Abstract
Aims: Biodegradable polymer-based drug-eluting stents are thought to be safer than durable polymer-based stents. However, the long-term vascular response remains unclear. The aim of this study was to compare the biocompatibility of durable polymer-based sirolimus-eluting (SES) and everolimus-eluting (EES) stents with biodegradable polymer-based biolimus-eluting (BES) stents in a porcine coronary model.
Methods and results: Stents were implanted in porcine coronaries. Acetylcholine challenge tests and optical coherence tomography (OCT) examination were performed at one month. Animals were sacrificed at three and six months (n=6 each), and the stents were analysed histologically. At one month, acetylcholine challenge tests revealed a trend towards greatest vasoconstriction in SES, less in BES, and least in EES, but the differences were not significant. OCT analysis demonstrated the highest incidence of uncovered struts in SES, followed by BES, while EES showed almost complete strut coverage (41.7±27.0%, 24.5±23.8%, 0.4±0.8%, respectively; p=0.004). Upon histological analysis at three months, SES showed a significantly higher inflammatory score than BES and EES (2.9±1.4, 0.8±0.9, 0.5±0.4, respectively; p=0.001), and this was maintained at six months (1.6±1.5, 0.3±0.3, 0.4±0.6, respectively; p=0.049).
Conclusions: While SES showed an increased inflammatory reaction, EES and BES showed minimal inflammation. These results indicate that the late inflammatory reaction does not necessarily depend on degradability of the polymer, if the combination of the drug, metal, and polymer is biocompatible.
Introduction
Since the advent of first-generation drug-eluting stents (DES), the occurrence of in-stent restenosis, which is the major limitation of intracoronary stent therapy, has been reduced with controlled drug release from durable polymers1,2. However, late thrombotic events have been noted with the use of DES, and have emerged as a concern3-5. Pathologic studies have reported various causes of late stent thrombosis in different phases after placement, such as delayed arterial healing, hypersensitivity reaction, excessive fibrin deposition with malapposition, and neoatherosclerosis6-9. One of the device-related complications includes hypersensitivity reaction, which is considered to occur due to a lack of biocompatibility of the polymer10. Therefore, the development of newer DES has focused on creating devices with a higher safety profile. Newer-generation DES employ more biocompatible polymer, and it is generally believed that the biodegradable polymers should be more favourable clinically than the durable ones since, after drug delivery and subsequent complete polymer degradation, only the biologically inert bare metal platform remains in the vessel wall after certain periods11. However, the long-term safety and superiority of this technology over durable polymers are unclear.
The porcine coronary model has been used to evaluate the efficacy and safety of DES, and appears to mimic the reaction to intracoronary devices in humans if the time points are properly chosen11,12. Previously, we reported a progressively increasing inflammatory reaction over time with placement of SES in the porcine coronary model11, resembling reactions found in human autopsies8, although the incidence of this phenomenon is considered to be much lower in humans. Therefore, using the porcine coronary artery model for three to six-month periods is suitable for the examination of long-term biocompatibility12.
The aim of this study was to evaluate the biocompatibility following implantation of durable polymer-based SES and EES compared to biodegradable polymer-based BES.
Methods
STUDY DESIGN
Twelve mini swine were used in the current study. Three different types of DES were employed: durable polymer-based SES (CYPHER SELECT™ 3.0×18 mm; Cordis Corp., Johnson & Johnson, Warren, NJ, USA), durable polymer-based EES (XIENCE V® 3.0×18 mm; Abbott Vascular, Santa Clara, CA, USA), and biodegradable polymer-based BES (Nobori 3.0×18 mm; Terumo Medical Corp., Tokyo, Japan), with one of each type implanted into each pig (one stent per one vessel). The biodegradable polymer-based BES (Nobori) is abluminally coated with a matrix containing Biolimus A9 and polylactic acid, which degrades over six to eight months. Animals were sacrificed at three and six months (n=6 each). Over six months, six animals received acetylcholine challenge tests and optical coherence tomography (OCT) examinations for each coronary artery at one month following stent implantation (Figure 1). Following euthanasia, stents were subjected to histologic examination to analyse vascular responses.
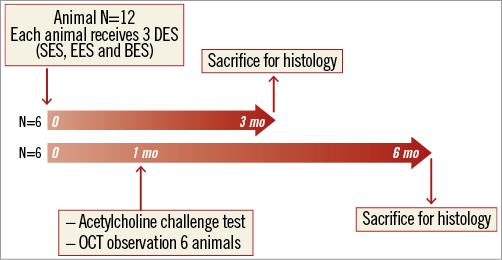
Figure 1. Study design of the current study. mo: months
ANIMAL PREPARATION AND PROCEDURES
All animals were premedicated with oral clopidogrel (75 mg/day) and aspirin (100 mg/day) at two days before the procedure, and these medications were continued until the day of euthanasia. After anaesthesia with isoflurane, surgical access was obtained via a femoral artery using general sterile techniques. During cardiac catheterisation, heparin (5,000-10,000 IU) was injected to maintain an activated clotting time (ACT) of 250-300 seconds. Vessel allocation to experimental groups was predetermined to distribute the different stent types equally in three different coronary arteries. Stent deployment was performed using a 1:1.1-1.2 stent-to-artery diameter ratio.
At three and six months, the animals were euthanised under general anaesthesia, by IV injection of pentobarbital euthanasia solution (100 mg/kg) and/or potassium (40 mEq). Hearts were excised and pressure perfused with 0.9% saline until cleared of blood, followed by pressure perfusion fixation in 10% neutral buffered formalin until hardening of the heart muscle was clearly perceptible.
ACETYLCHOLINE CHALLENGE TEST
At one month, the six animals assigned for the six-month study period underwent acetylcholine challenge tests after the initial angiography, as previously described13. In brief, doses of 10–6 mol/L and 10–5 mol/L of acetylcholine (1 ml/min) were administered via the intracoronary microcatheter. Coronary angiograms were performed at 2.5 minutes after the acetylcholine injections and recorded for each dose. The final angiograms were recorded after injection of 200 μg of nitroglycerine into each coronary artery. Coronary artery diameters within the 5 mm segments proximal and distal to the stents were measured using quantitative coronary analysis (Medis QCA CMS 6.0; Medis medical imaging systems bv, Leiden, The Netherlands).
OPTICAL COHERENCE TOMOGRAPHY (OCT) PROCEDURES AND ANALYSIS
Following the acetylcholine challenge test, animals underwent OCT examinations. Nitrates were given to all vessels in all animals prior to OCT examination to eliminate influence from the vasomotor tests. OCT (M3 system; LightLab Imaging Inc., Westford, MA, USA) imaging was performed in all stented vessels using the balloon occlusion technique.
OCT data were analysed in a core laboratory at Kobe University. Assessments were performed for every 1 mm slice through the stented segment. Parameters including stent area, lumen area, neointimal area (by calculation), % area stenosis (by calculation), and neointimal thickness were measured digitally. Percent uncovered struts were determined by visual assessments performed by experienced cardiologists (TS and HO). Parameters for each stent were obtained from the average of all measured variables through the entire stented segment (stent-based analysis). The results were compared among the three different types of DES.
HISTOLOGIC PREPARATION AND ASSESSMENTS
The stented arteries were embedded in methylmethacrylate resin. After polymerisation, sections were taken for every 2 mm of the stent (for a total of four to five sections) and stained with haematoxylin and eosin and Movat’s Pentachrome. Histomorphometric analysis was performed for quantifying neointimal growth and assessment of arterial injury and inflammation.
A vessel injury score was calculated according to the Schwartz method14. The cross-sectional areas (external elastic lamina [EEL], internal elastic lamina [IEL], lumen area, and neointimal thickness) of each section were measured with digital morphometry (WinROOF image-processing software, Version 6; Mitani Corp., Tokyo, Japan). Ordinal data were collected for each stent section on fibrin deposition, granulomatous reactions, and the presence of giant cells around the stent struts. Each was expressed as a percentage of the total number of struts in each section. An overall neointimal inflammation and fibrin value was scored for each section, as previously described15.
STATISTICAL ANALYSIS
Values were expressed as mean±standard deviation. One-way analysis of variance ANOVA was used to compare statistical differences in continuous values between the groups. Nonparametric score data, including injury, fibrin, and neointimal and adventitial inflammation, were compared using a Wilcoxon Kruskal-Wallis test. A value of p<0.05 was considered statistically significant (JMP software; SAS Institute Inc., Cary, NC, USA).
Results
STENT IMPLANTATION
Stent implantation was performed successfully in 12 pigs (three vessels per pig) with no differences in quantitative coronary analysis (QCA) data including in-stent minimum lumen diameter and stent length. All pigs survived the study period without illness.
ACETYLCHOLINE CHALLENGE TEST
Acetylcholine challenge tests were performed successfully in six mini swine at one month following stent implantation. All DES showed vasoconstriction in both the proximal and distal stent edge segments following acetylcholine administration. The extent of vasoconstriction in the proximal edge showed a trend towards being greatest in SES, less in BES, and least in EES. However, the differences were not significant (Table 1, Figure 2). The distal segments showed similar results, with a trend towards greater vasoconstriction with SES compared with EES and BES, but, again, the differences were not significant.
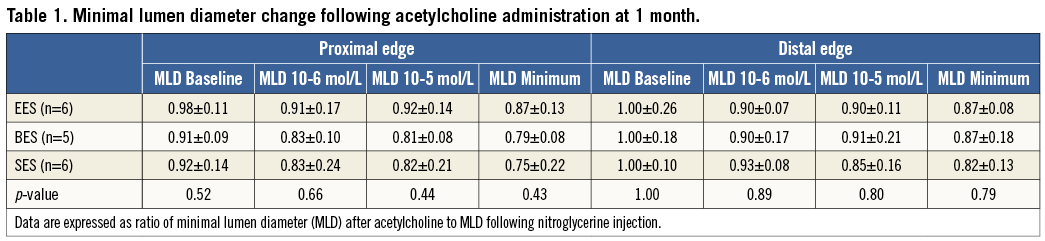
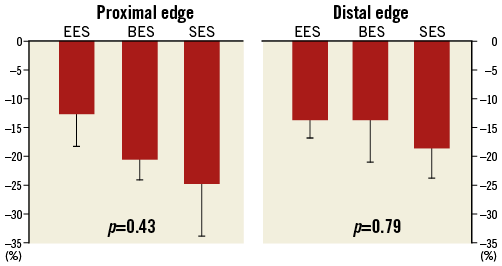
Figure 2. Change in minimal lumen diameter following acetylcholine administration. Observed lumen diameter reduction is expressed as the percentage of lumen diameter reduction observed after nitroglycerine administration.
OPTICAL COHERENCE TOMOGRAPHY EXAMINATION
OCT images were obtained successfully from all stented segments, but one lesion with BES showed poor image quality and was excluded from the analysis. Stent length and stent area were similar among the three groups; however, the average neointimal thickness was smallest in SES followed by BES and EES (0.10±0.63 mm, 0.20±0.10 mm, and 0.27±0.98 mm, respectively, p=0.006). On the other hand, as shown in Table 2, qualitative OCT analysis demonstrated the greatest incidence of uncovered struts in SES, followed by BES, while EES showed almost complete stent coverage (41.7±27.0%, 24.5±23.8%, and 0.4±0.8%, respectively, p=0.004) (Figure 3).
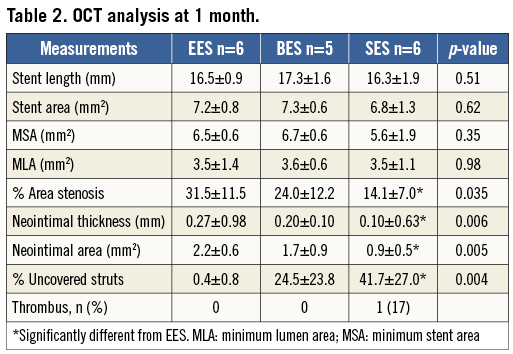
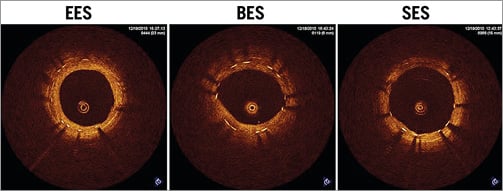
Figure 3. Representative images from OCT examination at one month. Note that uniform stent strut coverage was observed in EES, whereas BES and SES showed uncovered struts with relatively uneven neointimal growth.
HISTOLOGIC OBSERVATIONS
THREE-MONTH GROUP
All DES showed patent lumens with no thrombus formation at three months, except for one BES, which displayed total occlusion by an organised thrombus. A total of 32 sections for SES and 36 sections for BES and EES were analysed. Mild neointimal formation was observed in EES and BES, whereas SES showed a relatively large amount of neointimal formation which was significantly greater than that in EES and BES. The accelerated neointimal formation in SES was demonstrated by an increased inflammatory reaction including a significantly greater granulomatous reaction along with giant cell infiltration. A higher injury score was obtained in SES compared with EES and BES due to a greater inflammatory reaction which extended to the adventitial area. There were no significant differences between EES and BES in various parameters including inflammatory reaction and neointimal formation (Table 3, Figure 4).
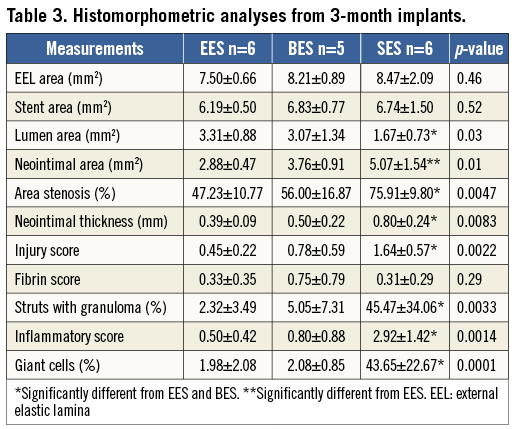
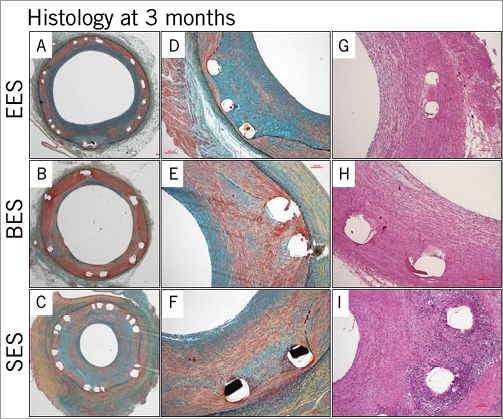
Figure 4. Representative histologic images at three months. Mild neointimal formation was observed in EES and BES, whereas SES showed a relatively large amount of neointimal formation (A-F). The accelerated neointimal formation in SES was accompanied by an increased inflammatory reaction compared with EES and BES (G-I).
SIX-MONTH GROUP
All DES showed patent lumens with no thrombus formation at six months. A total of 36 sections for each DES were analysed. Moderate neointimal formation was observed in all DES, but did not differ significantly among the groups. However, SES demonstrated a significantly increased inflammatory reaction compared with EES and BES, including a greater granulomatous reaction, which led to a greater injury score. Similar to the three-month results, there were no significant differences between EES and BES in various histologic parameters with a minimal inflammatory reaction in both groups (Table 4, Figure 5).
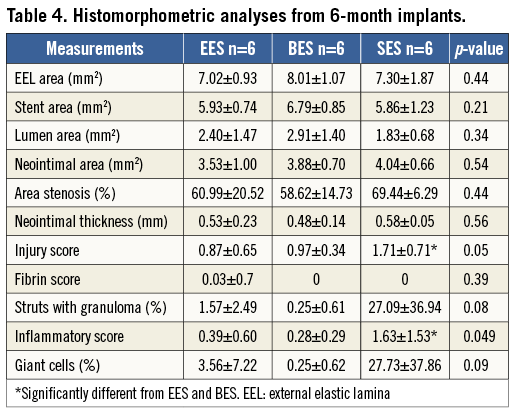
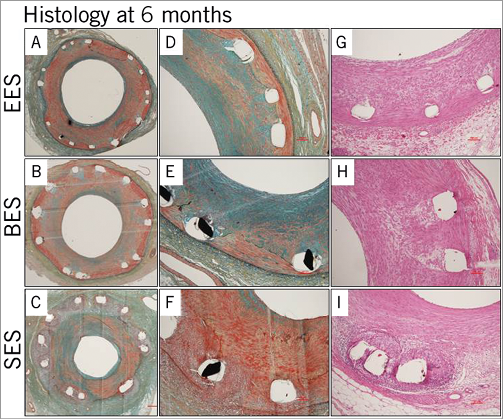
Figure 5. Representative histologic images at six months. Moderate neointimal formation was observed in all DES with no significant difference among the groups (A-F). SES demonstrated a significantly increased inflammatory reaction, including a greater granulomatous reaction, compared with EES and BES (G-I).
Discussion
The major findings of the current study were: 1) minimal uncovered struts by OCT examination at one month in EES compared to BES and SES; 2) a trend towards greater vasoconstriction following acetylcholine administration in SES compared to BES and EES; and 3) consistent increase in inflammatory reaction with SES, whereas EES and BES showed minimal inflammation even at six months.
ACETYLCHOLINE CHALLENGE TEST AND OCT EXAMINATION
In addition to carrying out a long-term biocompatibility investigation, we performed acetylcholine challenge testing and OCT imaging at one month in order to compare differences in early arterial healing among the DES devices tested. Despite limitations, it seems probable that the reaction of normal porcine coronary arteries to stent implantation at one month is reasonably similar to human coronary responses at about six to nine months when early arterial healing and device efficacy can best be evaluated16. Acetylcholine challenge testing showed an insignificant trend towards the greatest paradoxical vasoconstriction in SES with less in BES and the least in EES. Although the differences were not significant, these findings appear to correlate to some degree with stent strut coverage as examined by OCT imaging, in which SES showed the greatest incidence of “uncovered struts”, with fewer in BES and the least in EES. Paradoxical vasoconstriction following acetylcholine administration is considered to be a surrogate marker for endothelial dysfunction. Fujii et al have reported that in a clinical setting paradoxical vasoconstriction produced by acetylcholine was associated with stent strut coverage as determined by OCT examination in patients with DES implants17. Shinke et al demonstrated in a one-month porcine model that endothelium-dependent vasomotor function was diminished in first-generation DES compared with bare metal stents in association with less neointimal coverage and higher fibrin deposition assessed by histology and angioscopy. Their findings are consistent with those of the current study18.
In the current study, OCT examination confirmed that SES showed greater delayed arterial healing compared with second-generation DES, i.e., EES19-21. On the other hand, BES showed a relatively higher incidence of uncovered struts compared with EES, although the difference did not reach statistical significance. This finding may be related to thicker stent struts, a higher drug dosage, and longer drug release22. The rate of early vascular healing is determined principally by parameters such as strut thickness, drug type and dose, and drug release kinetics.
BIOCOMPATIBILITY EXAMINATION BY HISTOLOGY
Histologic examination revealed a consistent increased inflammatory reaction in SES at both three and six months, whereas EES and BES showed minimal inflammation during the study period. These findings confirm the lack of biocompatibility of SES, which is consistent with previous preclinical studies and clinical findings8,10,11,23. Together, these findings suggest that our study model was able to determine long-term biocompatibility. In this study, both BES and EES were far more biocompatible than SES. At the same time, it should also be stressed that durable polymer-based EES showed a similar safety profile compared with biodegradable polymer-based BES. Therefore, it is fair to mention that polymer degradation may be less critical in determining late inflammatory reactions than biocompatibility. Furthermore, Kolandaivelu et al reported recently that fluoropolymer applied in everolimus-eluting stents can be antithrombotic when compared to bare metal stents24.
As mentioned above, although the porcine coronary model used in the current study is considered suitable for biocompatibility testing, animal models are not perfect. The major limitation of device testing in preclinical studies is that the turnover and proliferation in animals is much faster than in humans and, although drug release kinetics in DES do not vary between species, the possibility of a different response between animals and humans does exist. In the current study, extending the study period to one year may be appropriate, since the completion of polymer biodegradation in BES takes six to nine months25. However, we believe that the six-month period was sufficient to evaluate long-term biocompatibility because, even in clinical settings, unfavourable vascular responses such as peri-stent contrast staining or malapposition rarely occur after eight months of follow-up if there are no such signs at earlier follow-up26. Given the fact that biological responses occur much faster in animals, the six-month period in the porcine model should be a much later time point than the eight-month follow-up in humans.
Recently, a network meta-analysis was performed which reported a significantly lower incidence of definite stent thrombosis in cobalt-chromium EES compared with other DES including SES at one year27. Similarly, in the LEADERS trial, biodegradable polymer BES showed a significantly lower rate of very late stent thrombosis compared with durable polymer SES, which was eventually associated with better composite clinical outcomes28,29. These clinical data clearly demonstrate that newer-generation DES have achieved a steady improvement in terms of safety as well as efficacy since the first-generation DES era. However, several studies, including the COMPARE II trial30, showed no difference in clinical outcomes between biodegradable-polymer DES and newer-generation durable-polymer DES at one year. Our results suggest similar biocompatibility between EES and BES, and both DES were found to be far more biocompatible than SES, which was consistent with the other clinical studies.
Limitations
There are limitations to the current study. We used a limited number of animals/stent (n=6 each) per time point. Therefore, some results, such as the acetylcholine challenge testing, did not reach statistical significance. However, this is a preclinical study using juvenile mini swine. The variability between individual pigs receiving stents is not as high as that of humans with a different extent and duration of coronary artery disease and more variable reactions.
Conclusion
While SES consistently showed a progressive increase in inflammatory reactions, EES and BES showed minimal inflammation even on long-term follow-up. The current study suggests that the reduced inflammatory reaction appeared to depend more upon biocompatibility of the combination of DES components rather than solely on the degradability of the polymer.
| Impact on daily practice It is generally believed that biodegradable polymer-based drug-eluting stents (DES) are safer than those with permanent polymer, since some permanent polymers are reported to induce inflammatory reactions while biodegradable polymers eventually disappear leaving the metal stent only. However, our findings suggest that the inflammatory reaction appears to depend more upon biocompatibility of the combination of DES components rather than solely on the degradability of the polymer. Based on the current study, biocompatible permanent polymer-based DES or biodegradable polymer-based DES can be equally used in daily practice. |
Funding
This study is in part supported by Abbott Vascular Japan.
Conflict of interest statement
G. Nakazawa is a consultant for Abbott Vascular Japan, Terumo Corp., and Japan Stent Technology. The other authors have no conflicts of interest to declare.

