Abstract
Aims: The efficacy of paclitaxel-coated balloons (PCB) for the treatment of superficial femoral artery (SFA) disease has been demonstrated in the clinical setting. Due to the high frequency of arterial calcification found in this vascular territory, the adjunctive use of atherectomy plus PCB has been proposed. In this study, we aimed to evaluate the biological effect on vascular healing and drug retention of this combination approach in the familial hypercholesterolaemic swine (FHS) model of femoral artery stenosis.
Methods and results: Eleven femoral arteries (six superficial and five profunda arteries) were included. Vessels were injured (x2) over a 28-day period and all animals were maintained on a high cholesterol diet for 60 days following initial injury. Vessels were randomised to PCB (n=5) or orbital atherectomy system (OAS) plus PCB (n=6). At 28 days following therapy, vessels were followed with angiography, intravascular ultrasound (IVUS) and optical coherence tomography (OCT). Vessels were harvested for histological and pharmacokinetic analysis. Angiographic findings were comparable at termination between both groups. The OCT findings were comparable at termination. There were no differences in the vascular healing profile between both groups. The paclitaxel levels at termination were comparable between both groups (PCB=5.16 vs. OAS+PCB=3.03 ng/mg).
Conclusions: In the experimental setting, the combination of OAS+PCB appears to be safe by demonstrating a vascular healing profile and drug tissue levels comparable to PCB only. The vascular effect of PCB may be enhanced by the use of OAS by decreasing plaque burden and cholesterol crystals.
Introduction
Paclitaxel-coated balloons (PCB) have emerged as the therapy of choice for superficial femoral artery disease1. Several clinical studies have shown that PCB significantly reduce restenosis compared to balloon angioplasty in relatively short and simple lesions2,3. Superficial femoral artery disease is characterised by a complex biomechanical behaviour, significant plaque burden and high levels of calcium in the media and intima4-6. Due to these unique biological characteristics, the development of successful technologies targeting this vascular territory has been hampered by multiple technical failures (i.e., stent fractures, stent malapposition) and biological failures (i.e., DES failures)7-12. In addition, restenosis cases following PCB therapy have been associated with highly calcified vessels located in complex long lesions13.
The use of atherectomy has been suggested to induce plaque debulking and lesion preparation and to increase periprocedural success in peripheral vascular interventions14. However, despite favourable acute periprocedural results, the long-term impact on restenosis and clinical outcomes is still controversial15. However, it is believed that, due to its favourable effect on plaque biomechanics (calcium reduction), the adjunctive use of this technology may enhance the biological effect of PCB technologies. In this study, we aimed to determine the biological effect on healing and paclitaxel retention of the synergistic use of PCB and an orbital atherectomy system (OAS) in a novel model of SFA stenosis in the familial hypercholesterolaemic swine (FHS).
Methods
TEST DEVICE DESCRIPTION
The Diamondback 360°® Peripheral Orbital Atherectomy System (OAS) (Cardiovascular Systems, Inc., St. Paul, MN, USA) is a percutaneous endovascular system that modifies calcific, non-compliant plaque in patients with stenotic or occluded peripheral arteries16. The system includes a single-use, 6 Fr catheter with an eccentrically mounted 30 µm solid diamond-coated crown mounted to a coil-wound drive shaft, control handle with control knob, flexible drive shaft, and protective sheath. The crown shaft tracks and rotates over a guidewire, which can be independently advanced. The crown sizes available range from 1.25 to 2.0 mm. The IN.PACT Admiral Paclitaxel-eluting PTA balloon catheter (Medtronic Invatec, Minneapolis, MN, USA) consists of an angioplasty balloon coated with antiproliferative paclitaxel at a 3.0 mcg/mm2 dose. The delivery system is 6 Fr sheath-compatible. The sizes used in this study were 5.0 and 6.0 mm in diameter by 20 mm in length.
SFA LESION DEVELOPMENT PROTOCOL
The study was approved by the Institutional Animal Care and Use Committee. The FHS bears a homozygous trait of LDL receptor deficiency displaying high total cholesterol levels (~300 to 400 mg/dL) despite being maintained on a normal diet. This model, originally described by Rapacz et al17,18, progressively develops atheromatous lesions within 18 months from birth19. All animals received standard care in accordance with the Guide to Care and Use of Laboratory Animals formulated by the Institute of Laboratory Animal Resources (National Research Council, NIH Publication No. 85-23, revised 1996). FHS (n=3; mean weight=76.6±7.1 kg) approximately 30 months of age were maintained on a high cholesterol diet starting three days prior to intervention. The diet included a mixture of Porcine Grower (Purina Lab Diet Porcine Grower Diet 5084) with a high cholesterol pig chow (Purina Test Diet Modified Laboratory Mini-Pig Diet #583V; Lab Diet Mini-Pig Grower Diet 5081 modified with 20% lard, 2% cholesterol, and 1.5% sodium cholate) and was provided daily in a controlled amount until the treatment procedure. After treatment, only the regular grower diet was provided until termination of the study. Once general anaesthesia and endotracheal intubation were achieved, arterial carotid access was obtained by cut-down. Anticoagulation with heparin was achieved to maintain an activated clotting time (ACT) ≥300 seconds.
Injury sites in the arteries (superficial and profunda femoral arteries) were selected in each animal using quantitative vascular angiography (QVA). After baseline angiography, all vessels were injured at day 0 using a Fogarty embolectomy catheter (Edwards Lifesciences, Irvine, CA, USA). At 28 days following initial injury, a second vascular injury procedure was performed. Following QVA and intravascular ultrasound (IVUS) evaluations, an angioplasty balloon was inflated to the appropriate pressure to achieve a target balloon-to-artery ratio (BAR) of 1.4:1 overstretch. A total of 11 peripheral vessels were reinjured; one vessel was excluded from second injury due to total occlusion.
Treatment randomisation was performed 60 days following initial injury. Treatments included PCB only versus OAS followed by PCB treatment. PCBs were inflated for 60 (±3) seconds to achieve a target ratio of 1.2:1 based on the manufacturer’s compliance chart. Follow-up imaging (QVA, OCT, IVUS) was performed on all animals and terminated 28 days after treatment. At termination, arteries were harvested for histological evaluation and pharmacokinetic analysis. Data generated from QVA, morphometry and IVUS were compared in both groups with specific focus on plaque volume and percent area stenosis (Figure 1).
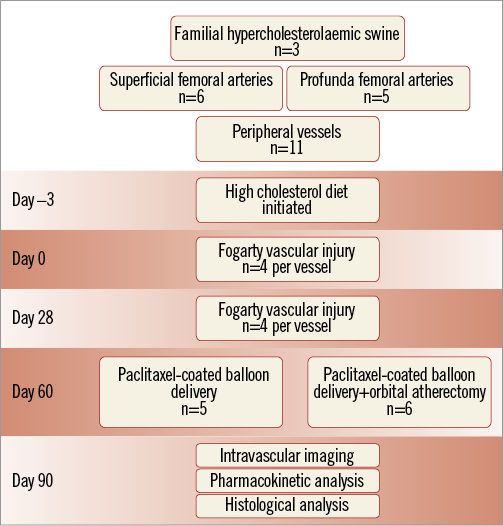
Figure 1. Study design.
INTRAVASCULAR ULTRASOUND (IVUS) IMAGING
IVUS pullback images were obtained and analysed using a coronary ultrasound catheter (Atlantis® SR Pro 40 MHz Coronary Imaging Catheter; Boston Scientific, Natick, MA, USA) and a commercially available measurement analytic system (iLab; Boston Scientific). Using fluoroscopy, the IVUS catheter was placed distal to the vascular segment of interest and an automated pullback performed at a speed of 0.5 mm/s covering 10 mm proximal and distal to the site of interest. The starting position of the IVUS catheter was determined by fluoroscopy and situated by anatomical landmarks in a live image during the pullback. The in-segment analysis in each vessel was performed using standard definitions20. IVUS studies were archived onto DVDs and analysis was performed by two operators blinded to the follow-up time points. Each individual pullback analysis was divided into proximal reference vessel, vascular interest segment and distal reference vessel. Volumetric measurements were performed using planimetry software (echoPlaque; INDEC Systems, Inc., Santa Clara, CA, USA). Quantitative analysis included measurement every 5 mm of the external elastic membrane (EEM) and lumen areas. Neointimal volume was calculated as internal elastic lamina (IEL) area minus lumen area. Volume measurements were automatically calculated using Simpson’s rule20.
OPTICAL COHERENCE TOMOGRAPHY (OCT) IMAGING
OCT images were recorded using the CX7 imaging system (ImageWire™; LightLab Imaging, Inc., Westford, MA, USA). All pullback images were stored on the system hard drive for off-line analysis. OCT analysis involved cross-sectional images analysed in three locations inside the treatment area. OCT morphometric measurements were performed by selecting a proximal, mid, and distal measurement in each treatment segment of each vessel in each animal. Lumen area and lumen diameter were measured using standard vascular microanatomy landmarks in each frame analysed.
HISTOLOGY PROTOCOL
The animals were sacrificed 28 days after treatment and a comprehensive necropsy was performed in which the major organs were examined. A total of 11 arterial segments were harvested and sent to Alizée Pathology (Thurmont, MD, USA) fixed in 10% neutral buffered formalin. Tissues were trimmed at each treatment site and sectioned six or seven times based on treatment maps and angiographic printouts with treatment tracings. The trimmed tissues were processed, embedded in paraffin, and stained with routine haematoxylin and eosin (H&E) and Movat’s Pentachrome (MP).
Lumen, neointima (internal elastic lamina area – lumen area) and medial (external elastic lamina – internal elastic lamina area) areas were measured using Image-Pro Plus (Media Cybernetics, Rockville, MD, USA). The histological parameters (cholesterol clefts deposition and inflammatory) were evaluated under a semi-quantitative score (0=absent histological feature, to 4=overwhelming presence of histological feature).
PHARMACOKINETIC ANALYSIS PROTOCOL
Briefly, the tissue segments that underwent pharmacokinetic paclitaxel evaluation were homogenised in a solution of HPLC grade water. A portion of the homogenate was then spiked with a solution of methyl-t-butyl ester and chloroform using a 90:10 ratio. The samples were then centrifuged, and the organic layer was transferred and dried under an inert gas atmosphere. The residue was reconstituted using a solution of octylamine, acetic acid, acetonitrile, and water. The analysis was performed using a liquid chromatograph/mass spectrometer (LC/MS). The chromatographic separation was performed with a C18 reverse phase column using acetonitrile and water. The paclitaxel compound was detected and quantified as the octylamine adduct in the single ion mass (SIM) mode of detection.
Results
QUANTITATIVE VASCULAR ANGIOGRAPHY
A total of 11 femoral vessels were included in the final analysis. There were no reports of treatment complications. At the time of therapy randomisation, one target vessel site was occluded and was excluded from the study; the rest of the vessels did not show reports of total occlusions or aneurysmatic dilatations. At termination, all treated femoral arteries remained patent with normal blood flow. The quantitative vascular angiography (QVA) assessment showed at day 0 a mean minimal lumen diameter of 3.91±0.55 mm and a percentage area of stenosis of 16.4±11% in the PCB group, and minimal lumen diameter and percent diameter of stenosis of 3.01±0.96 mm and 27.8±14.2%, respectively, in the PCB/OAS group (Figure 2A, Figure 2B). At 28-day follow-up, the minimal lumen diameter was 3.7±1.47 mm (–0.21 mm compared to day 0, resulting in a 5.3% decrease in MLD) in the PCB group. Conversely, in the PCB/OAS group, the mean minimal lumen diameter was 3.9±1.07 mm (0.84 mm compared to day 0, resulting in a 29.5% increase in MLD) (Figure 2A). Similarly, %DS was 27.2±22.41% in the PCB group (10.8±14.65% increase compared to day 0) versus 18.17±23.43% in the PCB/OAS group (-9.67±32.12% decrease compared to day 0) (Figure 2B).
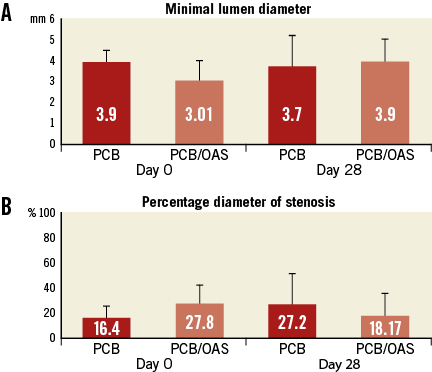
Figure 2. Quantitative vessel analysis. A) Minimal lumen diameter. B) Percentage diameter of stenosis.
ENDOVASCULAR IMAGING ANALYSIS
Prior to therapy randomisation (pretreatment, day 0), intravascular ultrasound (IVUS) analysis showed a slightly higher degree of plaque burden (plaque areas) in the PCB/OAS group (9.28±3.97 mm2) compared to the PCB group (7.63±2.59 mm2). At 28 days after treatment, the total plaque area as assessed by IVUS was comparable between both groups (PCB/OAS=8.93±3.07 mm2 versus PCB=9.55±4.08 mm2). However, considering pretreatment IVUS, the therapy combination of PCB/OAS represented a 4% decrease in total plaque area compared to an approximately 25% increase in total plaque area in the PCB group only (29% difference between groups) (Table 1, Figure 3).
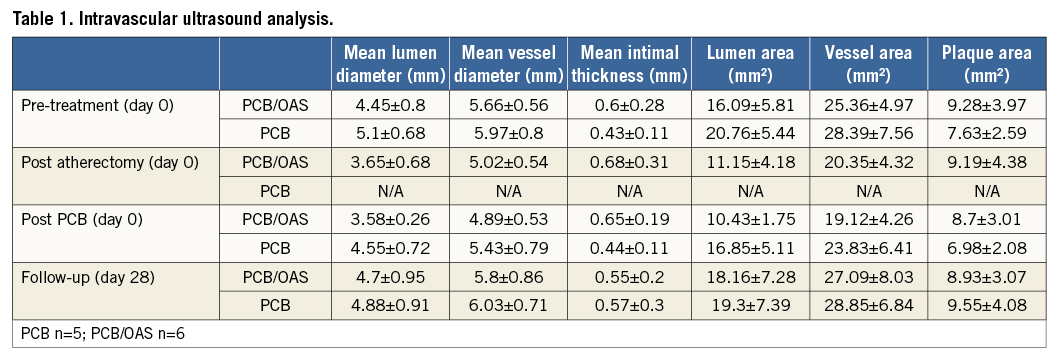
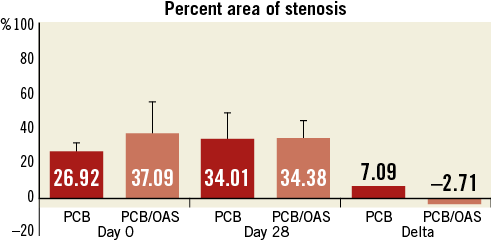
Figure 3. Percent area of stenosis by intravascular ultrasound.
Optical coherence tomography (OCT) analysis was performed with the objective of determining the level of vascular wall damage at termination (i.e., intimal dissection not visible in angiography or IVUS). At 28 days post treatment, there was no evidence of either vascular wall damage or aneurysm formation in any treated vessel. The morphometric OCT assessment showed similar lumen diameters and areas (Table 2, Figure 4).
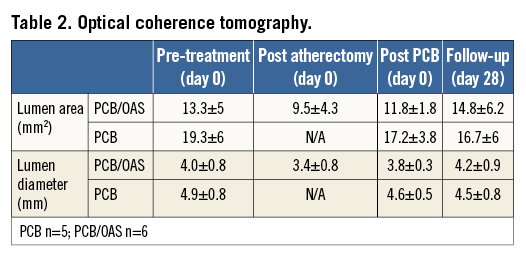
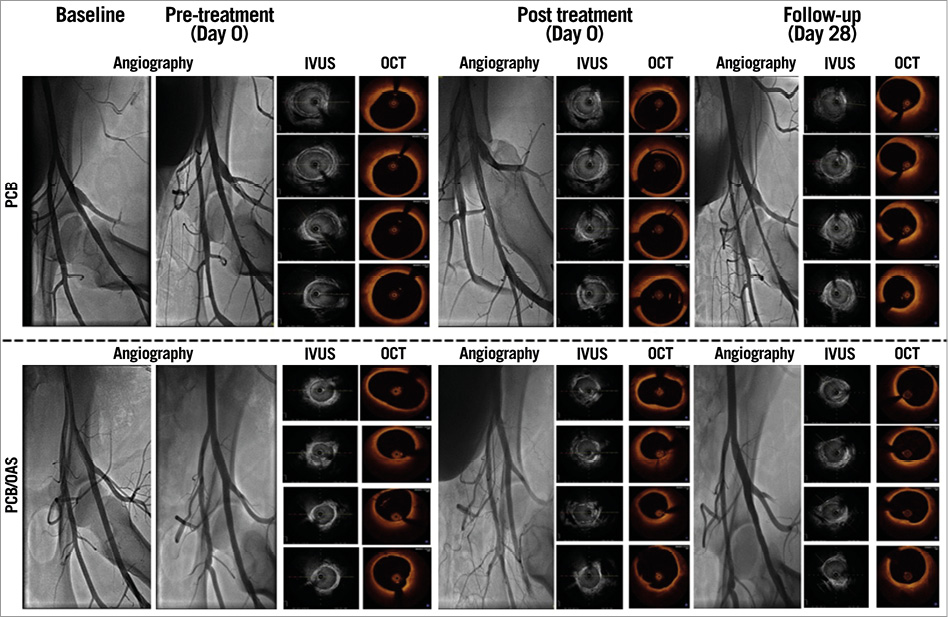
Figure 4. Representative images of the intravascular imaging.
HISTOLOGICAL ANALYSIS AND PHARMACOKINETICS
A total of 11 peripheral arteries were included for this analysis (PCB; n=5 versus PCB/OAS; n=6). Histomorphometry analysis showed comparable lumen (PCB=1.44±0.59 mm2 versus PCB/OAS=1.07±0.73 mm2), neointimal (PCB=6.7±4.34 mm2 versus PCB/OAS=6.58±2.91 mm2), and medial (PCB=5.74±1.55 mm2 versus PCB/OAS=5.38±1.37 mm2) areas within both groups (Table 3). Interestingly, scores for cholesterol crystals were lowest in the PCB/OAS treatment combination group (PCB=0.6±0.9 versus PCB/OAS=0.2±0.4). Furthermore, contrary to what was expected, the further manipulation that occurred in the PCB/OAS group did not increase the amount of inflammation compared to PCB only and neointimal inflammatory infiltration (PCB=1±0.7 versus PCB/OAS=0.3±0.5) in the PCB/OAS treatment group compared to the PCB only group. This neointima was characterised by prominent lipidic plaque formation and foam cell infiltration. Due to the advanced age of the animal model, the hypercholesterolaemic background and the vascular restenotic model, there was evidence of a cellular plaque area found in both groups with presence of cholesterol clefts (Table 3). The pharmacokinetic analysis showed a similar concentration of paclitaxel in both groups (PCB, 5.16±8.11 ng/mg versus PCB/OAS treatment, 3.03±2.59 ng/mg) (Figure 5).
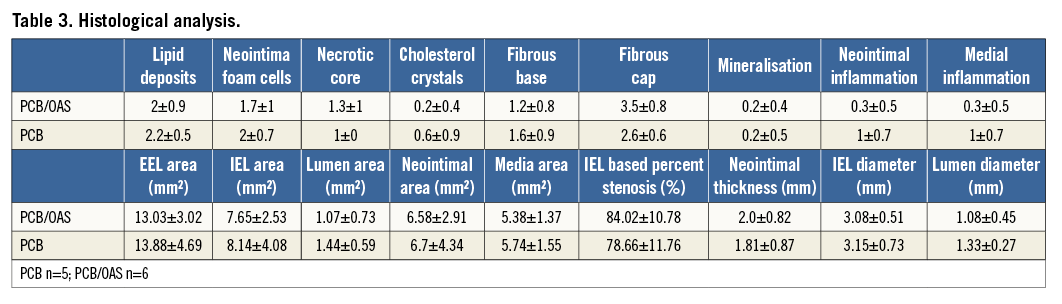
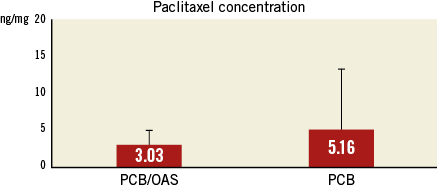
Figure 5. Pharmacokinetic analysis.
Discussion
Peripheral vascular disease is characterised by a complex biomechanical behaviour, significant plaque burden and high levels of calcification in the media4-6. Due to these unique biological characteristics, the development of successful interventional technologies has been hampered by multiple technical failures (i.e., stent fractures) and biological failures (i.e., DES failures)7-12,20. Paclitaxel-coated balloons are emerging as a potential therapy of choice for the interventional treatment of superficial femoral artery disease. Small clinical studies have shown that PCB reduce restenosis compared to balloon angioplasty in relatively short and simple lesions2,3.
Several studies have demonstrated that the use of atherectomy increases periprocedural success in peripheral vascular interventions by plaque debulking and changing vessel compliance prior to angioplasty (lesion preparation)14. However, despite these favourable acute periprocedural results, the long-term benefit in relation to restenosis and clinical outcomes is still controversial15. It has been proposed that, due to its favourable effect on plaque biomechanics (debulking and calcium reduction), the adjunctive use of atherectomy may enhance the biological effect of the antiproliferative drug provided by PCB delivery. While such a therapeutic approach appears to be make biological sense, important considerations regarding vascular safety and toxicity need to be comprehensively tested. First, due to the reduction in plaque burden induced by atherectomy, it is plausible that the paclitaxel transfer to the tissue is dramatically increased by PCB delivery. Second, due to the antiproliferative effect of the drug delivered, a significant impact on healing response may be elicited. Finally, the potential for aneurysm formation and thrombosis may be increased.
In this study, we aimed to determine the biological effect on healing and paclitaxel retention of the synergistic use of PCB and OAS in a novel model of SFA restenosis. Our laboratory has validated this model for the evaluation of drug-coated balloon technologies in the de novo21 and in-stent restenosis setting22. In this study, we induced double injury in order to increase the degree of neointimal proliferation. However, despite the degree of injury and the short follow-up period, the degree of angiographic stenosis was not significant compared to human disease. However, IVUS findings demonstrated a significant level of plaque burden amenable to interventional therapy. Following intervention, there was no significant change in acute lumen gain when both therapies were compared. The comparison of both approaches is challenging in the post-procedural setting due to associated spasm. At follow-up, there was no evidence of ectatic or aneurysmatic formation seen in either group. In addition, QVA and IVUS analysis showed a trend towards an increase of lumen gain and lower plaque burden in the PCB/OAS combination approach compared to the PCB only group. Histological analysis confirmed the safety of the combination vascular treatment. At the tissue level, there was no evidence of vascular necrosis related to the therapy or delayed healing in the PCB/OAS combination approach. Similarly, the paclitaxel tissue levels were comparable between both groups and similar to the levels reported by other investigators with a similar PCB technology22,23.
Limitations
The limitations of this study are multiple and deserve a detailed discussion. First, despite the fact that the development of this model represents a step forward towards the validation of interventional devices in a diseased vascular environment, the disease state displayed at this stage of development is still early and does not fully represent the degree or complexity seen in human atherosclerosis in the SFA territory. Second, the sample size is still limited, making the statistical comparison of the data difficult. Third, due to the long-term residence time of paclitaxel, long-term evaluation of vascular toxicity is required. Fourth, the nature of these findings may only be applied to the particular combination of technologies used in this study. Due to the technical (atherectomy devices) and pharmacological (PCB) differences of the devices used in this study, these data may not be generalisable to other similar devices. However, due to the controlled environment applied to this study we believe that evaluation of the safety of this approach can be properly investigated.
Conclusions
In summary, in the experimental setting, the combination of OAS/PCB appears to be safe at short-term follow-up. This combination approach did not display any safety concerns evaluated by both in vivo imaging and tissue analysis. In addition, we observed late lumen gain and decrease in plaque burden. However, these findings need to be confirmed in a larger sample size and at long-term follow-up. The validation of these findings needs to be investigated in the clinical setting.
| Impact on daily practice Patency in long-term follow-up after endovascular intervention depends on the vascular territory, but the femoropopliteal arterial segments have demonstrated the greatest need for reduced restenosis rates, which today are responsible for most of the lifestyle-limiting claudication present in clinical practice. Clinical trials (i.e., THUNDER trial) demonstrated a marked reduction in angiographic late lumen loss at six months by using DCB. However, these peripheral lesions are often significantly calcified and chronically occluded throughout their length. In our study, it was demonstrated that the use of orbital atherectomy for preparation of the lesion is not only safe, but may also provide benefit in drug delivery to the vascular wall. |
Conflict of interest statement
The present study was sponsored in part by Cardiovascular Systems, Inc. (St. Paul, MN, USA). R. Dattilo and J. Mustapha have consultant agreements with Cardiovascular Systems, Inc. (St. Paul, MN, USA). The other authors have no conflicts of interest to declare.

