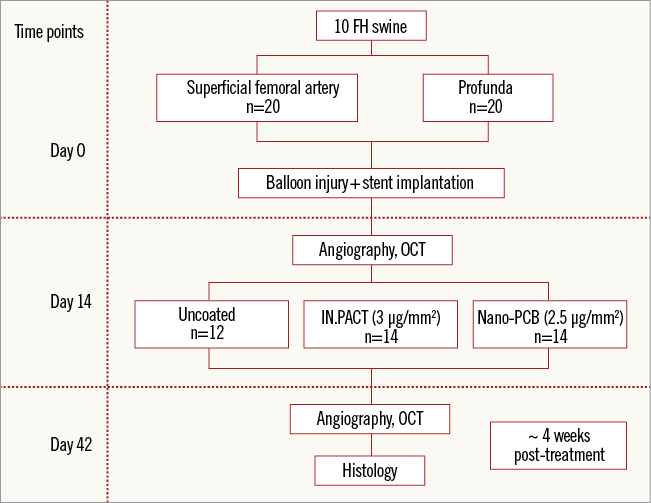
Figure 2. Study design.
TISSUE PHARMACOKINETICS
A total of 20 peripheral arteries of five domestic swine were included in the paclitaxel tissue transfer study. The tested Nano-PCB was inflated based on quantitative vascular angiography (QVA)-derived vessel reference diameters to achieve a balloon-to-artery ratio of 1.2:1.0 for 60 seconds. The animals were euthanised at one hour, seven days and 28 days after the procedure and the treated vessels were harvested, weighed, and snap frozen in liquid nitrogen for paclitaxel tissue content by BASi® (West Lafayette, IN, USA) using the high-performance liquid chromatography tandem mass spectrometry method.
FAMILIAL HYPERCHOLESTEROLAEMIC SWINE (FHS) MODEL
All swine (6.1±0.5 months of age, weight 36.1±4.2 kg) were obtained from the University of Wisconsin, Department of Animal Sciences. The baseline cholesterol levels of the animals varied from 327 to 548 mg/dl (mean cholesterol level: 433.5±66.4 mg/dl). The genotypic, clinical characteristics and use of this model in the evaluation of the efficacy of local drug delivery devices have been extensively published6-8. Animals were maintained on a low-grade cholesterol diet (0.6%) to increase the cholesterol levels and to accelerate the disease process for the whole study duration. The mean cholesterol level of the animals had increased by 77% (759.5±110.2 mg/dl) at treatment (day 14), and by 56% (672.5±100.5 mg/dl) at terminal procedure (day 42).
IN-STENT RESTENOSIS MODEL AND TREATMENT
The model development of ISR in the peripheral vasculature model of FHS has been described previously6-8. In brief, general anaesthesia was induced with xylazine and TELAZOL® (Zoetis, Florham Park, NJ, USA) (tiletamine/zolazepam). After reaching an adequate anaesthetic level, the animals were intubated and maintained with inhaled 1% to 3% isofluorane. Surgical access was obtained via the carotid artery with the general sterile technique. Heparin (5,000 to 10,000 U) was injected to maintain an activated clotting time >250 s. Intravascular ultrasound (ILAB™ IVUS system; Boston Scientific, Marlborough, MA, USA) was used for vessel sizing. Oversized balloon inflation using a 1.3:1.0 balloon-to-artery ratio was applied to the target area followed by self-expanding bare metal stent (6×40 mm) implantation. At 14 days, after baseline angiography and OCT imaging had been performed, the previously injured arterial segments were randomly treated with either a clinically proven PCB (IN.PACT, n=14), an experimental Nano-PCB (n=14), or a control uncoated balloon (n=12). The balloons (size 5.0×40 mm and 6.0×40 mm) were inflated within the stented segment for a total of 60±2 s with a target of 120% overstretch. Four weeks later (day 42), terminal angiography and OCT imaging were performed, then arterial tissue was sent for histological evaluation.
QUANTITATIVE ANGIOGRAPHY ANALYSIS
QVA was performed using QAngio® XA software (Medis medical imaging systems, Leiden, The Netherlands). The outer diameter of the contrast-filled catheter was used as the calibration standard and the minimum lumen diameter (MLD) was obtained from the single worst view, while the reference vessel diameter (RVD) was automatically calculated by the interpolation method. The percent diameter stenosis was calculated as: (1–[MLD/RVD])×100%, and the late loss was calculated as MLD at follow-up − MLD at post inflation.
OCT ANALYSIS
OCT images were obtained using the ILUMIEN™ PCI Optimization System (St. Jude Medical, St. Paul, MN, USA) and imaging was performed using continuous non-occlusive contrast-saline mixture as a flush. Motorised OCT pullbacks were performed at a rate of 20 mm/s. All images were acquired at 100 frames per second, displayed with a colour look-up table and digitally archived. Qualitative analyses were performed with commercial software (ILUMIEN OPTIS™; St. Jude Medical).
Cross-section area (CSA) measurements of the lumen and stent were applied to every 5 mm of the total 40 mm-long treated site. Neointimal hyperplasia CSA was calculated as stent minus lumen CSA, and percent of neointimal obstruction was calculated as neointimal divided by stent area. Neointimal thickness (NIT) was defined as the distance from the inner surface of the stent struts to the luminal border and automatically calculated by the software.
HISTOLOGICAL ANALYSIS
The histological analysis was conducted by an independent pathology laboratory (Alizée Pathology LLC., Thurmont, MD, USA). After terminal imaging, the animals were euthanised and all treated vessels harvested and immersed in 10% neutral buffered formalin. All vessels were first embedded in methyl methacrylate and then cut into 40 to 50 µm sections obtained from the proximal, mid and distal portions of each stented segment. These sections were stained with haematoxylin and eosin and elastic trichrome. The cross-sectional areas (external elastic lamina [EEL], internal elastic lamina [IEL], and lumen area) of each section were measured. These measurements were used to calculate vessel layer areas with the following formulas: medial=EEL−IEL; neointima=IEL−lumen; percent area stenosis (%AS)=(1–[lumen area/IEL area])×100. Vessel injury score was scored according to the method of Schwartz et al10. Neointimal inflammation (0-4) and fibrin deposition (0-3) were semiquantitatively scored for each section, as previously described11. The neointima maturity was evaluated using the following semiquantitative score: 0=not present; 1=light dispersed smooth muscle population; 2=heavier population throughout less than the entire thickness of the neointima; and 3=dense population throughout the entire thickness of the neointima.
STATISTICAL ANALYSIS
Statistical analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, NC, USA). All measurements were tabulated as mean±SD. A two-factor repeated measures ANOVA, with one factor of days and the second factor of three levels of treatment, was used to perform variance analysis, and a post hoc test (Bonferroni method) was used to compare differences among the three treatments. Additionally, two-way ANOVA was used to analyse the difference among distal, middle and proximal treated segments of the neointimal distribution. All tests were two-tailed with a significance level of p≤0.05.
Results
TISSUE PHARMACOKINETICS
Figure 3 illustrates the arterial tissue concentration of paclitaxel at one hour, seven days and 28 days after a single 60-second inflation of the Nano-PCB. The tissue uptake of paclitaxel peaked at one hour (120.7±105.6 ng/mg). Arterial tissue paclitaxel concentration was 22.6±29.2 ng/mg at seven days and the drug was still detectable in the tissue at 28 days (7.65±0.04 ng/mg).
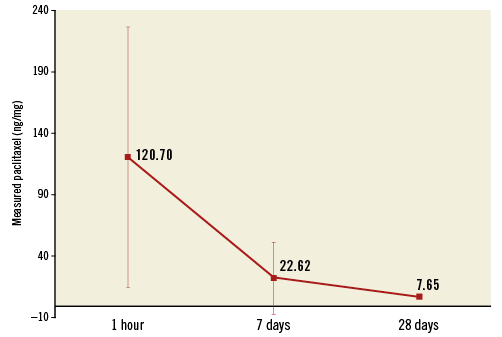
Figure 3. Drug release profile of the nanocrystalline paclitaxel-coated balloon as assessed in the peripheral vessels of domestic swine. Peak tissue uptake was demonstrated at one hour and the drug was still detectable in the tissue at 28 days.
ANGIOGRAPHIC ANALYSIS
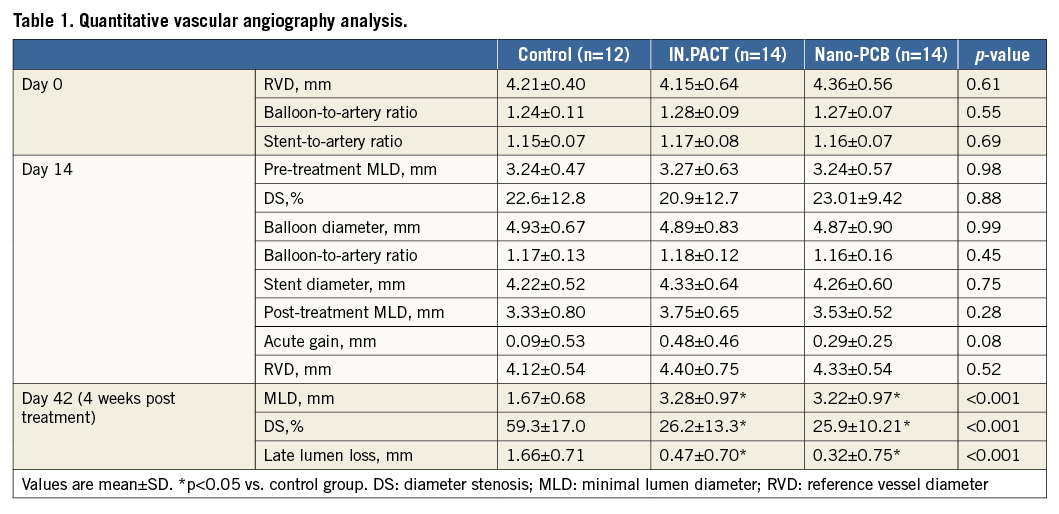
A summary of angiographic data is presented in Table 1. Before injury, the average angiographic vessel diameters were comparable among all groups. Also, the degree of balloon injury achieved in all groups was comparable (balloon-to-artery ratio=control: 1.24±0.11, IN.PACT: 1.28±0.09, and Nano-PCB: 1.27±0.07, p=0.55). The final stent-to-artery ratio was similar among all groups (control: 1.15±0.07, IN.PACT: 1.17±0.08, and Nano-PCB: 1.16±0.07, p=0.69). At the time of treatment (14 days), the mean pre-procedural %DS (control: 22.6±12.8%, IN.PACT: 20.9±12.7%, and Nano-PCB: 23.01±9.42%, p=0.88) and late loss (control: 1.28±0.43 mm, IN.PACT: 1.21±0.67 mm, and Nano-PCB: 1.41±0.46 mm, p=0.64) were similar in all groups. Following treatment randomisation, the mean final balloon inflation diameters were also similar, leading to a similar angiographic acute gain post vessel treatment (Table 1). Four weeks after treatment, the %DS was significantly lower in both PCB groups compared with control (control: 59.3±17.0%, IN.PACT: 26.2±13.3%, and Nano-PCB: 25.9±10.2%, p<0.001). There was a significant reduction in angiographic late loss in both PCB groups compared with the uncoated balloon group. No statistical differences in any of the angiographic endpoints were found between the IN.PACT and Nano-PCBs.
OCT ANALYSIS
A summary of OCT data is presented in Table 2. OCT analysis revealed that the percentage area stenosis at the time of PCB randomisation was comparable among the groups (control: 24.3±10.2%, IN.PACT: 24.6±12.3%, and Nano-PCB: 26.2±12.9%, p=0.90). At day 42, compared with the uncoated control group, both PCB groups displayed a larger lumen area and a lower percentage area stenosis (control: 67.9±15.1% vs. IN.PACT: 36.0±14.2%, and Nano-PCB: 29.3±9.2%, p<0.001). There were no statistical differences in mean neointimal area between the IN.PACT and Nano-PCB. However, OCT analysis showed that the distribution of neointima in the Nano-PCB group was more homogenous (mean NIT [mm] proximal: 0.54±0.21, mid: 0.54±0.34, distal: 0.56±0.29; p=0.90) as compared to the IN.PACT group (mean NIT [mm] proximal: 0.48±0.19, mid: 0.42±0.16, distal: 0.33±0.13; p=0.01) (Figure 4). The IN.PACT DCB achieved higher neointimal inhibition at the distal portion compared to the Nano-PCB (p=0.01).
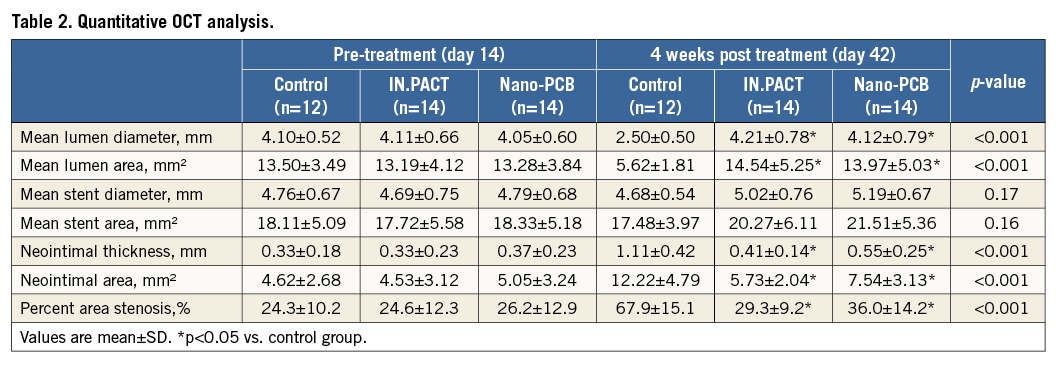
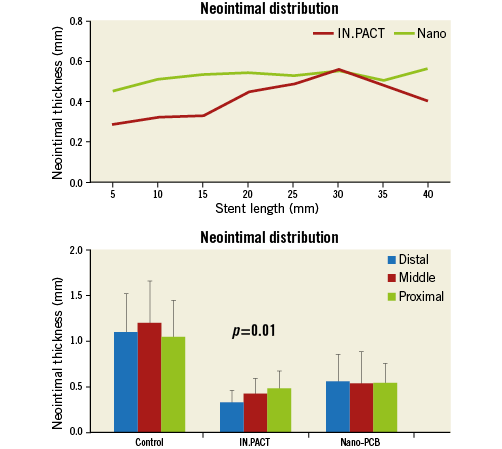
Figure 4. Pattern of in-stent neointimal distribution along the treated segments analysed by OCT. The neointimal distribution was irregular in the IN.PACT group, lower at the distal segment but higher at the proximal part.
HISTOLOGICAL ANALYSIS
The histological data are summarised in Table 3. Correlating with the angiography and OCT findings, the variables representing neointimal hyperplasia such as neointimal thickness, neointimal area, and %AS were comparable between both PCB groups and significantly lower than in the uncoated control group. The neointimal inhibition at the proximal, middle and distal segments throughout the implanted stent is presented in Figure 5. The degree of vessel wall injury was comparable between the control group (0.18±0.23) and both PCB groups (IN.PACT: 0.12±0.14, and Nano-PCB: 0.13±0.12, p=0.56). There was a higher degree of fibrin deposits in the PCBs (IN.PACT: 2.29±0.65, and Nano-PCB: 2.12±0.88) as compared with the control group (0.17±0.27, p<0.001). The uncoated balloon group showed a higher score of peri-strut inflammation compared to the PCBs. The neointimal maturity and endothelialisation scores were lower in both PCB groups compared to the uncoated control group (Table 3).
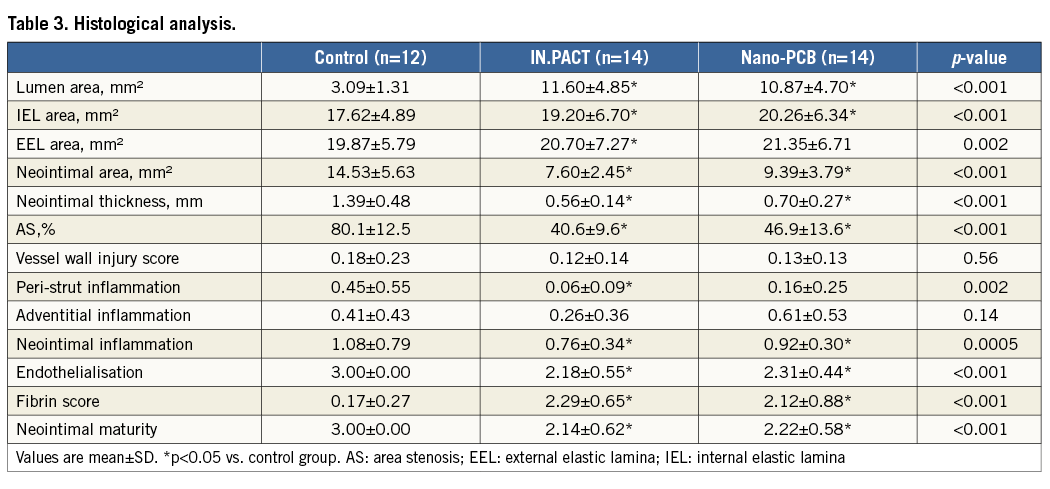
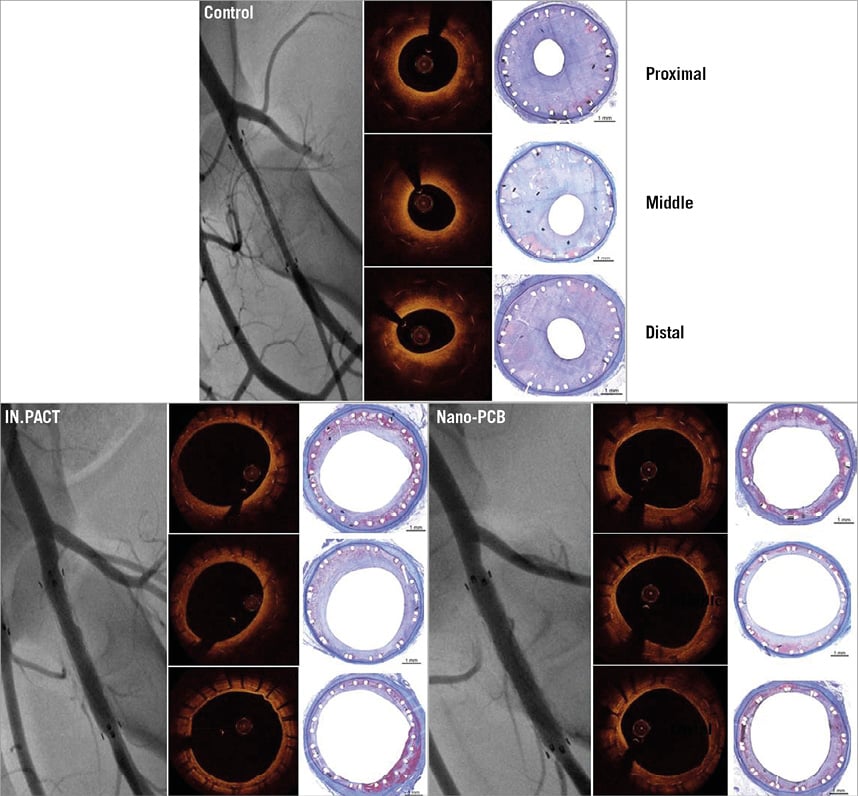
Figure 5. Representative images at four weeks post treatment on angiography, OCT and histology. The uncoated balloon group showed the highest degree of neointimal proliferation within stents. An evident decrease in neointimal formation was observed in both the IN.PACT and Nano-PCB groups.
Discussion
Several studies have shown that paclitaxel inhibits neointimal formation efficaciously in coronary and peripheral arteries following balloon delivery12-14. The biological effect of drug-coated balloon (DCB) technologies relies on the rapid and efficient transfer of paclitaxel to the vessel wall following balloon inflation15. First-generation PCB technologies used a crystalline coating formulation and showed significant decrease in angiographic restenosis among patients undergoing percutaneous intervention of the superficial femoral artery6,16. However, bench data suggest that DCB are not generally efficient at transferring paclitaxel into the vessel wall. It has been estimated that during balloon transit to the target lesion paclitaxel losses of up to 26% and 36% were accounted for in urea-based and iopromide-based PCB, respectively17. In the Nano-PCB technology, the drug is encapsulated in a phospholipid bilayer and coated on a hydrophilic balloon surface which delivers paclitaxel nanocarriers by wetting of the hydrophilic part. The nano-crystalline-based coating allows better adhesion to the balloon surface, resulting in lower levels of drug loss during device tracking to the target site (~2%) as well as lower particulate formation during balloon inflation. In contrast, due to their inherent coating characteristics, first-generation coatings raised concerns about the potential for tissue toxicity and distal particle embolisation following balloon inflation, especially in vessels with poor distal vascular run-off9.
Although non-crystalline coatings are perceived to be safer, clinical data suggest that they may not be as effective compared to first-generation crystalline DCB technologies18,19. Paclitaxel-coated technologies are evolving towards the use of lower drug concentrations or non-crystalline forms of paclitaxel aiming to improve the safety profile of PCB technologies. In particular, the encapsulation of paclitaxel nanocrystals offers a novel opportunity to take advantage of the crystallinity profile of paclitaxel and the higher solubility achieved by the size and encapsulation achieved at the end of the nanocarrier formation process. Then, the resulting coating has the potential to provide a more homogeneous long-term drug distribution and lower particulate formation compared to standard crystalline PCB technologies. Nanoparticle encapsulation also offers a unique opportunity to improve clinical outcomes as it provides a higher potential for particle uptake and distribution into the tissue, leading to less systemic side effects and toxicity20,21 as cellular uptake of nanoparticles occurs very rapidly. Xu et al22 reported that treatment with paclitaxel-loaded multi-ligand nanoparticles suppressed neointimal stenosis in injured rat carotid arteries. However, size alone is not the only factor governing particle uptake. Previous studies23 have shown that the modification of the nanoparticle surface may markedly increase uptake and retention in the arterial wall. Lemos et al24 showed that, using a similar technological principle, sirolimus tissue levels could be maintained for up to 14 days in a rabbit model. Their data showed that the in-tissue uptake of sirolimus at one, seven and 14 days had a concentration of 140.6, 15.5 and 5.5 ng/mg, respectively. Confocal microscopy showed homogeneous fluorescent tagged nano sirolimus distribution into the artery wall with intima to adventitia flow.
In our study, the tissue pharmacokinetics study revealed that the Nano-PCB (2.5 µg /mm2) had higher early tissue transfer levels (121 ng/mg) compared to a clinically validated crystalline PCB technology using a higher concentration of paclitaxel (48.7 ng/mg in IN.PACT)8. In addition, there was a more sustained paclitaxel tissue level (7.65 ng/mg at 28 days) compared to the other published pharmacokinetics (PK) curves (2.8 ng/mg in the IN.PACT and 0.9 ng/mg in Lutonix® PCB [BARD Peripheral Vascular, Tempe, AZ, USA] at 28 days)8. This is an important finding as it was previously proposed that highly crystalline paclitaxel was needed to maintain tissue levels over time following balloon delivery. Next, this approach provides an opportunity to decrease total paclitaxel concentration exposed to the vessel wall and distal tissues but maintain the clinical effect of paclitaxel observed with first-generation DCB coatings.
Moreover, we evaluated the biological effects of nanocrystal encapsulation in neointimal proliferation and vascular healing. Previous studies have shown that inhibition of neointimal growth by paclitaxel is dependent on the dose and tissue pharmacokinetics produced by the balloon coating25. In turn, coating properties are the result of the type of excipient and manufacturing process used to develop the coating. In general, crystalline coatings result in prolonged tissue level retention as compared to amorphous coatings; however, crystalline coatings are limited by heterogeneous drug tissue uptake and neointimal inhibition5. In this study, IN.PACT showed higher neointimal inhibition in the distal segment (NIT: 0.33±0.13 mm vs. Nano-PCB: 0.56±0.29 mm, p=0.01), and the neointimal distribution in the low-dose Nano-PCB followed a more constant pattern along the treated section (NIT [mm] proximal 0.54±0.21, mid: 0.54±0.34, distal: 0.56±0.29; p=NS). Due to the nature of the coating and the fact that the particles are in the nano range size, the effect on neointimal inhibition is more predictable and uniform. In contrast, crystalline coatings result in heterogeneous and more powerful inhibition. Also, it is expected that the amount, quality and distribution of neointima will be more predictable in the Nano-PCB group. Our results support the notion that a more uniform nanoparticle balloon coating results in more homogeneous drug distribution along the treated site. This is an important finding because the variation in tissue concentration of paclitaxel appears to influence the overall healing response after PCB treatment.
The most important findings seen in the histological evaluation were the bioequivalence of neointimal inhibition of both PCB technologies and the more homogenous pattern of neointimal distribution elicited by the Nano-PCB. These findings are important as they prove for the first time that the safety of these technologies could be improved whilst maintaining biological efficacy. The presence of fibrin in the tissue is a typical finding for technologies which use antiproliferative agents, reflecting the biological effects of drug transfer to the vessel wall. The constant presence of fibrin in the long term has been considered a marker of delayed healing, and has been associated with adverse clinical events26. In the present study, although no statistical difference was found among the PCB groups, there was a trend towards lower fibrin deposits and greater neointimal maturity and endothelialisation in the Nano-PCB group as compared with the IN.PACT group.
Limitations
Several limitations were present in the current study. First, the experimental nature of the study may limit the translation of our findings to the human condition. Second, we used the 28-day follow-up time point for the evaluation of efficacy. It is possible that some of the differences seen in tissue levels among all devices could reflect important differences at later time points not evaluated in this study. Finally, the lack of a significant atherosclerotic burden found in this model takes away important biological components known to be critical for the success of local drug delivery devices in humans.
Conclusion
In conclusion, compared with the uncoated control group, treatment with PCBs showed great efficacy for neointimal inhibition, with less neointimal hyperplasia. Additionally, the nano-crystalline PCB achieved comparable levels of neointimal inhibition compared to a commercially available PCB, despite its reduced paclitaxel concentration. Also, endovascular imaging and histological evaluation suggest that neointimal proliferation may be more homogenous and mature compared to the PCB used as a control in this study.
| Impact on daily practice Novel paclitaxel balloon coatings may improve clinical safety and decrease particulate formation following balloon inflation. The present study shows that novel lower-dose encapsulated paclitaxel nanocrystals delivered via a coated balloon displayed comparable biological efficacy with superior healing features compared to a clinically validated PCB technology. These emerging coating improvements have the potential to expand the clinical indications of drug-coated balloon technologies. |
Funding
Vascular Nanotransfer Technologies (VNT, Auburndale, MA, USA) is an inactive company which holds the intellectual rights to this technology and partially funded this study in collaboration with Concept Medical Inc. (Surat, India).
Conflict of interest statement
J. Granada has, in the past, acted as Chief Scientific Advisor of VNT. M. Doshi and P. Sojitra are employees of Concept Medical Inc. The other authors have no conflicts of interest to declare.

