Abstract
Aims: The concept of fully biodegradable stents has emerged as an attractive alternative to current permanent metallic stents, mainly as a potential solution to avoid late stent thrombotic events. We sought to evaluate a novel, fully bioabsorbable sirolimus-eluting stent (SES) synthesised entirely from a unique salicylic-acid polymer, in a clinically relevant animal model.
Methods and results: Fully biodegradable balloon-expandable stents (n=45) were implanted in a porcine coronary arteries using quantitative coronary angiography (QCA) and intravascular ultrasound (IVUS) to optimise stent apposition. Dose density of sirolimus was 8.3 µg/mm of stent length with in vitro studies demonstrating elution over 30 days and complete stent degradation over 12 months. Animals were terminated at 7, 14, 30, 90, and 180 days for complete histological analysis. Optical coherence tomography (OCT) was also performed for the 90- and 180-days samples.
All stents were deployed successfully without notable mechanical difficulties. Angiographic diameter stenosis (DS) was 20±16%, 24±4%, and 23±17%, at one, three, and six months, respectively. In parallel, IVUS showed good stent apposition with DS of 21±9%, 25±7%, and 18±3%; and area stenosis (AS) of 35±13%, 33±7%, and 32±4% at one, three, and six months,respectively. OCT further demonstrated good stent apposition with DS of 28±7% and 20±6%, and AS of 37±10% and 33±13% at three and six months, respectively. OCT showed reduction of stent thickness by 23% from three to six months. Histologic analysis confirmed these in vivo findings and revealed a favourable healing process of absorbable stent incorporation into the arterial wall, without excessive thrombotic or inflammatory reactions.
Conclusions: This study shows favourable vascular compatibility and efficacy for a novel fully bioabsorbable salicylate-based SES. This device has good mechanical performance during deployment and stays well-apposed to the vessel wall at long-term follow-up. These initial results are highly encouraging and support progress into more extensive preclinical studies as well as early clinical testing.
Introduction
Recent advances in bioabsorbable stent technology make these devices potentially attractive alternatives to current metallic drug-eluting stents (DES). Although the concept of fully degradable stents, which provide luminal scaffolding for the required duration and subsequently dissolve, is not new, many novel materials and designs have been proposed and evaluated. The specter of late stent thrombosis (LST) and requirement of prolonged dual antiplatelet therapy have further fueled and accelerated recent research in this arena.
Although many factors such as patient, lesion, as well as procedural characteristics are likely contributory, clinical, histopathological, and pathophysiological studies have implicated that delayed arterial to healing and poor re-endothelialisation may play a major role in the pathogenesis of LST1-3. Stents composed of bioabsorbable/ biodegradable material represent an alternative revascularisation modality, with avoidance of the potential long-term complications of metal stents4,5. Compared with their metal counterparts, the bioabsorbable stents offer several theoretical advantages, including absence of permanent endovascular prostheses, restoration of vasomotion, artifact-free lesion imaging with CT and MRI, and perhaps most importantly, decreased LST and need for prolonged antiplatelet therapy.
Preliminary studies involving bioabsorbable polymerics stents are promising. However, issues such as degree of recoil, rate of polymer degradation, inflammatory response to the polymer, and degree of the neointimal formation remain unclear6-13. Nonetheless, in terms of the short-term need for vessel scaffolding and avoidance of the potential long-term complications of metallic stents, bioabsorbable stents appear to be an ideal alternative candidate material.
Unique polymers have been synthesised from poly(anhydride-esters) comprised of salicylic acid as novel degradable biomaterials14-17. Salicylic acid, the active ingredient in aspirin, has well-known anti-inflammatory properties as well as antiplatelet effects. An important feature of this homopolymer is its complete degradation into biocompatible compounds, salicylic acid and adipic acid. This polymeric platform offers the unique ability to chemically incorporate the anti-proliferative drug directly into the polymer backbone, rather than attachment as a side group or physically admixed. Therefore, drug release is directly dependent on the hydrolytic cleavage of the anhydride and ester bonds.
Our previous work18 demonstrated favourable vascular compatibility and efficacy for a new bioabsorbable salicylate-based polymer as a DES coating in pig coronary artery stent implants. The aim of the present study was to evaluate a novel, fully bioabsorbable SES synthesised entirely from this unique salicylic-acid polymer, in a clinically relevant porcine coronary artery model.
Methods
Study design
Seventeen domestic pigs (30 to 50 kg) were enrolled in this study. Experimental procedures were performed in a standard fashion without notable difficulties according to the recommendations of the consensus advisory panel to the Food and Drug Administration19 and in compliance with the Association for Accreditation of Laboratory Animal Care. A total of 45 balloon-expandable biodegradable salicylate-based SES were implanted in 17 pigs. All the stents were 3.0 mm in diameter and 13 mm in length.
Animals, stent implant, terminal restudy, and sample preparation
Animals received 81 mg aspirin and 75 mg clopidogrel three days before stent implant and daily until termination. Cardiac catheterisation was performed with full heparinisation (200 units/kg) and stents were implanted using QCA to obtain a stent:artery ratio ≈1.1:1. Post implant angiography with QCA and IVUS was performed to determine stent size, complete apposition, and patency.
All animals underwent angiographic restudy with QCA and IVUS and were terminated at seven (six stents), 14 (six stents), 30 (15 stents), 90 (nine stents), and 180 (nine stents) days for histomorphometric and histopathological analyses. IVUS imaging was performed on all stented vessels using a 40-MHz single-element mechanically rotating transducer (ClearView, CVIS, Boston Scientific, Natick, MA, USA) and automated transducer pullback (0.5 mm/sec). The images were recorded on s-VHS 0.5-in. tapes and were manually analysed off-line by independent observers. Optical coherence tomography (OCT) was also performed for the 90- and 180-day samples. The OCT system utilised in this study consisted of an electro-optical imaging engine, a computer, monitor displays, a probe interface unit (Model M2 Cardiovascular Imaging System, LightLab Imaging, Inc., Westford, MA, USA) and a 0.356-mm (0.014-inch) wire-type imaging catheter (ImageWire, LightLab Imaging, Inc., Westford, MA, USA). A motorised pull-back system at 1.0 mm/s was used, and OCT images were acquired at 15 frames per second. The axial resolution capacity of this system was 10 µm. A guiding catheter was introduced into the target coronary artery by transfemoral approach. An over-the-wire type occlusion balloon catheter (Helios™, LightLab Imaging Inc., Westford, MA, USA) was advanced to the distal end of the stent implantation site. The guide wire was removed and the OCT imaging probe was inserted (ImageWireTM, Light-Lab Imaging Inc., Westford, MA, USA) through the over-the-wire lumen of the occlusion balloon catheter and positioned distal to the stent. To clear blood from the field of view and acquire clear images, the occlusion balloon was inflated to 40.5-60.8 kPa (0.4-0.6 atm); and warm lactated Ringer’s solution was infused through the balloon catheter into the arterial lumen at 0.5-1.0 ml/s. The internal fibre optics within the ImageWire™ were pulled back from distal to proximal with an automatic pullback device at 1 mm/s. The entire stent was imaged, and all OCT images were stored digitally for subsequent observation and analysis. The observers were blinded to the results of angiography and IVUS.
At explant, coronary arteries were perfusion-rinsed with phosphate buffered saline and perfusion-fixed in situ at physiologic pressure with 5% formalin/1.25% glutaraldehyde for 15 to 20 minutes. Stented vessels were trimmed free from the hearts and embedded in methyl methacrylate. Sections from proximal, middle, and distal vessel regions were cut using a heavy-duty microtome, collected on glass slides, de-plasticised, and stained with hematoxylin-eosin and Verhoeff-Masson elastic-trichrome.
Polymeric stent formulation and drug loading
The BTI stent (Bioabsorbable Therapeutic Inc, Menlo Park, CA, USA) (Figure 1) was synthesised entirely from salicylic acid bioabsorbable polymer derivates; the top coat, utilised for drug elution, was comprised of salicylate #1 + sirolimus; and the core, providing mechanical scaffolding, was comprised of salicylate #2 (Figure 2). Salicylate #1 was a trimer of two salicylic acid molecules joined by adipic acid as a linker molecule, whereas salicylate #2 was synthesised from polylactide anhydride #21 and a trimer of two salicylic acid molecules joined by a sebacic acid as a linker molecule. This hybrid compound was then polymerised to achieve the desired overall molecular weight of ≈3 kDa. As the polymer degraded, salicylic acid moieties were released and became available to arterial wall cells. The stent eluted approximately 10 µg of salicylic acid. The adipic acid moieties are biocompatible, non-inflammatory and enter into endogenous biochemical pathways through the Krebs cycle. Stents were fabricated to allow drug coating layer absorption over one month, as determined by measurement of coating weight loss after exposure to 37°C buffer in a closed-loop system. The biodegradable polymeric stent had a 9 µm coating thickness and contained a 1:1 polymer-to-sirolimus ratio for a total dose density of 8.3 µg/mm of stent length, similar to the Cypher stent (Cordis, Johnson and Johnson Corporation, Miami Lakes, FL, USA). In vitro studies have suggested that the radial strength of this novel absorbable stent is almost twice that of Cypher and that degradation begins at 50 days post-implantation (Figure 3).

Figure 1. The BTI stent: fully bioabsorbable balloon expandable stent made entirely from salicylate-based polymer.
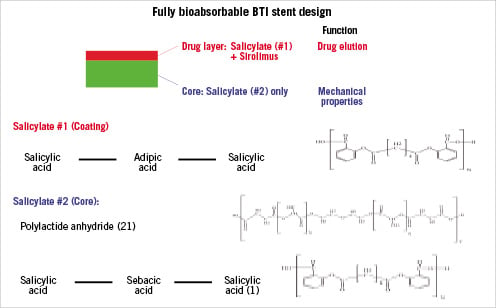
Figure 2. Molecular structure of the BTI stent.
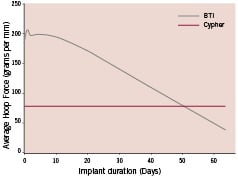
Figure 3. Radial strength over time.
Histopathology
Distal, middle, and proximal sections from each of the stented coronary arterial segments were evaluated for inflammation, necrosis, intramural haemorrhage, and mural thrombus. The nature of the extracellular matrix in the neointima and adventitia was assessed and described. An overall inflammation score was assigned according to the following semi-quantitative grading scale: 0=none; 1=mild, including the presence of minimal infiltrated inflammatory cells; 2=moderate, either increased number of inflammatory cells appearing as diffuse infiltrates or small foci of more intense infiltrates; 3=severe, especially large clusters of inflammatory cells with granulomatous morphology20. A scoring scale was used to grade the extent of vessel injury determined by stent strut penetration into the vessel wall as follows: 0=no injury, intact internal elastic lamina (IEL), 1=strut contacting IEL with profile in neointima, 2 = strut penetrating IEL and profile in media, 3=strut penetrating media and contacting external elastic lamina (EEL), 4=strut in adventitia21.
Histomorphometry
Sections were imaged with 20X instrument magnification. Morphometric analysis was performed by computerised planimetry at all levels of all stents. Lumen, IEL, and EEL were traced and area measurements obtained; areas of neointima and media were obtained by subtraction. Neointimal thickness at each stent strut was measured, and histologic percent area stenosis (1- [luminal area/IEL area] X100) was calculated.
Statistical analysis
Descriptive statistics were generated for all quantitative data and expressed as mean + SEM. Angiographic and histomorphometric data were evaluated with One-way analysis of variance (ANOVA). All-pairwise multiple comparisons with the Tukey method were performed if ANOVA probability was <5%. For non-continuous data (i.e., histopathologic scoring), Kruskal-Wallis One-way ANOVA on ranks was used. A critical value of P<0.05 was considered to indicate a significant treatment effect.
Results
In vitro drug-release pharmacokinetics
Elution of sirolimus from BTI stents was determined using the USP4 methodology, Sotax CE7 equipment (Sotax AG, Basel, Switzerland) mated to HPLC, and inorganic media. Procedures followed FDA Guidance Documents for Dissolution Testing. Media flow rate conditions were set to achieve approximately 4-to-1 elution acceleration (i.e., one day of in vitro testing approximates four days in vivo). The elution profiles of BTI and Cypher stents were similar, exhibiting a «burst» over the first 48 hours, followed by more gradual elution afterwards (Figure 4). In addition, salicylic acid eluted from the BTI stents in a gradual, linear fashion.
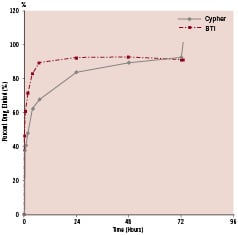
Figure 4. In vitro elution profile of sirolimus from salicylate-based fully bioadsorbable (BTI) and biostable polymeric sirolimus-eluting stent (Cypher).
Procedural success and macroscopic analysis
All BTI stents were deployed successfully, without notable mechanical difficulties or acute recoil. All animals survived the scheduled follow-up period without any signs of stent thrombosis or other related adverse events. Gross examination of the hearts showed no signs of macroscopic abnormalities along the coronary arteries, no epicardial haemorrhage, or aneurysms.
Angiographic follow-up: QCA, IVUS, and OCT data
At all follow-up time points, stented vessels were patent without any signs of stent migration, distal embolisation, malapposition, dissections, or thrombosis. Angiographic diameter stenosis (DS) was 20±16%, 24±4%, and 23±17%, at 1, 3, and 6 months, respectively (Figure 5). In parallel, IVUS showed good apposition of the stent to the vessel wall with DS of 21±9%, 25±7%, and 18±3%; and area stenosis (AS) of 35±13%, 33±7%, and 32±4% at 1, 3, and 6 months, respectively (Figure 6). OCT demonstrated good apposition of the stent with DS of 28±7% and 20±6%, and AS of 37±10% and 33±13% at 3 and 6 months, respectively. Furthermore, OCT showed reduction of stent thickness by 23% from 3 to 6 months (Figure 7).
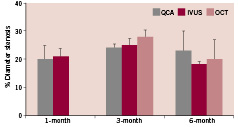
Figure 5. Angiographic % diameter stenosis measured over time by QCA, IVUS, and OCT.
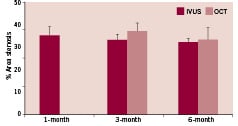
Figure 6. Angiographic % area stenosis measured over time by IVUS and OCT.
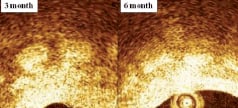
Figure 7. OCT showed significant reduction of stent thickness from three to six months.
Histological assessment
On histological cross-sections, stented segments demonstrated clearly visible struts or strut voids. In all animals, most stented sections revealed morphology typical of pig coronary arteries implanted with stents using a diameter over sizing of 10%. Stent strut injury score was low at all follow-up time points.
At seven days, stents had thin mural thrombi coverage with scattered leukocyte infiltration. Light microscopy photomicrographs indicate that the stents were completely expanded and intact. There were no signs of under-deployment or immediate recoil (Figure 8). At 14 days, early thrombus organisation, including invasion with round, spindle-shaped, and stellate cells were seen, along with collagen deposition, and mild inflammatory reaction. At 30 days, the neointima increased in size (0.26±0.14 mm) and demonstrated a well-healed fibro-cellular composition with only minimal residual thrombus components; inflammation was mild and mostly in the form of multinucleated foreign-body giant cells. None of the arteries analysed had incomplete stent apposition, excess of intimal thickening at the stent edges, or intraluminal thrombi. The in-stent neointima of all animals appeared fully healed, with abundant smooth muscle cells (SMCs).
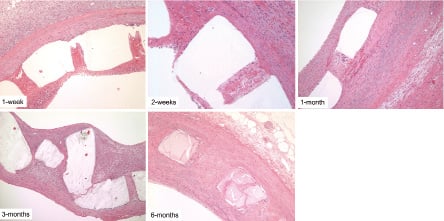
Figure 8. High-magnification micrographs of pig coronary arteries harvested 7, 14, 30, 90 and 180 days after BTI stent implant.
At 90 days, healing had progressed so that only rare intramural thrombus was seen, and intimal thickness was 0.4±0.17 mm. The stent appeared at this time to have undergone considerable absorption, mostly in the form of surface erosion, with some cellular infiltration into the stent space. At 180 days, arterial wall incorporation of the absorbable stent involved a highly organised and stable fibrocellular neointima with thickness of 0.31±0.10 mm. There was no evidence of any residual thrombus, fibrinoid deposits, or haemorrhage. At this stage, endothelialisation was complete, and inflammatory reaction was minimal and stable, being represented almost entirely by giant cells. The appearance of the stent profiles suggested further erosion and cellular infiltration, as compared to 90-day findings (Figure 8).
Discussion
Permanent metallic stents are well established components of modern interventional cardiology, and their use is recommended in official guidelines. The main goals of stent implantation include temporary scaffolding; sealing of dissections; and prevention of acute and chronic recoil, a transient event occurring in the first hours to weeks after angioplasty. However, impaired access and flow to side branches, attenuation of vascular vasomotion, long-term risk of stent thrombosis, inflammatory allergic reactions, coronary aneurysm formation, and technical difficulties for future surgical anastomoses are some of the drawbacks of permanent stents.
Polymeric stents have the potential to act as local drug delivery systems. These materials, especially biodegradable polymers, have been widely utilised for the controlled release of drugs. Therefore, it is possible to design a biodegradable polymer stent, not only offering a physical barrier to the vessel wall, but also presenting a pharmacological approach in the prevention of thrombus formation and intimal proliferation. However, there are some common and practical challenges of any biodegradable stent technology including radial force, acute and chronic recoil, time of degradation, neointimal inhibition, and biocompatibility with the vessel wall.
Biocompatibility of degradable biomaterials is largely dependent upon the solubility of the released degradation products. Their local toxicity is related to the local concentration of the elements over time. Nevertheless, important drawbacks of polymer stents relate to their intrinsic mechanical properties. Polymeric stents may not afford the same radial force and limited recoil, as compared to metal platforms; and their relative bulkiness could limit application in small vessels.
The present study demonstrated favourable vascular compatibility and efficacy for a novel, fully bioabsorbable salicylate-based SES. This device displayed good mechanical performance during deployment, with preserved wall apposition at long term follow-up. Salicylic acid released from the BTI stent has potential anti-inflammatory and antiplatelet properties. Of note, the radial strength of the BTI stent was maintained over time.
Although the current version of this absorbable stent needs further development, our preliminary study suggests that the implantation is safe and feasible, achieving results consistent with those of bare metal stents in a well-established animal model. The absence of a rigid intravascular prosthesis subsequent to complete absorption is expected to restore vasomotion and is the target of ongoing studies.
Study limitations
Lack of control group and relatively small numbers of animal subjects should be considered. Normal porcine coronary arteries do not precisely simulate responses to stents, bare-metal or drug-eluting, in human atherosclerotic coronary arteries. Therefore, it remains uncertain if the BTI stent can demonstrate similar effects in human coronary artery disease.
Conclusions
Our findings demonstrate that a novel, fully bioabsorbable salicylate-based SES has favourable vascular compatibility and efficacy in a porcine model up to six months. This device has good mechanical performance during deployment and retains vessel wall apposition at long-term follow-up. While these initial results are highly encouraging, further work is required to determine long-term safety and efficacy of this technology, as well as its clinical applicability.
Acknowledgments
I would like to thank Audrius Abrutis for his assistance with the project management, Cindy Baranowski, Lian Dorsey, and Connie Micko for their expert technical assistance with the histologic section preparation; Ashley Strong, Musa Hasan, Bobby Price, and Jourdan Davis for their support with the stent implant procedures; Addrena Taylor for her assistance with the necropsy and tissue preparation. I also thank Patrick Rivelli, Olex Hnojewyj, and Tony Shaffer for their valuable contribution to this investigation.
References

