Abstract
Aims: This study evaluated vascular compatibility of the novel platinum chromium alloy Element™ stent platform delivering abluminal everolimus from a poly-lactide-co-glycolide bioabsorbable polymer (SYNERGY™ stent), currently undergoing clinical trial, compared with the PROMUS® (XIENCE V) and bare metal and polymer-only Element™ stents.
Methods and results: Stents (n=161) were implanted one per coronary artery in 72 swine at a stent-to-artery ratio of 1.1:1. Similar numbers of each device group were explanted at each of 30, 90, 180, and 360 days (except no PROMUS® (XIENCE V) stent at 360 days) for pathological analysis. There was no stent thrombosis, myocardial infarction, or strut fractures in any group. Vascular response was similar between the SYNERGY™ and PROMUS® (XIENCE V) stents, with no thrombi and complete endothelialisation on both scanning electron microscopy and histology at 30, 90 and 180 days. There were no significant differences for the morphologic parameters of luminal thrombus, endothelial cell coverage, strut tissue coverage, inflammation, internal elastic lamina (IEL) disruption, external elastic lamina (EEL) disruption and medial smooth muscle cell loss across device groups or between time points, but there was mild but greater (p<0.0001) para-strut fibrin at 30 days for both drug-eluting stents (DES) compared with the bare and polymer-only controls; this difference completely dissipated by 90 days. Inflammation was predominantly minimal to mild for all device types. No morphometric parameters, including intimal thickness, stent profile-based area stenosis, and EEL area were significantly different when comparing the SYNERGY stent with the bare metal Element™ and polymer-only Element™ control stents at 90, 180 and 360 days.
Conclusions: In this non-injured porcine coronary artery model, the bioabsorbable polymer SYNERGY™ stent demonstrated vascular compatibility equivalent to the PROMUS® (XIENCE V) stent and to the bare metal and polymer-only Element™ stents.
Abbreviations
ANOVA: analysis of variance
DES: drug-eluting stent(s)
EEL: external elastic lamina
IEL: internal elastic lamina
LSD: least significant difference
PLGA: poly-lactide-co-glycolide
SEM: scanning electron microscopy
SMC: smooth muscle cell
Introduction
The SYNERGY™ everolimus-eluting stent (Boston Scientific Corporation [BSC], Natick, MA, USA) consists of the platinum chromium alloy Element™ stent platform delivering abluminal everolimus1 from a poly-lactide-co-glycolide (PLGA) biodegradable polymer. This stent is currently undergoing clinical investigation in the EVOLVE trial (ClinicalTrials.gov NCT01135225). The SYNERGY stent design concept provides the minimal amount of drug necessary to be effective, delivered via a biodegradable polymer, such that the polymer is resorbed once drug elution is complete. Limiting the polymer to the abluminal aspect of the stent allows a bare metal luminal surface, reduces total polymer burden, and eliminates chronic polymer exposure. The Element stent platform has been designed to provide increased radiopacity, deliverability, conformability, radial strength and fracture resistance compared to earlier generation stents, while maintaining low stent recoil2.
The objective of this study was to evaluate safety and time-dependent vascular responses to SYNERGY stents explanted over a wide range of follow-up in a non-injured porcine coronary artery model. The SYNERGY stent was compared with the PROMUS® (XIENCE V) stent, which also elutes everolimus, and both a bare metal Element™ stent and a polymer-only (no drug) coated Element™ stent that acted as controls.
Methods
This study complied with the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health Publication 85-23, revised 1996). Stent implants were performed at MPI Research, Inc., Mattawan, MI, USA, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, Frederick, MD, USA. Study procedures were conducted in compliance with the study protocol and all applicable test facility standard operating procedures.
Study devices
Control devices (3.0 and 3.5±12 mm) included the bare metal Element stent (BSC, Natick, MA, USA), the polymer-only coated Element stent (BSC), and the PROMUS (XIENCE V) everolimus-eluting stent (Abbott Vascular, Santa Clara, CA, USA). The test device (3.0 and 3.5±12 mm, BSC, Natick, MA, USA) was the SYNERGY abluminal everolimus-eluting stent with PLGA biodegradable polymer on the platinum chromium alloy Element stent platform. The Element stent platform is made of a novel platinum chromium alloy and was designed to have improved radiopacity, deliverability, conformability, radial strength and fracture resistance compared to earlier generation stents while maintaining low stent recoil. The SYNERGY stent coating contains the antiproliferative agent everolimus applied abluminally to the Element stent using a PLGA biodegradable polymer with a total coat weight of approximately 150 µg. The everolimus cumulative release profile over time for the SYNERGY stent is similar to the PROMUS (Xience V) stent. Figure 1 illustrates the PLGA biodegradable polymer applied only to the abluminal surface of the stent struts (roll coat) and demonstrates the durable polymer applied around the entire strut perimeter to a thickness of approximately 7 µm for the PROMUS (XIENCE V) stent compared with 3-4 µm for the SYNERGY stent. The abluminal drug delivery and biodegradable polymer provide more targeted drug delivery with complete absorption of drug and polymer by four to six months.
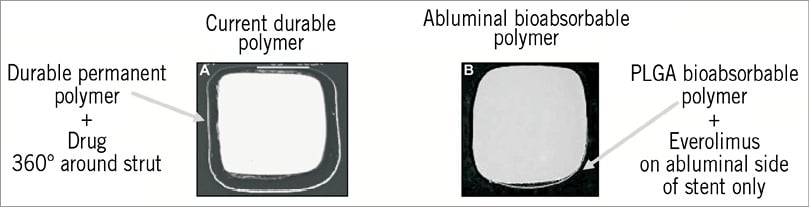
Figure 1. The PLGA biodegradable polymer. Comparison of the polymer applied around the entire strut perimeter to a thickness of about 7 µm for the PROMUS (XIENCE V) stent (left) versus the abluminal poly-lactide-co-glycolide (PLGA) polymer of about 3-4 µm in thickness for the SYNERGY stent (right).
Study protocol
Success criteria defined in the study protocol included statistically equivalent outcomes between the SYNERGY and PROMUS (XIENCE V) stents in the non-injured swine model for vascular responses (assessed by histomorphology and histomorphometry) including luminal thrombus, endothelialisation, inflammatory response, neointimal formation, and vascular stability (lack of substantial positive or negative remodelling of the arterial wall). Twelve millimetre-long stents were implanted one each in the left anterior descending, left circumflex, and/or right coronary non-injured arteries of swine following a previously described implantation protocol and antithrombotic regimen4. Targeted stent-to-artery diameter ratio was 1.1:1 using quantitative coronary angiography, with no vessel pre-injury.
A total of 161 stented coronary artery specimens from 72 animals (including three unscheduled deaths) were received for analysis. Of these, a total of 133 stented coronary artery specimens explanted at 30 days (33 stents), 90 days (33 stents), 180 days (36 stents) and 360 days (31 stents) post-implantation were examined by histopathology and 21 stented coronary artery specimens (eight each at 30 and 90 days and five at 180 days) were examined by scanning electron microscopy (SEM). An additional seven stented vessels from three unscheduled deaths were also examined histologically. Similar numbers of devices for the drug-eluting stents (n=11-13) and bare and polymer-only controls (n=8-10) were explanted at each of the follow-up time points. The PROMUS (XIENCE V) stent was not evaluated at 360 days because the purpose of the long-term evaluation was to compare the vascular response to the Synergy biodegradable polymer stent, both with and without everolimus, with the same bare metal stent platform acting as a control.
At scheduled follow-up, animals were anaesthetised and final angiography performed to document stent patency. Endpoints included cardiac death, myocardial infarction, histopathology on any abnormal tissue seen at necropsy, clinical chemistry, and haematology as well as morphology, morphometry, strut fracture, and SEM of stented segments. Tissue harvesting and processing for histology and SEM were performed as described previously4. Histomorphological analysis of three in-stent sections (proximal, middle, and distal) was performed using an ordinal grading scale of 0 up to 4 (with higher numbers indicating a worse outcome) of the following parameters: luminal thrombus, endothelialisation, strut tissue coverage, para-strut leukocytes, disruption of the internal elastic lamina (IEL), disruption of the external elastic lamina (EEL), medial smooth muscle cell (SMC) loss, and para-strut fibrin4. Histomorphometric and SEM evaluation of stent explants were performed as described previously4. The study pathologist (GJW) was blinded to the treatment group until completion of the histopathology evaluations for each time point.
Statistical analysis
All histomorphological and histomorphometric data were statistically analysed using SAS® System Software Version 8.2 (SAS Institute, Cary, NC, USA). P-values less than 0.05 were considered significant. The only stented vessels excluded from statistical analysis were those from three unscheduled deaths that occurred outside the designated follow-up time points. Median morphologic scores of all three sections for each stent were determined for each time point and one-way analysis of variance (ANOVA) was performed for group comparisons. Similarly, differences across time points for each device type were tested. The least significant difference (LSD) method was used to perform pairwise comparisons of these ranks for time points within each device group and for device types at each time point. For morphometric parameters, means were determined by averaging all three sections for each stent and a two-sample t-test was performed to compare device groups at each time point. Differences across device groups at each time point and comparisons across time points within each device group were assessed with one-way ANOVA. Pairwise comparisons of time points for each device group and comparisons of device types at each time point were made using the LSD method.
Results
Examination of the myocardium from every animal showed no significant pathology or evidence of ischaemic changes downstream of the stented vessels. All stents were angiographically patent (TIMI flow 3) at all time points examined. There were no stent occlusions or strut fractures. The unscheduled death of one animal found dead approximately three hours after implantation was possibly due to a procedurally-related event at implantation as a vessel wall dissection was discovered in the left circumflex (LCX) coronary artery immediately distal to the stent (polymer-only Element) upon histologic examination. The unscheduled deaths of two other animals at nine days and 155 days post-implantation were not device-related. Stented vessels from these animals were found to be widely patent with unremarkable tissue responses but were not included in the statistical analysis. Otherwise, there was no mortality or stent-related morbidity in the entire study.
Comparative vascular response
Remarkably similar vascular responses were seen histologically for the two drug-eluting stents (DES), SYNERGY™ and PROMUS® (XIENCE V), across all time points (30, 90 and 180 days) and on examination by SEM at 30, 90 and 180 days. Comparison of the SYNERGY stent with the bare metal Element™ and polymer-coated Element™ control stents revealed only subtle differences for all assessment parameters. Representative low magnification images comparing the SYNERGY, bare metal Element, polymer-only Element and PROMUS (XIENCE V) stents at 30, 90, 180 and 360 days (no PROMUS [XIENCE V] stent at 360 days) post-implantation are presented in Figure 2.
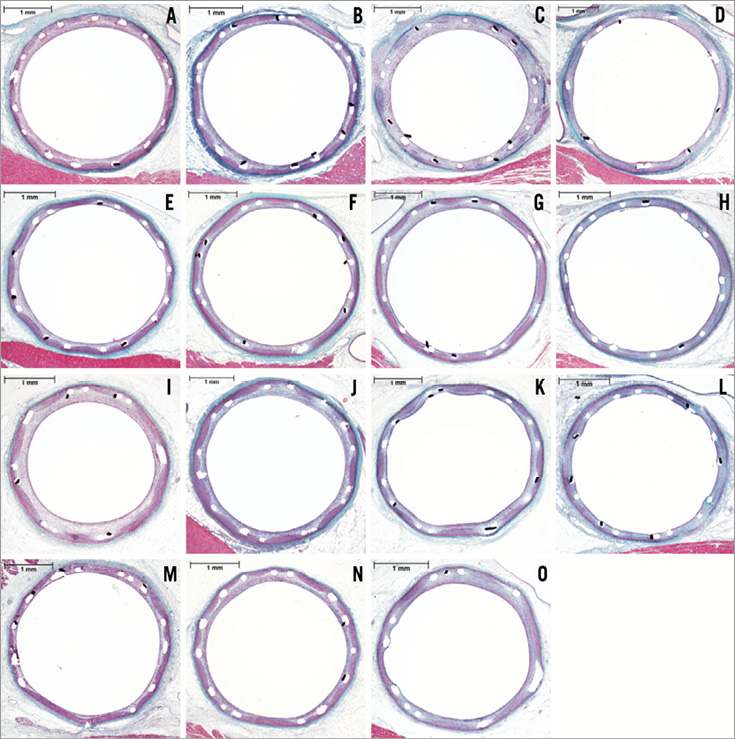
Figure 2. Representative histology. Representative elastic trichrome staining (20x total magnification) of the SYNERGY (A-D), bare metal Element (E-H), polymer-only Element (I-L) and PROMUS (XIENCE V) (M-O) stents at 30 days (A, E, I, M), 90 days (B, F, J, N), 180 days (C, G, K, O) and 360 days (D, H, I) post-implantation. The difference in percent area stenosis between the group mean and the representative section is not greater than 1.1%.
Morphology and scanning electron microscopy
Statistical analysis comparing the four device types showed no significant differences for luminal thrombus, endothelial cell coverage, strut tissue coverage, inflammation (para-strut leukocytes), IEL disruption, EEL disruption and medial SMC loss at any time point. All scores were 0 for luminal thrombus (none) and endothelial cell coverage (>90%), and almost all were 0 for strut tissue coverage (all struts covered) indicating preferred healing responses. Scores for IEL disruption and medial SMC loss were more variable but only para-strut fibrin at 30 days showed statistical significance in these comparisons.
Endothelialisation was complete (grade 0, >90%) at 30 days and there was a complete absence of luminal thrombus in every section of all stented vessels. Strut tissue coverage was complete (grade 0, all struts covered) in all sections from all device groups at all time points with the exception of only one section from a 30-day PROMUS (XIENCE V) stent in which a single strut was not covered. By 90 days, there was complete endothelialisation and complete strut tissue coverage for all stent types. At 90, 180 and 360 days all histologic sections from all stented vessels demonstrated complete endothelialisation with tissue coverage of every strut and a complete absence of luminal thrombus.
By SEM at 30, 90 and 180 days there was complete endothelial cell coverage of all struts (the only exceptions being incomplete endothelial coverage of a few struts crossing side branches) of all device types and no luminal thrombus was seen in any specimen. Figure 3 shows representative SEM views of the flow surface of vessels which received each of the four stent types at 30 days.
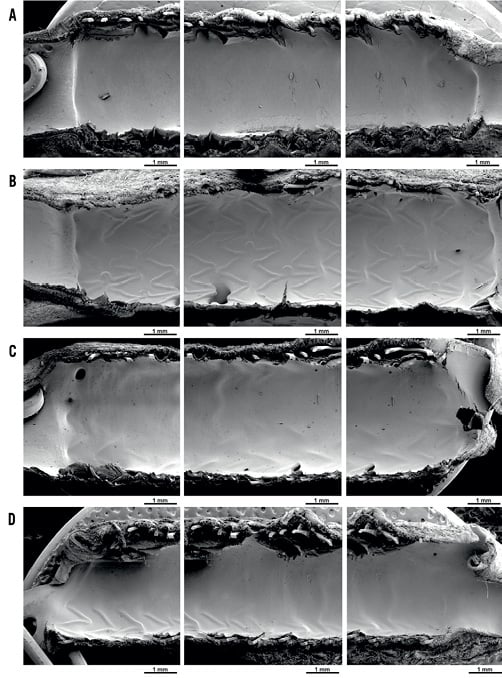
Figure 3. Representative scanning electron microscopy (SEM). Representative low magnification (X20) SEM for SYNERGY (A), PROMUS (XIENCE V) (B), polymer-only Element (C) and bare metal Element (D) stents at 30 days post-implantation, showing proximal, mid and distal segments (left to right) for each.
Scores for para-strut fibrin at 30 days were significantly different (p<0.0001) across the four device types. The two DES were not significantly different from each other but did have more fibrin than either the bare metal Element or polymer-only Element stents. The pairwise comparisons between the polymer-only Element and the bare metal Element stents indicated significantly more fibrin with the polymer-only Element stent at 30 days. All sections from the SYNERGY stent and all but one section from the PROMUS (XIENCE V) stent at 30 days were grade 1 (mild) for para-strut fibrin; one section from the PROMUS (XIENCE V) stent at 30 days showed grade 2 (moderate) fibrin. By 90 days, scores for the four devices were not significantly different (p=0.19) and this continued at 180 days. Most DES scores were 0 (no fibrin) and none exceeded mild. Representative high magnification images comparing para-strut fibrin deposition between the SYNERGY and PROMUS (XIENCE V) stents at 30, 90 and 180 days are presented in Figure 4. The two DES were associated with an equivalent deposition of modest amounts of fibrin at 30 days, which had almost entirely cleared by 90 days.
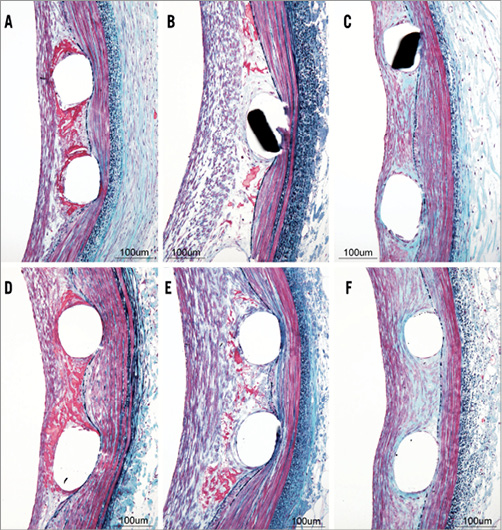
Figure 4. Para-strut fibrin deposition with the SYNERGY and PROMUS (XIENCE V) stents at 30, 90 and 180 days. Representative elastic trichrome staining (200x total magnification) for the SYNERGY (A-C) and PROMUS (XIENCE V) (D-F) stents at 30 days (A, D), 90 days (B, E) and 180 days (C, F) post implantation.
There were no statistically significant differences in inflammation across device types at any time point, although there were infrequent instances of severe inflammatory reactions, including granuloma formation with abundant eosinophils, for all groups except the bare metal Element stents. Of 140 histologically-examined stented vessels in 68 pigs (not including the four pigs from which all stented vessels went for SEM analysis), only four (2.9%) were so affected, with two instances occurring in one pig (two vessels in the same pig). Only three pigs exhibited severe inflammatory response, which was seen for the SYNERGY, PROMUS (XIENCE V) and polymer-only Element stents. All histologic sections showing severe (grade 3) inflammation also demonstrated granuloma formation with abundant eosinophils. This prevalence of severe inflammation is an anticipated response when conducting DES studies in the porcine coronary artery model3.
Disruption (loss) of the IEL or EEL indicates either mechanical injury at implantation or loss of elastica due to elastase activity associated with inflammatory/remodelling activity. Such injury and/or inflammation involving the vessel wall results in fibrosis and medial SMC loss. All instances of IEL disruption, EEL disruption and medial SMC loss (i.e., grades 1-4) were associated with either vessel wall injury or with para-strut inflammation, or with both. No evidence of drug-related medial SMC loss was seen in either of the two drug-eluting stent types. The majority of sections at all time points from all device groups demonstrated either none to minimal (grade 0) or mild (grade 1) IEL disruption and medial SMC loss and showed no disruption (grade 0) of the EEL. No statistically significant differences in IEL disruption, EEL disruption or medial SMC loss among the four devices were found at any time point.
Morphometry
Statistical analysis comparing morphometric parameters across the four device types showed no significant differences for all measured morphometric parameters assessed –lumen, IEL, EEL, medial and intimal areas, neointimal thickness (average of measured strut-to-lumen lengths), and area stenosis– at 90, 180, and 360 days. There were no significant differences for any morphometric parameter comparing the two DES –SYNERGY and PROMUS (XIENCE V)– at any time point.
At 30 days, the polymer-only Element stent demonstrated significantly greater (p<0.0001) intimal area, neointimal thickness, and percent area stenosis compared with the other device types but none of the values involved was indicative of substantial luminal narrowing and the differences from the other devices were relatively small. Percent area stenosis (stent profile-based) for the polymer-only Element stent at 30 days ranged from 26.41% to 47.25%, as compared with 15.47% to 23.28% for the bare metal Element stent, 14.05% to 31.24% for the PROMUS (XIENCE V) stent and 15.11% to 38.86% for the SYNERGY stent. Neointimal thickness at 30 days ranged from 0.23 mm to 0.49 mm for the polymer-only Element stent as compared with 0.13 mm to 0.23 mm for the bare metal Element stent, 0.13 mm to 0.27 mm for the PROMUS (XIENCE V) stent and 0.13 mm to 0.36 mm for the SYNERGY stent. Similarly, intimal area ranged from 1.52 mm2 to 4.08 mm2 for the polymer-only Element stent at 30 days as compared with 0.70 mm2 to 1.73 mm2 for the bare metal Element stent, 0.78 mm2 to 1.54 mm2 for the PROMUS (XIENCE V) stent and 0.77 mm2 to 2.20 mm2 for the SYNERGY stent.
The stent profile-based percent area stenosis at 180 days was 23.3±7.1% (mean±standard deviation) for the SYNERGY stent, 26.8±8.6% for the PROMUS (XIENCE V) stent, 20.0±7.5% for the bare metal Element stent and 20.1±5.8% for the polymer-only Element stent (not significantly different across the four groups).
Comparisons across stent types and time points of the key morphometric indices of neointimal thickness, percent area stenosis (indicative of neointima formation), and EEL area (indicative of vascular stability) are shown in Figure 5. Morphometric measurements thus confirmed the similar histology across all groups from 30 through 360 days.
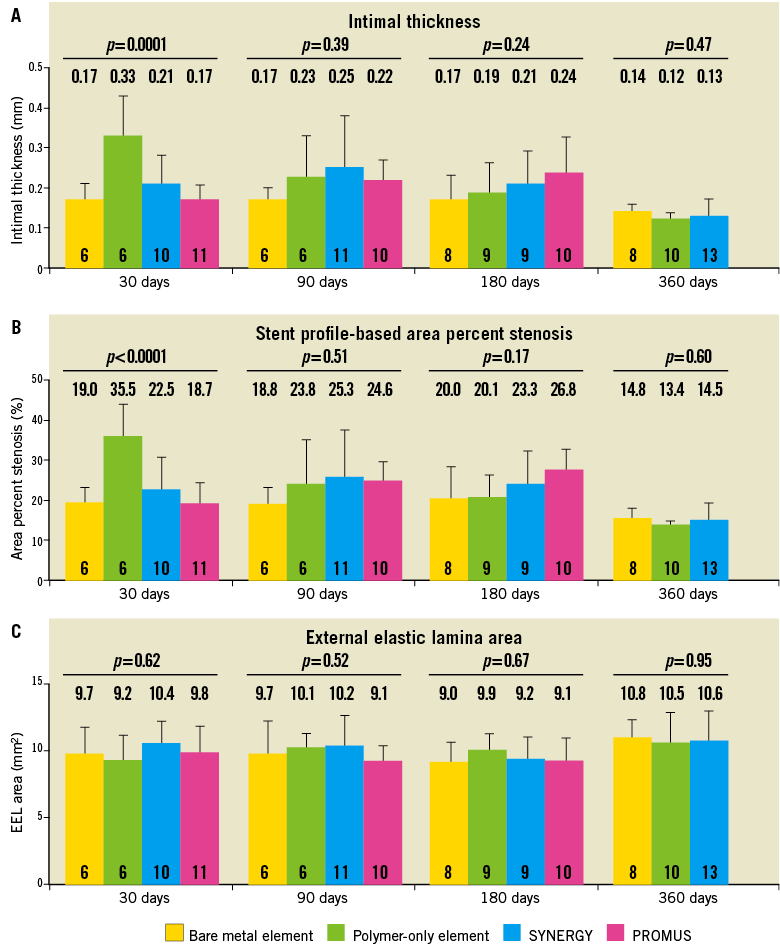
Figure 5. Comparison of morphometric parameters across stent types and time points. Intimal thickness and stent profile-based area percent stenosis measuring neointima formation, and the external elastic lamina area measuring vessel stability are plotted for 30, 90, 180, and 360 days. All data are mean ± standard deviation. P values are from one-way analysis of variance. N for each group is shown on the bar.
Discussion
This study evaluated safety and vascular responses over a wide range of implant durations for to the everolimus-eluting SYNERGY stent in a non-injured porcine coronary artery model. The SYNERGY stent, which elutes everolimus via an ultrathin abluminal biodegradable polymer, was compared to the everolimus-eluting PROMUS (XIENCE V) stent, which delivers everolimus via a conformal durable polymer, at 30, 90, and 180 days and to the bare metal Element and polymer-only Element stents, as controls, at 30, 90, 180, and 360 days. Comparison of the SYNERGY stent with both the bare metal Element and polymer-only Element control stents revealed only subtle differences in the morphological assessment parameters. While a logical choice in sharing the same stent platform and drug but not polymer as the SYNERGY stent, the PROMUS Element stent was not included as an additional control device as a safety evaluation of PROMUS Element in the same animal model using identical pathology methodology and the same control devices as the present study has been previously published in EuroIntervention2. Comparing studies, similar vascular responses were seen in the non-injured porcine coronary artery model for the SYNERGY and PROMUS Element stents.
Mild, and equivalent, para-strut fibrin deposition was observed for both the SYNERGY and PROMUS (XIENCE V) stents at 30 days (Figure 4) and was the only morphologic characteristic which separated these DES from both the bare metal and polymer-only control stents. The peak amount of fibrin deposition was considerably less than observed in previous studies with paclitaxel4,10, and had substantially dissipated by 90 days in contrast to the much slower clearing of paclitaxel-induced para-strut fibrin.
As expected in this porcine coronary artery model3,11, severe inflammatory responses, including para-strut granuloma formation with abundant eosinophils, were seen in a small proportion of DES and polymer-only coated stents in the study, and their occurrence in this model does not detract from safety. Granuloma formation with abundant eosinophils is characteristic of a hypersensitivity reaction that is a recognised response in humans to certain DES but, fortunately, in only a very small proportion of patients12. It is much more prevalent in pigs3,11,13 than in humans and the low rate of hypersensitivity reactions observed in the more sensitive porcine model of this study is reassuring for the clinical utility of these stents. The scoring system for para-strut inflammation used in the present study is illustrated in Wilson et al13.
Upon morphometric evaluation, the intimal area, neointimal thickness and area stenosis at 30 days was significantly greater for the polymer-only Element stent group compared with the other device types, but none of the observed values (i.e., percent stenosis ranged from 26.41% to 47.25%) was indicative of substantial luminal narrowing and the differences from the other device 30-day cohorts were relatively small. All four stent types evaluated are considered to be safe based on all of the data from this animal model.
All measures of neointima formation, including neointimal thickness and stent profile-based percent area stenosis (Figure 5), were similar at 30, 90, and 180 days among both the SYNERGY DES and the bare metal Element stents. Thus, efficacy in reduction of neointima for either DES was not demonstrated in this non-injured model. This lack of efficacy has also been reported for the PROMUS Element stent as compared to the PROMUS (XIENCE V) and bare metal Element stents implanted in an overlapping configuration in the same non-injured porcine coronary artery model at 30, 90 and 180 days follow-up2. In contrast, efficacy in neointima reduction, as measured by both intimal thickness and percent area stenosis by both optical coherence tomography and histomorphometry, has recently been demonstrated for both the SYNERGY and PROMUS Element stents compared to the bare metal Element stent in familial hypercholesterolaemic swine14. A rabbit hypercholesterolaemic model has also been reported to demonstrate reduced neointimal area with DES compared to bare metal stents (BMS) at 28 days15. The use of hypercholesteromic models14,15, or a diabetic model16, holds great potential for predicting the clinical efficacy for new DES designs, and may even be able to provide direction-relative clinical efficacy for competing DES designs while in preclinical prototype evaluation. However, in this study, the non-injured porcine coronary artery model is the standard for safety assessment required by the regulatory authorities, and allows this well-characterised model to be compared to a very large database. The higher of the two doses of everolimus used in the Evolve clinical trial17 was used, as is appropriate, in a preclinical safety study.
Inhibition of neointima formation has been described for paclitaxel-eluting stents (TAXUS® Liberté®; Boston Scientific Corporation, Natick, MA, USA) in the same non-injured porcine coronary artery overlapping stent model at 30 days compared to the bare metal control stent, but greater neointima than in the control stent was seen at 90, 180, 360 and 580 days4. The lack of sustained inhibition of neointima formation by DES was also reported in the Yucatan mini-pig model for non-overlapping CYPHER stents5 and for both CYPHER and TAXUS overlapping stents implanted into rabbit iliac arteries6. Therefore, the present results are consistent with observations on both paclitaxel and limus drugs used to coat stents in both pigs and rabbits, and are distinctly different from the substantial and sustained reduction in late loss observed clinically for both paclitaxel and everolimus-eluting stents7-9.
Limitations of this study include the use of a non-injured pig coronary artery model as a surrogate for human coronary arteries clinically. In addition to not demonstrating the sustained reduction in neointima formation observed clinically after implantation of various DES, as discussed above, this porcine model is not a sensitive indicator of differences in the progression of strut endothelial cell coverage due to rapid endothelial cell proliferation18. The rabbit iliac artery, in which endothelialisation of stents is slower than in porcine arteries, may be a better model to evaluate the endothelialisation process in DES19. Nevertheless, the non-injured porcine coronary artery model is a well-accepted standard for preclinical safety evaluation of DES.
Conclusions
In the non-injured porcine coronary artery model SYNERGY stents, delivering abluminal everolimus via a biodegradable polymer demonstrated vascular compatibility equivalent to the PROMUS (XIENCE V) stents, which deliver everolimus via a conformal durable polymer, and the bare metal Element and polymer-only Element control stents.
Acknowledgements
The authors gratefully acknowledge Mohammad Eskandarian, Sue Omar, Gordana Svajger, and Peter Wilson in Dr. Wilson’s group who assisted in pathological evaluations and manuscript preparation; Yun Lu and Hong Wang for assistance with statistical analyses; and Vicki M. Houle (BSC) for manuscript review and formatting.
Funding sources
This study was funded by Boston Scientific, Natick, MA, USA.
Conflict of interest statement
G.J. Wilson is a consultant for Boston Scientific Corporation and his institution was funded to perform the pathology on this study; B.A. Huibregtse, D.E. Pennington, and K.D. Dawkins are employees and stockholders of Boston Scientific Corporation.

