Abstract
Aims: The routine use of paclitaxel-coated balloons (PCB) in combination with bare metal stents (BMS) in de novo coronary lesions has been questioned. In this study, we aimed to compare the vascular response of BMS implanted using a second-generation PCB (BMS+PCB) with the TAXUS stent (PES) and a BMS control (BMS) in the familial hypercholesterolaemic swine (FHS) model of coronary injury.
Methods and results: A total of 17 stents (PES=6, BMS+PCB=6, and BMS=5) were implanted in the coronary territory of 10 FHS using a 20% overstretch injury ratio. Imaging evaluation (QCA and IVUS) was conducted in all animals at baseline and 28 days following stent implantation. Following terminal imaging all animals were euthanised and stented coronary segments harvested for histological evaluation. At 28 days, the lowest degree of percentage diameter stenosis by QCA was achieved by the PES (2.9±9%) followed by the BMS+PCB (9.5±16.4%) and the BMS group (25.65±18.7%, p<0.05). In histology, percentage area of stenosis (BMS+PCB=29.6±6.4% vs. PES=21.5±3.3% vs. BMS=55.2±12.9%; p<0.01) and neointimal thickness (BMS+PCB=0.26±0.1 mm vs. PES=0.21±0.1 mm vs. BMS=0.59±0.2 mm; p<0.01) were significantly reduced in both paclitaxel groups in comparison to BMS controls. Both BMS+PCB and BMS groups had higher endothelialisation scores (PES=1.50±0.9 vs. BMS+PCB=2.73±0.4 vs. BMS=3.00; p<0.05) and lower peri-strut inflammatory scores (PES=0.83±0.4 vs. BMS+PCB=0.20±0.2 vs. BMS=0.43±0.6, p<0.05) when compared to PES. Neointima maturity (PCB+BMS: 2.00 [2-2.4] vs. PES: 1.00 [0.3-1] vs. BMS: 3.00, p<0.05) and fibrin deposition (PCB+BMS: 1.40±0.3 vs. PES: 2.17±0.7 vs. BMS: 0.27±0.3, p<0.05) scores in PCB+BMS appeared to fall between the PES and the BMS ranges.
Conclusions: In the FHS coronary injury model, BMS implantation using a PCB yields a degree of neointimal inhibition comparable to the PES. The BMS+PCB combination presented lower degrees of inflammation and fibrin deposition; however, signs of delayed healing were still present.
Introduction
Paclitaxel-coated balloons (PCB) have emerged as an alternative therapeutic tool for the treatment of in-stent restenosis (ISR). In small clinical trials of coronary ISR, a single time transfer of paclitaxel using PCB has been shown to be effective in the prevention of restenosis1,2. Following these promising results, further utilisation of PCB has been extended to other applications such as de novo lesions. Although positive clinical outcomes have been observed in the peripheral territory3,4, the use of this technology unaided and in conjunction with BMS in the coronary arteries for de novo lesions has been challenged5. On the other hand, these trials incorporated the first-generation in-fold coatings, a manufacturing process which contributed to inconsistent drug concentrations, high particulate formations and shedding during intervention. Therefore, in the last few years, developments in coating formulation have led to more consistent dosing and tissue release kinetics. We hypothesise that, due to the differences in the pharmacokinetic behaviour of paclitaxel delivered by two different methods (stent versus balloon), differences in vascular healing could be appreciated in vivo. In this study, we aimed to evaluate the variations in vascular healing of BMS delivered using a second-generation PCB technology (PCB+BMS) compared to PES and BMS controls using a novel model of a coronary artery overstretch injury in the familial hypercholesterolaemic swine.
Material and methods
DEVICE DESCRIPTION
The PCB used in this study was the second-generation Cotavance® balloon catheter (3.0 or 3.5×20 mm) (Bayer Pharma AG / MEDRAD Inc., Indianola, PA, USA) coated with a paclitaxel-iopromide formulation at a dose of 3 μg paclitaxel/mm2. The coating of this device is achieved by precise microsyringe surface deposition allowing a higher degree of coating uniformity in comparison to the original PACCOCATH® technology (data on file, MEDRAD6). Commercially available stainless steel bare metal stents (Meo:FlexST®, 3.0-3.5×18 mm; MeoMedical GmbH, Munich, Germany) were pre-mounted on the PCB. The reference stents were 3.0-3.5×18 mm paclitaxel-eluting stents (TAXUS Liberté®; Boston Scientific, Natick, MA, USA) while control devices were BMS (Liberté®; Boston Scientific) in similar sizes.
HYPERCHOLESTEROLAEMIC SWINE MODEL
A total of 10 male familial hypercholesterolaemic swine obtained from the University of Wisconsin, Department of Animal Sciences, were used in this study. This animal model has the liver LDL receptor deficient bearing a homozygous mutation in one allelic mutant gene, Lpb5 at the apo B locus, and as a consequence naturally develops hypercholesterolaemia (>240 mg/dl) and atherosclerosis even if maintained under low-cholesterol, low-fat diet7,8. By two years, these animals develop eccentric lesions that consist predominantly of macrophage-derived foam cells with admixed smooth muscle cells. By the third year, large areas of necrosis, fibrous cap formation, mononuclear cell infiltration and intraplaque haemorrhage are commonly seen in these lesions9. All animals included in the study ranged from six to eight months of age with an average weight of around 42 kg at the time of enrolment.
STUDY DESIGN
Seventeen coronary sites in 10 FHS, preferentially located in the middle arterial segments of the left and the right coronary artery were selected after IVUS screening for stent implantation and randomised in 1:1:1 fashion. A total of six PES (reference group), six BMS pre-mounted on PCB (study group) and five BMS controls were implanted targeting up to 20% overstretch. All animals were followed up for 28 days and then control coronary angiography and IVUS were performed before sacrifice. All arterial segments were dissected and harvested for histology and histomorphometric analysis.
EXPERIMENTAL PROCEDURES
The study was approved by the Institutional Animal Care and Use Committee. All animals received standard care outlined in the study protocol and in accordance with the Animal Welfare Act and the “Principles of Care of Laboratory Animals”10. The mean age of the FHS was eight months and mean weight was 42 kg. One intramuscular (IM) muscarinic anticholinergic dose (glycopyrrolate, 0.2 mg/ml, dosage 0.005-0.02 mg/kg) was given prior to procedure. Induction of anaesthesia was achieved with a rapid-acting general anaesthetic (Tiletamine+Zolazepam, Telazol™ 100 mg/ml, dosage 2-5 mg/kg). All animals underwent endotracheal intubation and were maintained with a continuous inhalation of 1-3% Isoflurane. Arterial carotid access was obtained under general anaesthesia using a cut-down technique. Anticoagulation with heparin was achieved (3,000-10,000 U) to maintain a coagulation time ≥250 seconds. Following coronary angiography, all coronary vessels were sized for proper stent implantation after prior IVUS guidance. After final angiography, Isoflurane was discontinued and the animals were extubated when the gag reflex returned. Buprenorphine 0.01-0.02 mg/kg IM and Flunixin 1-2 mg/kg IV was injected for routine pain management. Animals received Cefazolin as prophylaxis (1g IV). All pigs were terminated at 28 days following stent implantation: animals were anaesthetised and prepared in the same fashion as described above for stent implantation. Terminal angiography and endovascular imaging (IVUS) were acquired.
QUANTITATIVE CORONARY ANALYSIS (QCA)
Coronary artery angiographies were obtained using General Electric Innova digital flat-panel angiographic units. QCA analysis was performed in a blinded fashion utilising QAngio XA Software version 7.1.14.0 (Medis Medical Imaging Systems, Leiden, The Netherlands). The baseline and 28-day follow-up reference vessel diameters (RVD) were taken from the proximal and distal portions of the treated segments using the guiding catheter as a standard for measurement. The balloon-to-artery ratio was calculated. Percent diameter stenosis (%DS) at follow-up was calculated as: (1-[MLD/RVD])×100%.
INTRAVASCULAR ULTRASOUND (IVUS) IMAGING
IVUS pullback images were obtained and analysed using a coronary ultrasound catheter (Atlantis® SR Pro 40 MHz Coronary Imaging Catheter; Boston Scientific) and a commercially available measurement analytic system (iLab; Boston Scientific). Using fluoroscopy, the IVUS catheter was placed distal to the stented vascular segment and an automated pullback performed at a speed of 0.5 mm/sec covering 10 mm proximal and distal to the implantation site. The starting position of the IVUS catheter was determined by fluoroscopy and situated by anatomical landmarks in a live image during the pullback. The in-stent analysis in each vessel was performed using standard definitions11. The analysis was performed by two operators blinded to the treatments. Each individual pullback was divided into 10 sections to determine neointimal distribution along stented segments. Volumetric measurements of each section were performed using planimetry software (echoPlaque; Indec Systems Inc., Santa Clara, CA, USA). Quantitative analysis included measurement of the external elastic membrane (EEM), stent (SA) and lumen areas (LA). Neointimal area was calculated as stent area minus lumen area. Volume measurements of the stented segments were calculated by applying the Simpson’s rule12.
HISTOLOGICAL ANALYSIS
Following vessel harvesting, stented segments were immersed in normal buffered formalin 10% (NBF). For light microscopy all treated vessels were embedded in methylmethacrylate and then 40-50 micron sections from the proximal, mid and distal portion of each stented segment were obtained. These sections were stained with haematoxylin and eosin (H&E) and elastic trichrome (ET). The cross-sectional areas (external elastic lamina [EEL], internal elastic lamina [IEL] and lumen area) of each section were measured. Neointimal thickness was measured as the distance from the inner surface of the stent struts to the luminal border and then the neointimal area, the media area and % stenosis calculated. These measurements were used to calculate vessel layer areas with the following formulas: media = EEL-IEL; neointima = IEL-lumen; % stenosis=(1- [lumen area / IEL area])*100. All sections were evaluated using semi-quantitative scoring criteria. To evaluate the amount of injury and inflammation, criteria defined by Schwartz et al13 and Kornowski et al14, respectively, were utilised. A detailed methodology of descriptive histopathological analysis is provided in the Online Appendix.
STATISTICAL ANALYSIS
Normally distributed parametric data are expressed as average and standard deviation (SD) or variance, while skewed are expressed as median and interquartile range (IQR). For qualitative data, Levene’s equal variance and Shapiro-Wilk normality tests were initially performed. When equal variance and normality were observed, one-way analysis of variance (ANOVA) with Holm-Sidak post-ANOVA tests were used to test for differences in variables between stent types. When either an equal variance test or a normality test failed, a Kruskal-Wallis test (with Dunn’s method for post hoc group comparison) was conducted. A value of p≤0.05 was considered statistically significant.
Results
QUANTITATIVE CORONARY ANALYSIS
A summary of QCA results is shown in Table 1. The overstretch ratio and the reference vessel diameters at baseline and post stent implantation were not significantly different between groups. At 28 days, PES significantly reduced both %DS (89% reduction, p=0.03) and angiographic late lumen loss (77% reduction, p=0.01) when compared to BMS. In addition, there was a 63% reduction in %DS (p=0.08) and 38% in LL loss (p=0.12) in the PCB+BMS group in comparison to the BMS control group. There was no difference in any of the angiographic parameters between the PES and PCB+BMS groups.
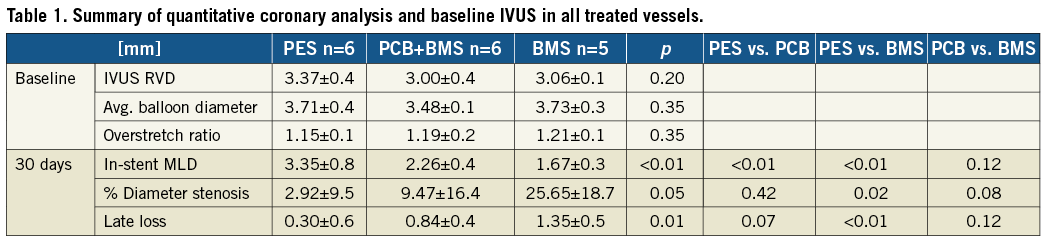
INTRAVASCULAR ULTRASOUND ANALYSIS
In vivo IVUS evaluation analysis at 28 days is presented in Table 2. The PES group showed the largest final lumen area in comparison to the PCB+BMS (32% increase) and the BMS group (55% increase). There was a significant decrease in in-stent neointimal area in the PES (78%) and the PCB+BMS groups (49%) in comparison to the BMS group (Table 2). The uniformity of neointima distribution within the stent is shown in Figure 1. In the BMS group there was a higher degree of variability of neointimal distribution throughout the length of the stent. Both PES and PCB+BMS groups displayed a comparable degree of in-stent neointimal distribution (variance: PES=0.02, PCB+BMS=0.14, BMS=0.57). The Levene’s test showed equality of variance in each group.
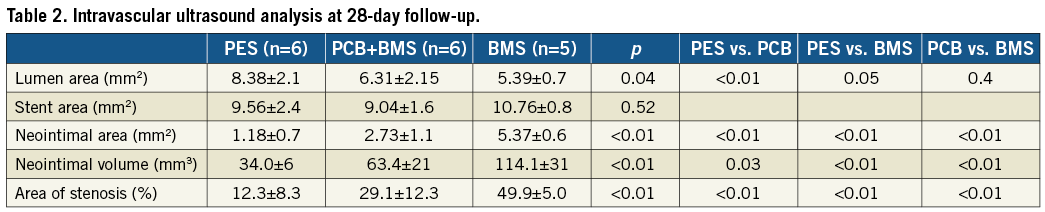
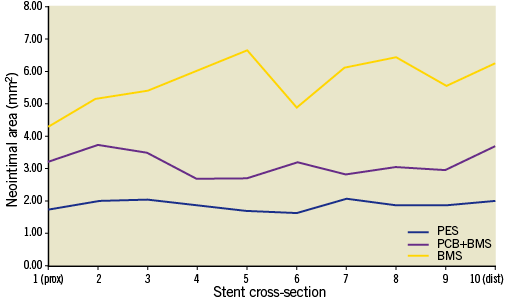
Figure 1. Neointimal distribution along stented segments assessed by IVUS.
HISTOLOGICAL EVALUATION
In the histological analysis (Table 3) the vessel areas expressed as EEL were similar among groups (p=0.13). The parameters of neointimal growth and restenosis such as neointimal thickness, neointimal area and percent area stenosis were statistically comparable between both paclitaxel groups and significantly lower when compared to BMS (Figure 2). Stents in all groups were completely covered with neointima. The representative vessel sections are presented in Figure 3. In the qualitative histology analysis (Figure 4), the injury scores were mild and comparable among all studied groups. Endothelialisation was considered complete in both the PCB+BMS and the BMS groups (p=0.22). On the other hand it appeared to be reduced in the PES group. The neointima in PCB+BMS was significantly more mature than in PES (p<0.01) and less mature than in BMS (p<0.01). The peri-strut inflammation was significantly lower in the PCB group when compared to PES (p=0.02) and similar to BMS (p=0.35). There were no significant differences in adventitial inflammation among groups. The fibrin deposition in the PCB group was significantly higher when compared to BMS (p<0.01) and lower when compared to PES (p=0.02). No differences were noticed in media cell loss among groups.
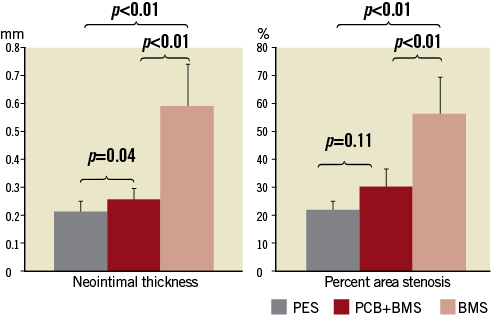
Figure 2. Histomorphometric parameters representing device efficacy.
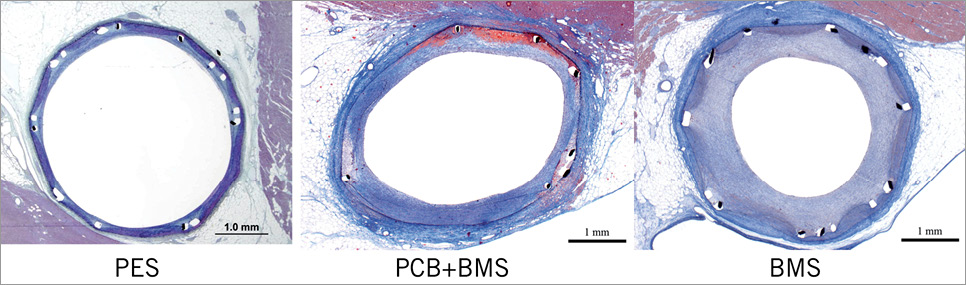
Figure 3. Representative histopathological vessel sections.
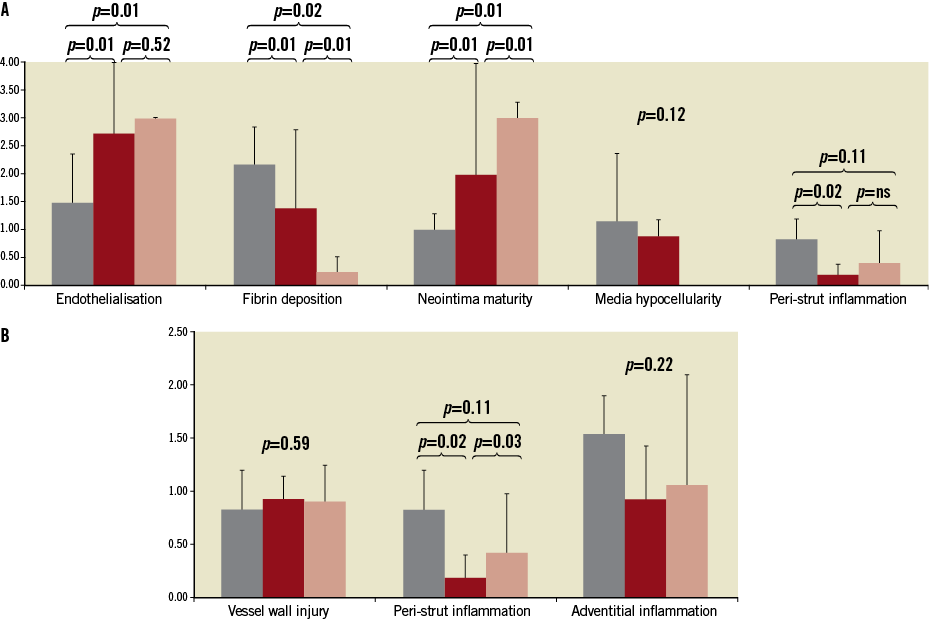
Figure 4. Qualitative histopathological analysis representing vessel healing (A) and biocompatibility (B).
Discussion
Small clinical trials have demonstrated the safety and efficacy of PCB in the treatment of coronary in-stent restenosis and de novo peripheral artery disease1-4. On the other hand, treatment of de novo lesions with PCB and BMS combination in the coronary territory has been questioned5,15-17. In the last few years, PCB have improved with regard to their coating characteristics and device performance. Due to their particular pharmacokinetic characteristics, we hypothesised that the vascular healing profile of polymer-free, bare metal stents delivered using a second-generation PCB technology may differ favourably from the well characterised PES technology.
Most drug-coated balloon technologies have been tested using the healthy porcine model of restenosis18-21. Although well validated for evaluation of safety, these models are limited in the evaluation of efficacy and vessel healing following drug delivery, especially in the SFA territory22. Therefore, in this study, we decided to use the familial hypercholesterolaemic swine (FHS) model displaying a vascular territory similar in vascular diameters, lengths and anatomic configuration to the human coronary arteries. This strain of pigs maintains high cholesterol levels despite regular diet supplementation. Several validation studies have described the progression of disease over time8,9,23. By 18 months, these animals develop complex atherosclerotic lesions with pathological features similar to human disease8,9. We have shown that by eight months (the age of animals included in the study) the nature of the disease found (fatty streaks and occasional pathological intimal thickening) would be sufficient to demonstrate an efficacy signal following overstretch stent implantation with local drug delivery either from a stent or from a balloon24.
In our study, we demonstrated a statistically comparable degree of efficacy (neointimal formation and distribution) of BMS implanted using a PCB (drug and polymer-free device concept) and a commercially available paclitaxel-eluting stent in a coronary injury model of the FHS. At last follow-up, both paclitaxel technologies proved to be effective in reducing neointimal proliferation as shown by angiographic, IVUS and histological evaluation. Neointima distribution was similar to the PES, thus showing consistent uniform drug balloon coating. However, despite this comparable degree of neointimal inhibition, PES appeared to have a more profound effect on delayed healing. In general, BMS delivered using PCB appeared to have more mature neointima and less peri-strut inflammation compared to the PES group. However, delay in the healing process expressed by somewhat impaired endothelialisation and some fibrin deposition was still present21.
The biological reasons for these results may be explained by the pharmacokinetic profile displayed by PCB technologies. Following initial drug transfer, most of the paclitaxel is deposited on the surface of the vessel and washed off over time25. In contrast to PES, most of the paclitaxel transferred to the vessel wall via PCB technologies disappears over time. Therefore, it is biologically plausible that the delayed healing response observed with PES could be ameliorated by PCB delivery. This could perhaps explain the promising results of utilisation of PCB unaided in the treatment of de novo lesions in small vessels26. However, it is important to note that, in the presence of a stent, signs of delayed healing such as fibrin deposition and delayed endothelialisation are still present. Therefore, the results of this study demonstrate how different methods of local paclitaxel delivery can affect the final vessel healing response. It seems that improved pharmacokinetic profile, overall balloon design, and lack of permanent polymer can contribute positively to an overall early result. Additionally, in our study we used a second-generation PCB, which is automatically coated and provides more homogenous paclitaxel-iopromide distribution. This technology provides more uniform drug distribution in the vessel wall and, as a result, improved vessel healing with sustained efficacy when compared to first-generation technology, which deposited the drug in the folds27.
Limitations
There are several limitations in this study. First, although a disease animal model was used, the lack of an atherosclerotic plaque component neglects the impact of tissue characteristics in drug uptake and retention. It is possible that in the presence of a plaque the biological behaviour of the PCB+BMS combination may be diverse. Different bare metal stents were used among the groups; however, their design, material and strut thickness were comparable. The study included a small sample size, which may hide the possibility that PCB+BMS may be inferior to PES in a larger cohort. Longer follow-up (90 days) is required to exclude the occurrence of the well described catch-up phenomenon seen in PES, which could also have been seen in the PCB group.
Conclusions
In summary, in a novel animal model of coronary injury, BMS delivered with a second-generation PCB technology displayed a degree of efficacy comparable to PES at short-term follow-up. The degree of vascular healing of this technology fell in between the BMS and the PES groups. However, biological signs of delayed healing were still present. The biological effects of this therapeutic alternative deserve further investigation using larger sample sizes and longer follow-up times.
Funding
MEDRAD Interventional/ (Indianola, PA, USA) provided financial support and materials for this study.
Conflict of interest statement
At the time of experiment, Mark Stenoien was a full-time employee of MEDRAD. The other authors have no conflicts of interest to declare.
Appendix
DESCRIPTIVE HISTOPATHOLOGICAL ANALYSIS
To evaluate the amount of injury, criteria defined by Schwartz et al10 were utilised: 0=IEL intact, 1=IEL lacerated, 2=media lacerated, 3=EEL lacerated. To evaluate the extent of peri-strut inflammatory reaction the following grading by Kornowski et al11 was used: 0=minimal inflammatory response around strut, 1=few inflammatory cells around strut, 2=mild to moderate inflammation, can extend into but not efface surrounding tissue, 3=dense and thick peri-strut aggregate of inflammatory cells, effacing surrounding tissue. Each strut in the section was scored and the mean inflammation and injury score for each section was calculated and reported. Adventitial inflammation score is based on the following criteria: 1=mild peripheral inflammatory infiltration in less than 25% of the relevant vessel compartment, 2=moderate peripheral inflammatory infiltration or focally marked in 25-50% of the relevant vessel compartment, 3=heavy peripheral inflammatory infiltration or focally marked in greater than 50% of the relevant vessel compartment. The endothelialisation score was described as percentage endothelial coverage of the arterial circumference: 0≤25%, 1=25-75%, 2=76-95%, 3=complete. The extent of fibrin deposits was assessed as follows: 0=none to focal, 1=mild involving <10% of artery circumference, 2=moderate involving of 10-25% artery circumference, 3=heavy, involving >25% of artery circumference. The neointimal maturity was defined as: 0=not present, 1=light dispersed smooth muscle population, 2=heavier population throughout less than the entire thickness of the neointima, 3=dense population throughout the entire thickness of the neointima. The media hypocellularity was a resultant of severity of cellular loss and its extension within arterial circumference graded from 0-3.

