Abstract
Aims: In a non-injured porcine coronary artery model, the aim was to evaluate vascular compatibility of the novel platinum chromium everolimus-eluting PROMUS Element stent as compared to the following control stents: everolimus-eluting PROMUS (XIENCE V), bare metal Element, and polymer-only Element.
Methods and results: Stent pairs (n=228) evenly distributed among the four stent types were implanted in overlap configuration in 79 pigs at a targeted stent-to-artery ratio of 1.1:1. Similar numbers were explanted at each of 7, 30, 90, 180, and 270 days for pathological analysis. No stent-related mortality or morbidity was observed. There were no stent occlusions or strut fractures. The PROMUS Element was more radiopaque than PROMUS (relative densities 9.9 and 9.1, respectively) and demonstrated at all time points vascular compatibility similar to that of the control stents for endothelial cell coverage, inflammatory response, and neointima formation. At 30 days, parastrut fibrin was mild but greater (P<0.0001) for the drug-eluting stents than either for the bare metal or the polymer-only Element; however, by 90 days the fibrin had dissipated.
Conclusions: In the non-injured porcine coronary artery model, the PROMUS Element demonstrated vascular compatibility equivalent to PROMUS and the bare metal and polymer-only stents.
Introduction
The everolimus-eluting PROMUS™ (XIENCE V™) stent is one of a new generation of coronary artery drug-eluting stents (DES) that has demonstrated impressive efficacy and safety in clinical trials.1-4 The PROMUS Element™ stent has the same drug and polymer as PROMUS on a novel platinum chromium alloy stent platform, which was designed to have increased radiopacity compared to previous platforms.
The objective of this study was to evaluate safety and time dependent vascular responses to overlapping stents implanted over a wide range of durations from 7 to 270 days in a non-injured porcine coronary artery model. We report on results obtained with the PROMUS Element, bare-metal Element, polymer-only Element, and PROMUS devices.
Methods
This study complied with the Guide for the Care and Use of Laboratory Animals (U.S. National Institutes of Health Publication 85-23, revised 1996). Stent implants were performed at MPI Research, Inc., Mattawan, MI, USA, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Study procedures, including pathologic evaluation, were performed in compliance with Good Laboratory Practices (GLP) as defined in the U.S. Code of Federal Regulations, 21CFR Part 58.
Study devices
Control devices (3.0 and 3.5×8 mm) included the bare metal Element stent (Boston Scientific Corporation [BSC], Natick, MA, USA), the polymer-coated Element stent (BSC), and the PROMUS (XIENCE V) everolimus-eluting stent (Abbott Vascular, Santa Clara, CA, USA). The test device was the PROMUS Element everolimus-eluting stent (3.0 and 3.5×8 mm, BSC, Natick, MA, USA). The Element stent platform is made of a novel platinum chromium alloy and was designed to have improved deliverability and increased radiopacity compared to earlier generation stents while maintaining low stent recoil. Element deployment recoil (bench tested under ASTM standard F2079) is 3.6% (95% confidence interval [CI] 3.2-4.0%, n=15) compared with 4.6% (95% CI 4.2-5.0%, n=10) for Vision, the platform for PROMUS (Xience V)5. The Element stent consists of 33% platinum homogeneously alloyed into a 316L stainless steel base material. The chromium oxide rich surface is corrosion resistant and the nominal nickel content is lower than that found in stainless steel and the available cobalt chromium stents. Thinner stent struts have been associated with reduced restenosis after stenting in humans6-8 and the platinum chromium alloy provides enhanced radial strength and fracture resistance to allow a thinner strut (81 microns) than that of some earlier generation DES. Relative radiopacity (density) is 9.9 for the Element and 9.1 for the cobalt chromium Vision platform5. The higher density of platinum versus iron or cobalt enhances the radiopacity of the platinum chromium alloy compared to stainless steel or cobalt chromium (Figure 1).

Figure 1. Comparison of radiopacity of the PROMUS and PROMUS Element stents. The difference in radiopacity between the (A) PROMUS/Xience V (3×28 mm) and (B) PROMUS Element (3×32 mm) stents reflects increased density of the Element platinum chromium alloy compared to the cobalt chromium alloy of the Vision platform.
The stent has a continuous cell geometry design to provide uniform drug delivery along its length and is mounted on a delivery system based on the APEX™ dilatation balloon catheter. The PROMUS Element contains the antiproliferative agent everolimus9 applied to the Element stent using the same combination of polymer layers found in the PROMUS everolimus-eluting stent4 with a drug dose density of 100 µg/cm2. The identical polymer coatings, consisting of a primer layer (n-butyl methacrylate) and a drug matrix layer (vinylidene fluoride-co-benaxfluoro-propylene) blended with everolimus, provide a similar drug elution profile with near complete drug release at 90 days.
Study protocol
Success criteria defined in the study protocol included statistically equivalent outcomes between the PROMUS Element and PROMUS stents in the non-injured swine model for responses (assessed by histomorphology and histomorphometry) including luminal thrombus, endothelialisation, inflammatory response, neointimal formation, and vascular stability (lack of substantial positive or negative remodelling of the arterial wall). Stents were placed in an overlap configuration (stent pair) in the left anterior descending (LAD), left circumflex (LCX), and/or right coronary (RCA) arteries of non-injured swine following a previously described implantation protocol and antithrombotic regimen.10 One overlapping pair of the same device type was implanted per artery. Stent pairs were evenly distributed among the four stent types with similar numbers explanted at each of the follow-up durations of 7, 30(±2), 90(±3), 180(±3) and 270(±3) days. With only three available coronary arteries, the four stent types were rotated in balanced distribution with most animals receiving three of the four types. Animals in five implant groups (15-17 animals per group) received one to three overlapping control or test device stent pairs; targeted stent-to-artery diameter ratio was 1.1:1 using quantitative coronary angiography, with no vessel pre-injury. Of 79 pigs, 19 received the PROMUS Element, bare metal Element, and polymer-coated Element stent pairs; 19 received the PROMUS Element, bare metal Element, and PROMUS; 15 received the PROMUS Element, polymer-coated Element, and PROMUS; three received the PROMUS Element and polymer-coated Element; four received the PROMUS Element and PROMUS; and 19 received only control (PROMUS, polymer-coated Element, bare metal Element) stent pairs.
At scheduled follow-up, animals were anesthetised and final angiography performed to document stent pair patency. Endpoints included cardiac death, myocardial infarction (MI), histopathology on any abnormal tissue seen at necropsy, clinical chemistry, and haematology as well as morphology, morphometry, strut fracture, and scanning electron microscopy (SEM) of stented segments. Tissue harvesting and processing for histology and SEM were performed as described previously.10 Histomorphological analysis of in-stent sections was performed using an ordinal grading scale10 of zero to up to four (with higher numbers indicating a worse outcome) of the following: luminal thrombus, endothelialisation, strut tissue coverage, parastrut leukocytes, disruption of the internal elastic lamina (IEL), disruption of the external elastic lamina (EEL), medial smooth muscle cell (SMC) loss, and parastrut amorphous material (PAM, including fibrin and potentially cellular debris). Histomorphometric and SEM evaluation of stent pair explants were performed as described previously.10 The study pathologist (G.J.W.) was blinded until completion of the histopathology evaluations for each time point.
Statistical analysis
All histomorphological and histomorphometric data were statistically analysed on a segment-by-segment basis using SAS® System Software Version 8.2 (SAS Institute, Cary, NC, USA). P values less than 0.05 were considered significant. The only stented vessels excluded from statistical analysis were those from unscheduled deaths that occurred outside the designated time points. Sections were excluded from statistical analysis on technical grounds if a mid-section did not show a double row of struts (indicating stent overlap) or a proximal or distal section did show a double row of struts (indicating a misplaced section location within the overlap zone).
The frequency distribution of morphologic ordinal grades was reported by group at each time point and location (i.e., proximal, overlap and distal sections). Morphologic values were ranked within each location and time point and one-way analysis of variance (ANOVA) was performed for group comparisons. Similarly, differences among locations within each group and time point and differences across time points within each section were tested. The Ryan-Einot-Gabriel-Welsh method was used to perform pair-wise comparisons on these ranks for time points within each group and location and for pair-wise comparisons of sections within each group and time point.
For morphometric parameters, a 2-sample t-test was performed to compare groups within each location and time point. Differences across locations within each group and time point and comparisons across time points within each group and across sectioning locations were assessed with one-way ANOVA. Pair-wise comparisons of time points within a group and location and comparisons of sectioning locations within each group and time point were done using the Ryan-Einot-Gabriel-Welsh method.
Results
There was no stent-related mortality or morbidity (including abnormal histology or haematology/blood chemistry abnormalities). Examination of the myocardium from every animal showed no significant pathology or evidence of ischaemic changes downstream of the stented vessels. All stents were angiographically patent (TIMI flow 3) at all time points examined. There were no stent occlusions or strut fractures. There were three unscheduled deaths due to non-cardiac conditions (lameness due to limb injuries in two cases; perforation of the spiral colon in the third). Stented vessels from these animals were found to be widely patent with unremarkable tissue responses but were not included in the statistical analysis. Of 549 in-stent sections examined histologically, seven were excluded from statistical analysis on technical grounds (see Methods).
Comparative vascular response
Remarkably similar vascular responses were seen histologically for the two drug-eluting stents (DES), PROMUS Element and PROMUS, at proximal, overlap, and distal section locations across all time points (7, 30, 90,180, 270 days) and on examination by SEM at 7, 30, and 90 days. Comparison of the PROMUS Element with the bare metal Element and polymer-coated Element controls revealed only subtle differences for almost all assessment parameters. The data presented below come from overlap sections (unless otherwise stated) where there is a theoretical doubling of both drug release and tissue contact with coating polymer. They are, however, generally representative of results obtained in the proximal and distal sections. Results of statistical analysis for comparisons of eight morphology and seven morphometry parameters across proximal, overlap, and distal sections and across follow-up durations are not presented in detail because the inferred differences were subtle and not adverse from a safety perspective.
Morphology
Statistical analysis comparing overlap sections (seven to 11 per stent type per parameter at each time point) across the four device types showed no significant differences for luminal thrombus, endothelial cell coverage, strut tissue coverage, inflammation (parastrut leukocytes), IEL disruption, and medial SMC loss at any time point. Almost all scores were zero for luminal thrombus (none), endothelial cell coverage (>90%), and strut tissue coverage (no strut uncovered) indicating preferred healing responses. Scores for IEL disruption and medial SMC loss were more variable but only PAM at 30 days and EEL disruption at 270 days showed statistical significance in these comparisons.
In every section (proximal, overlap and distal) at 30, 90, 180, and 270 days the bare metal Element stents demonstrated the best possible (grade 0) scores for endothelial cell coverage (>90%), strut tissue coverage (no struts uncovered), and luminal thrombus (a single microthrombus in one section is described below). Parastrut fibrin was almost always absent and never more than mild. Disruption (loss) of the IEL or EEL indicates either mechanical injury at implantation or loss of elastica due to elastase activity associated with inflammatory/remodelling activity. IEL disruption was almost always grade 0 or 1 (<25% of circumference) and never exceeded grade 2 (25-50% of circumference). The EEL disruption grade was most often 0 and never exceeded grade 1 (<25% of circumference). Medial SMC loss was usually grade 0 or 1 (<25% of media), did not exceed grade 2 (25-50% of media) at 90 days and reached grade 3 (50-75% of media) or grade 4 (75-100% of media) in a few sections at 180 and 270 days.
Table 1 presents the distribution of 7-day scores by device for luminal thrombus and percentage of struts not covered by endothelium.
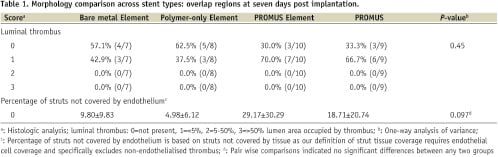
Healing at seven days was incomplete for all four device types, as expected. Limited amounts of incomplete endothelial cell coverage of thrombus that formed soon after implantation on and between stent struts was seen with all devices. In almost every section of every device this thrombus did not protrude noticeably into the lumen. Exceptions included one thrombus each in five of 101 in-stent sections examined with the following maximum dimension and percent of lumen area: two PROMUS (0.19 mm, 0.13% and 0.65 mm, 1.50%); one PROMUS Element (0.25 mm, 0.29%); one bare metal Element (0.14 mm, 0.08%); and one polymer-coated Element (0.11 mm, 0.10%). Parastrut thrombotic material, which is part of the early healing process following stent placement,11 was similar across all groups. There was not a statistically significant difference in strut endothelial cell coverage across the four stent types or between any two on pair wise comparisons. There was a trend (P=0.097) towards less strut endothelial cell coverage in the DES versus the bare metal Element and polymer-only Element but no significant difference between the PROMUS and PROMUS Element.
Endothelialisation was complete (grade 0, >90%) at 30 days and there was a complete absence of luminal thrombus in every section of all stented vessels. Strut tissue coverage with endothelialised neointima was complete in all sections for all devices with the exception of one section from PROMUS which showed a single strut not covered with tissue. By 90 days there was complete endothelialisation and complete strut tissue coverage for all stent types. A single thrombus (0.39 mm; 0.05%) was seen in one bare metal Element section. At 180 and 270 days all histologic sections from all stented vessels demonstrated complete endothelialisation with tissue coverage of every strut and a complete absence of luminal thrombus.
Scores for PAM at 30 days, which consisted essentially entirely of fibrin, were significantly different in the overlap sections (P<0.0001) across the four device types. The two DES were not significantly different from each other but they did have more fibrin than either the bare metal Element or polymer-only Element. Most scores at 30 days were grade 1 (mild) and none exceeded grade 2 (moderate) for the PROMUS Element or PROMUS. By 90 days, scores for the four devices were not significantly different (P=0.38). Most DES scores were zero (no fibrin) and none exceeded mild. Representative high magnification images comparing parastrut fibrin deposition between the PROMUS Element and PROMUS at 30 and 90 days are presented in Figure 2.
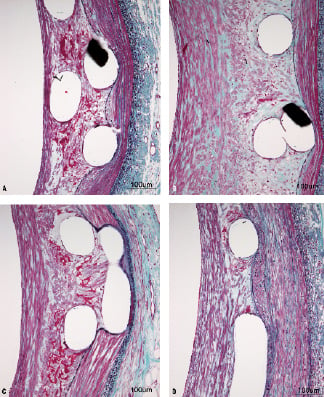
Figure 2. Parastrut fibrin deposition with the PROMUS Element and PROMUS stents at 30 and 90 days. Representative images of parastrut fibrin deposition (stained dark red, elastic-trichrome stain at 200× magnification) in overlap sections compare the PROMUS Element (a,b) and PROMUS (c,d) at 30 days (a,c) and 90 days (b,d) after device implantation. Fibrin deposition is equivalent between the PROMUS Element and PROMUS at both time points, declining from small amounts at 30 days to trace amounts at 90 days. a. 4667 RCA-C, b. 4629 LCX-C, c. 4666 LAD-C, d. 4627 RCA-C.
The two DES were associated with equal deposition of modest amounts of fibrin at 30 days, which had almost entirely cleared by 90 days. This was attributed to everolimus, as almost no fibrin was seen with either the bare Element or polymer-only Element stents.
Scores for EEL disruption (focal loss) at overlap at 270 days were significantly (P=0.042) different across device types but there was no statistical significance for any of the paired comparisons between the device groups. Mean scores were identical at 0.91 for the PROMUS Element, PROMUS, and polymer-only Element; the mean bare metal Element score was 0.10.
There were no statistically significant differences in inflammation across device types at any time point, although there were occasional instances of severe inflammatory reactions, including granuloma formation, in all but bare metal Element stents. Of 149 histologically examined stented vessels in 59 pigs (not including the 7-day time point, which is too early for a severe inflammatory response to develop), 13 (8.7%) were so affected, with six instances occurring in three pigs (two vessels/pig). This prevalence of severe inflammation is an expected response when conducting DES studies in the porcine coronary artery model.12
By SEM (three test and three of each control) at seven days, all PROMUS Element, PROMUS, and bare metal Element stented vessels and one polymer-coated Element vessel showed grade 0 (>90%) endothelial cell coverage and none showed any evident thrombus on the lumen surface. Two polymer-coated Element stent pairs could not be scored by SEM for endothelial cell coverage due to mechanical damage by surgical scissors at bisection of the stent pairs prior to processing, a problem to some extent for all stented vessels at seven days. Nevertheless, most observed struts of all four stent types were well covered by endothelium at seven days. At 30 days, healing had progressed so that there was complete endothelial cell coverage of all struts of all stent types explanted for SEM, the only exceptions being incomplete endothelial coverage of a few “jailed” struts crossing side branches. By 90 days there was complete endothelial cell coverage of all struts. No luminal thrombus was seen on SEM at 30 or 90 days.
Morphometry
Statistical analysis comparing morphometric parameters across the four device types at 30, 90, 180, and 270 days (seven days was too early for these parameters to be meaningful) for the overlap sections showed no significant differences for all measured morphometry parameters assessed –lumen, IEL, EEL, medial and intimal areas, neointimal thickness (average of measured strut-to-lumen lengths), and IEL-based area stenosis– with one exception. Intimal area at 30 days was significantly different (P=0.043) across device types due to greater intimal thickness in the polymer-only Element group, which was linked to a single outlier. A comparison across stent types and time points of the key morphometric indices neointimal thickness, IEL-based area percent stenosis indicative of neointima formation, and EEL area indicative of vascular stability is shown in Figure 3. Morphometric measurements thus confirmed the similar histology across all groups from 30 through 270 days.
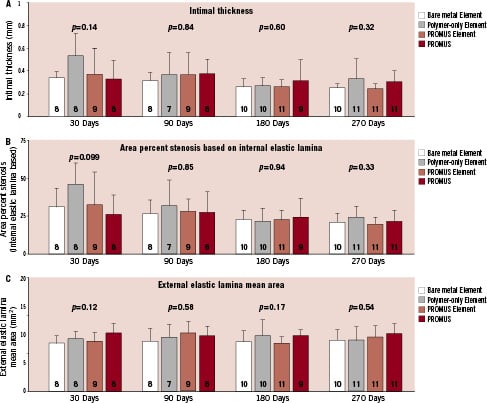
Figure 3. Comparison of morphometric indices across stent types and time points (overlap region). Intimal thickness and internal elastic lamina based area percent stenosis (mean±standard deviation) measuring neointima formation, and the external elastic lamina area measuring vessel stability are plotted for the four durations at 30, 90, 180, and 270 days. There were no statistically significant differences across the 4 device types at any of the time points. P values are from one-way analysis of variance. N for each group is shown on the bar.
Representative histology
Figure 4 shows representative low magnification views of the entire stented arteries from areas of stent overlap for the PROMUS Element, PROMUS, and the bare metal Element at 30, 90, and 270 days. Results at 180 days were similar to the 270-day images. Results with the polymer-only Element stent were similar to that with the PROMUS Element. Again, most remarkable are the similarities among the stent types, also evident from the aforementioned comparisons of eight morphology and seven morphometry parameters. Although a detailed review has not been presented here for the proximal and distal sections, those findings were similar to that of the overlap sections. On paired comparisons, the PROMUS Element was not found inferior for any morphologic or morphometric parameter at 30, 90, 180, or 270 days for proximal, overlap or distal sections.

Figure 4. Representative histologic examples (overlap region) at 30, 90, and 270 days. Images (elastic-trichrome staining, 20× magnification) representing average luminal stenosis of overlapped sections are shown for the PROMUS Element (A,B,C), PROMUS (D,E,F) and the bare metal Element (G,H,I) stented vessels at 30 days (A,D,G), 90 days (B,E,H), and 270 days (c,f,i) after device implantation. The vascular response to all three stent types is remarkably similar. a. 4655 RCA-C, b. 4636 LAD-C, c. 4640 RCA-C, d. 4618 LCX-C, e. 4633 LAD-C, f. 4639 LAD-C, g. 4659 LAD-C, h. 4626 LAD-C, i. 4632 RCA-C.
Discussion
This study evaluated safety and vascular responses over a wide range of implant durations to the everolimus-eluting PROMUS Element stent in a non-injured porcine coronary artery model. The PROMUS Element was compared to the everolimus-eluting PROMUS (XIENCE V) stent and to the bare metal Element and polymer-only Element stents. Equivalent results for all histological parameters assessing vascular compatibility were obtained with the PROMUS Element and PROMUS at proximal, overlap, and distal section locations across all time points (7, 30, 90,180, 270 days). Comparison of the PROMUS Element with the bare metal Element and polymer-only Element controls revealed only subtle differences for the assessment parameters. All four stent types evaluated are judged to be safe based on all of the data from this animal model.
All measures of neointima formation, including intimal thickness (Figure 3A), and IEL-based area percent stenosis (Figure 3B) were similar in the overlap sections at all time points among both DES and the bare metal Element stent. Thus, efficacy in reduction of neointima for either DES was not demonstrated in this non-injured animal model. In a previous study in the same porcine coronary artery overlapping stent model10, paclitaxel-eluting stents (TAXUS Liberté,) demonstrated a lower intimal thickness and less IEL-based area stenosis at overlap at 30 days compared to the bare metal control but greater neointima, based on both these morphometry parameters, at 90, 180, 360, and 580 days. The lack of sustained inhibition of intimal hyperplasia by DES has also been seen in the Yucatan mini-pig coronary model for non-overlapping CYPHER stents13 and for both CYPHER and TAXUS overlapping stents in the rabbit iliac artery model.14 Thus, for the-limus family of drugs and paclitaxel in both pigs and rabbits, one cannot expect results predictive of the remarkable profound and sustained reduction in late loss observed in the clinic, including that with everolimus in the PROMUS (XIENCE V) stents.2-4
Equivalent mild parastrut fibrin deposition was observed with both the PROMUS Element and PROMUS (Figure 2) and was the only morphologic characteristic that distinctively separated these DES from the bare metal and polymer-only controls. The peak amount of fibrin deposition was, however, considerably less than seen with paclitaxel and had substantially dissipated by 90 days in contrast to the slower clearing of paclitaxel-induced parastrut fibrin (see online supplement of reference 10 for detail). 10,15
Severe inflammatory responses with parastrut granuloma formation were seen in a small proportion of DES and polymer-only coated stents in this study, which is an expected finding in the porcine coronary artery model12,16 and does not detract from safety. The scoring system for parastrut inflammation in the present study is illustrated in Wilson et al17 which described a substantially greater prevalence and extent of inflammatory response to CYPHER sirolimus-eluting stents than that observed with everolimus in the present study in the same overlapping porcine coronary artery model.
It should be noted that the vascular response to the novel platinum chromium alloy bare metal Element stent demonstrated an excellent safety profile that was remarkably similar to that of the Liberté stainless steel platform evaluated in the identical model using the same evaluative parameters.10 Thus, the new platinum chromium alloy with enhanced radiopacity and improved radial strength for adequate stent expansion had no negative impact on biocompatibility. This is in accord with the experience of the use of platinum in other human implants including aortic stents18 and intra-cranial electrodes.19 While most published experience in animal models with BMS or DES does not extend beyond 180 days, follow-up well beyond six months with both bare metal Liberté10 and bare metal Element (present study) has indicated that the observed progressive medial SMC loss with BMS is not a safety concern as vascular stability (e.g., stability in EEL area) was maintained.
Limitations of this study include the use of a non-injured pig coronary artery model as a human clinical surrogate. Struts of implanted stents are quickly covered with a rapidly proliferating endothelial cell covered neointima in the porcine model.11,16 In addition to not predicting the sustained reduction in neointimal proliferation seen clinically with several DES, as discussed above, this porcine model is not a sensitive indicator of differences in strut endothelial cell coverage. Furthermore, in this study the evaluation of endothelialisation was limited to SEM and conventional histology and did not include immunochemical evaluation of endothelial cell function or other molecular and microscopic techniques. The rabbit iliac artery, in which endothelialisation is slower than in porcine coronary arteries, may be a better model to evaluate the endothelialisation process in DES.20 Nevertheless, the non-injured porcine coronary artery model is an accepted model for preclinical DES evaluation and provides valuable insights in safety profiling of new-generation stents.
Conclusion
In the non-injured porcine coronary artery model the PROMUS Element demonstrated vascular compatibility equivalent to PROMUS and the bare metal and polymer-only stents. The Element platform had better radiopacity than PROMUS.
Acknowledgments
The authors gratefully acknowledge Mohammad Eskandarian, Sue Omar, Gordana Svajger, Peter Wilson, and Andrea Chiaramida in Dr. Wilson’s group who assisted in pathological evaluations and manuscript preparation; Alan Yu and Yun Lu (Boston Scientific Corporation [BSC]) for assistance with statistical analyses; and Dennis Boismier and Tim Mickley (BSC) for manuscript review.

