Introduction
Since the introduction of percutaneous coronary interventions in 1977, refinements in material, technique and adjunctive medication made it the most frequently practised procedure of mechanical coronary revascularisation. Stents have eliminated the fear of periprocedural abrupt vessel closure and drug-eluting stents reduced the restenosis rate but they have also created new “iatrogenic” concerns, from the fear of late or very late stent thrombosis to permanent metal coverage of long segments precluding or limiting options of further revascularisation. These problems led to an increased interest for the development of a fully biodegradable stent. After favourable results from experimental studies on porcine coronary arteries and the first clinical experience with the fully-absorbable PLLA Igaki-Tamai stent, progresses in biodegradable polymers and design allowed favourable initial results in patients with simple coronary artery lesions, calling for the need of further larger trials. In this article we report a critical analysis of results with the new fully bioabsorbable magnesium AMS stent (Biotronik, Bulach, Switzerland) and the biodegradable BVS stent (Abbott Vascular, Santa Clara, CA, USA).
Background
During the last 15 years, advances in interventional cardiology have led to a wide use of drug-eluting stents (DES), which actually represent the most frequent choice for treatment of coronary artery lesions. DES significantly reduce the rate of in-stent restenosis and target lesion revascularisation compared with bare metal stents (BMS)1, but the enthusiasm for their use has been dampened over the last years by the risk of stent thrombosis2,3. Despite dual antiplatelet therapy with aspirin and clopidogrel which gives the possibility of reducing the risk of sub-acute stent thrombosis4, a persistent incidence of late stent thrombosis of 0.5% per year exists, confirmed both in randomised studies and large registries5,6. This unpredictable event may develop >1 year after stent implantation and has often fatal consequences2. Causes of late stent thrombosis have been described as related to delayed endothelialisation and subsequently longer exposure of uncovered stent struts in lumen vessel, chronic inflammatory response and localised hypersensitivity reactions7-9. All these adverse reactions can be potentially prevented by implantation of a fully biodegradable stent.
There are many potential practical advantages to a biodegradable stent. While covering a side branch with a stent, it would no longer be necessary to routinely open stent struts traversing the ostium of an uncompromised side branch. Following ostial stenting, struts protruding into the aorta or the parent vessel would no longer be a permanent potential source of embolism and of obstruction to future vessel instrumentation. Degradation of struts would remove the inflammatory stimulus for ongoing intimal hyperplasia and late positive remodelling would no longer be prevented from offsetting lumen encroachment. The problem of late stent strut malapposition would be eliminated and no longer would a “full metal jacket” preclude subsequent coronary surgery. Cardiac magnetic resonance (MR) and multislice computed tomography (MSCT) imaging - the likely future non-invasive alternative to angiography for coronary imaging – will not be prevented. In the current era of drug-elution, a biodegradable substance represents the ideal vehicle to ensure complete drug delivery, and the temptation may arise to extend treatment to “normal” or “near normal” vessel segments with minimal lumen narrowing, but severe atherosclerotic burden at risk of progression of destabilisation (rupture). On a more human level, how often do our patients ask us about the long-term fate of stents and whether they can be removed?
These reasons focused the interest of cardiologists and bioengineers on the development of a fully biodegradable stent. Optimal characteristics of a fully biodegradable stent are: a scaffolding-structure to ensure enough radial strength against vessel recoil for 3-6 months, a complete and progressive absorption within six months, and a low chronic induced inflammatory response (Figure 1).
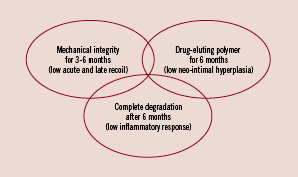
Figure 1. Optimal characteristics of a fully biodegradable stent.
Experimental pioneers
Since the first experimental studies on biodegradable polymers in porcine models10, important improvements in design and characteristics of polymeric stents have been made. In 1996, Van der Giessen and colleagues reported the negative sequelae of implantation of the Wiktor coil stent coated with five different polymers in porcine coronary arteries11. All were associated either with marked inflammation leading to intimal hyperplasia or with thrombotic occlusion. Clearly not all biodegradable compounds were proven to be biocompatible. Subsequently, the poly-L-lactic acid (PLLA) polymer was found to produce more encouraging results. Lincoff et al demonstrated that low-molecular-mass (80 kDa) PLLA causes an intense inflammatory neointimal response, whereas a high-molecular-mass PLLA polymer (321 kDa) is well tolerated within the coronary arteries with no inflammation, low degree of intimal hyperplasia and no sub-acute thrombosis reported12. Moreover, they reported the feasibility of drug-elution (dexamethasone) from this PLLA polymer coat for at least 28 days after stent implantation, although without any inhibitory effect on restenosis. Successively, the intramural delivery of a specific tyrosine kinase inhibitor with a PLLA biodegradable stent was able to overcome the proliferative stimulus three weeks after stent implantation, resulting in suppression of the restenotic changes of the coronary artery, with excellent biocompatibility and minimal vessel inflammation13. These exciting examples of progress achieved in experimental studies with new high-molecular-weight PLLA polymer coronary arteries supported its investigation in humans.
The pioneer: the Igaki-Tamai stent
The Igaki-Tamai stent (Kyoto Medical Planning Co. Ltd, Kyoto, Japan) has been the first fully biodegradable stent tested in humans14. It is a balloon-expandable stent, composed of a high-molecular-weight PLLA monofilament (321 kDa) with a zigzag helical design and a stent strut thickness of 170 µm. Structural limits of the Igaki-Tamai stent were the excessive thickness, the difficulty to have it securely crimped, the excessive sensitivity of the polymer from temperature with the potential risk of diameter growth and uncontrolled vessel injury. In fact, its deployment required a covered sheath system through an 8 Fr guiding catheter and a balloon temperature of 50° for a complete and safe deployment in about 30 seconds. The stent tends to continue to expand gradually to its original size after deployment, until equilibrium between the circumferential elastic resistance of the arterial wall and the dilating force of the PLLA stent is reached. The only reported experience nine years ago (25 Igaki-Tamai stents implanted in 19 simple de novo coronary artery lesions) demonstrated the safety and feasibility of implantation of this biodegradable stent in humans, without any procedural complications during stenting14. At six months follow-up, no stent thrombosis, MACE or adverse biocompatibility responses were observed after implantation in 15 patients. The rate of restenosis was similar to permanent BMS (10.5%), but the large acute vessel recoil (22%) and the uncontrolled enlargement of stent area at three months (+10.2%), due to its thermo-dependent auto-expandable properties, discouraged interest for this stent. Moreover, persistent hyperechoic struts have been observed by intravascular ultrasound at three and six months from its deployment, suggesting incomplete degradation within this period. In addition, the contemporary promising results on major efficacy of dual antiplatelet therapy with aspirin and clopidogrel in the first phase after coronary stenting dismissed the spectrum of sub-acute thrombosis4 and temporarily reduced the interest on biodegradable stents. The development of biodegradable polymers for drug delivery around metallic struts appeared as a more practical solution, eliminating the concerns of the persistent polymer and maintaining the mechanical advantages of strong well-tested stents, but the risk of stent thrombosis could not be excluded15.
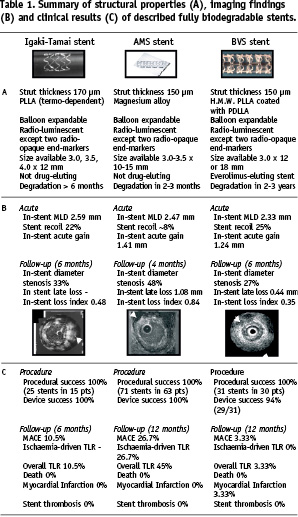
So far, only two biodegradable stents have provided exciting initial results – with the promise of excellent results in future larger trials – the bioabsorbable magnesium (AMS) stent (Biotronik, Bulach, Switzerland) and the biodegradable everolimus eluting (BVS) stent (Abbott Vascular, Santa Clara, CA, USA). Table 1 summarises their characteristics.
Bioabsorbable magnesium (AMS) stent
The introduction of magnesium material during stent development has been encouraged by the fact that its degradation “per se” generates an electronegative and thrombotic resistance surface16. Heublein et al tested a magnesium alloy (AE21) containing also 2% aluminium and 1% rare earth metals (Ce, Pr, Nd) in porcine coronary arteries17. Twenty slotted tube stents, with a length of 10 mm and uneven strut thickness of 150-200 µm, were implanted in 11 porcine coronary arteries of reference diameter 2.5-3.5 mm. An overstretch injury was produced with a stent: artery ratio of 1.3:1. There was no stent thrombosis or myocardial infarction, but there was one unexplained death four days following the procedure. Follow-up angiography, IVUS and histology were performed at 10, 35 and 56 days. Expansion of this stent was asymmetrical, and struts positioned within the adventitia caused inflammation and exaggerated intimal hyperplasia. More pronounced inflammation was also observed in areas with the greatest concentration of degradation products corresponding with corroding struts of greater thickness. Mean neointimal area was 1.41 mm2 at 35 days and 2.71 mm2 at 56 days. Strut biocorrosion had begun at 35 days, and it was estimated by extrapolation that this would be complete by 98 days. The natural process of arterial growth expected in young pigs was not prevented by the stent and the in-stent area increased from 3.28 mm2 at 35 days to 6.15 mm2 at 56 days. Degradation kinetics was rapid, and more uniform construction with thinner struts might further accelerate the process to the point of permitting recoil of a diseased coronary segment.
Successively, Biotronik developed the Lekton Magic coronary stent constructed from a magnesium alloy (WE43), also containing Zirconium (<5%), Yttrium (<5%) and rare earths (<5%). This alloy seems to have “per se” antiproliferative effects on smooth muscle cells. The stent has a novel design, characterised by circumferential noose shaped elements connected by unbowed cross-links along its longitudinal axis, it is balloon-expandable and mounted on a 6 Fr compatible rapid exchange delivery system. In a preclinical study, 33 minipigs were each implanted with two Lekton Magic stents and one control stent (Lekton Motion®; Biotronik, Berlin, Germany)18. After four weeks, the angiographic minimal luminal diameter (MLD, corrected for reference diameter) of the Lekton Magic group was higher than in the control group (1.49 mm vs. 1.34 mm). During the following two months, the MLD in the control group remained nearly unchanged (12 week follow-up: 1.33 mm), whereas the MLD in the Lekton Magic group revealed significant remodelling: it increased from 1.49 mm at week four, to 1.68 mm at week 12 (p<0.001). Despite the observed inhibitory effect on the smooth muscle cell growth of the absorbable metal stent, homogeneous and rapid endothelialisation of the Lekton Magic stent was observed in the porcine coronary arteries, and necroscopy after six days showed a nearly complete thin layer of neointima already covering the struts of the magnesium alloy stent.
Initial clinical experience with the absorbable metal stent has been gained in a peripheral application19. Twenty patients (10 diabetics), with a mean age of 76 years, have been treated with absorbable metal stents for critical lower limb ischaemia. A total of 23 stents were used to treat lesions, with a reference diameter of 2.7 mm and a lesion length of 11 mm. Angiographic and IVUS guidance were used. No adverse events were reported during the procedures. Post-procedural colour flow Doppler and MR demonstrated accurate positioning and expansion of the stents with unobstructed blood flow, indicating the absence of early recoil, despite angiographically visible calcifications present in 14 cases. Moreover, this study reported the compatibility of this stent material with MR imaging procedures. At one month follow-up with Doppler and MR, normal flow was present in 18 patients whilst indices suggested 30-40 % stenosis in two patients. At a later follow-up, two late occlusions have been reported. In one patient, this was the result of occlusion of a femoro-fibular venous bypass graft and the stent in the receiving vessel remained patent. No patient showed any symptoms of allergic or toxic reactions to the stent material.
After these encouraging results in peripheral circulation, the bioabsorbable magnesium stent was evaluated for the first time in human coronary arteries in the PROGRESS study20. This prospective, multicentre, consecutive, non-randomised study reported the successful implantation of 71 AMS stents in 63 patients with single de novo coronary artery lesions, without procedural complications or subacute stent thrombosis observed before discharge. It showed that AMS stents can be safely delivered and expanded at high pressure in simple coronary artery lesions, providing good mechanical scaffolding and achieving an immediate lumen enlargement (in-stent acute gain 1.41 mm) similar to that obtained with conventional BMS. Angiographic results four months after AMS implantation showed an in-stent late loss of 0.83 mm and a net luminal gain of 0.33 mm, with high restenosis rate (47.5%), mainly due to the neointima hyperplasia and negative remodelling. Strikingly, despite myocardial infarctions, stent thromboses or deaths were not observed within one year clinical follow-up, a wide number of ischaemia-driven target lesion revascularisations have been reported, mainly within four months from implantation (15/63 patients, 23.8%), while only two more events were observed at 12 months. This suggests that the ischaemia-driven target lesion revascularisations, due to restenosis rate, could be avoided by a slower degradation process to guarantee a longer mechanical support against the vessel recoil. In fact, unlike the Igaki-Tamai stent which continues to grow after implantation because it self-expands, the magnesium alloy stent presents a smaller area at four months, mainly due to the vessel recoil and neointima hyperplasia. Intravascular ultrasound examination showed the almost completed stent degradation at four months, with small echo-reflective remnants of the original struts without shadow, well embedded into the intima layer. These findings are concordant with results of experimental histological studies on porcine coronary arteries21, which showed by photomicrography the progressive degradation of the AMS struts with large cracks replaced at three months follow-up, and a trivial degree of vessel inflammation. Even if all these results indicate that an advanced stage of degradation already present in the first three months after AMS stent deployment, the high restenosis rate observed indicates that a more slow degradation is preferable to ensure a longer longitudinal and radial strength against vessel recoil.
Recent published intravascular ultrasound and angiographic findings in eight patients followed for 28 months from procedure, and not requiring further revascularisation after AMS implantation, demonstrated a late positive remodelling (median increase of in-stent minimal luminal diameter by intravascular ultrasound from 1.87 to 2.17 mm and a median reduction of luminal late loss by quantitative coronary angiography from 0.62 to 0.40 mm) and the absence of late adverse events22. This confirms that a slower degradation of this stent is warranted to provide sufficient radial force to improve long-term patency rates after its implantation.
Therefore, after the promising results from the PROGRESS-AMS trial, we await the results on the BVS stent, a fully biodegradable stent which disappears within a longer time to overcome the acute recoil and negative remodelling processes, but which might also release antiproliferative drugs to prevent restenosis (like a DES), an attractive idea. On the other hand, the controversial concern about the possibility of distal embolisation of residual struts soon after biodegradable stent implantation has already been dismissed by the PROGRESS-AMS trial. In fact, no increment of CK-MB values or new Q waves in the ECG have been observed after delivery of the AMS stent, suggesting that the risk of bulky stent particles forming emboli plugging the microcirculation is unfounded, and the corrosion of magnesium alloy starts within the wall layer when struts are already covered by a thin neointimal layer.
Biodegradable everolimus eluting (BVS) stent
The ABSORB trial23 investigated the feasibility of implantation of the first bioabsorbable eluting drug stent in humans, with an acceptable in-stent late loss and minimal intrastent neointima hyperplasia, six months after its deployment. The BVS stent is composed of a high-molecular-weight PLLA backbone, with serpentine rings connected by links. The stent body is coated with a matrix of everolimus and poly-D,L-lactic acid (PDLLA) in a 1:1 ratio. PLLA and PDLLA are fully metabolised and totally absorbed by macrophages. Its body is completely radio-luminescent, with only two radio-opaque platinum markers on both ends of its surface, to facilitate the identification of the prosthesis, as the AMS stent. The stent for the trial was the 3.0 mm x 12 or 18 mm, mounted on the VISION RX balloon catheter, which is identical to the everolimus eluting stent delivery balloon catheter. Experimental studies showed that BVS stent is fully metabolised to carbon dioxide and water and fully absorbed within 2-3 years from implantation. This stent presents structural characteristics similar to the first biodegradable stent implanted in humans, the Igaki-Tamai stent (both made of PLLA, strut thickness respectively 150 and 170 µm), that resulted in a similar acute behaviour of the polymer after its deployment (acute recoil 25% vs 22%, defined as the difference between the maximum balloon diameter and the minimal luminal diameter post stent implantation). The BVS stent has been successful implanted in 30 patients, with only one case of non-Q-wave myocardial infarction observed 46 days after implantation and no any cases of ischaemia-driven target lesion revascularisation or stent thrombosis reported until one year follow-up. The introduction of a biodegradable drug-eluting polymer provided good results in terms of in-stent late loss (0.44 mm) at six months, similar to data reported for the paclitaxel eluting stent (0.39 mm)24, and for the permanent everolimus-eluting polymer on a metallic platform stent (0.12 mm)25, and better than classic BMS (> 0.8 mm), mainly due to a reduction of stent area (– 11.8%) and a minimal intra-stent neointimal hyperplasia (5.5%). Definitely, the acute stent recoil was prominent like the Igaki-Tamai stent, but its clinical consequences were limited by the blunted hyperplasia due to the release of the potent antiproliferative drug everolimus, suggesting that the everolimus eluting bioabsorbable polymer of the BVS stent is able to suppress restenosis as effectively as when the drug is eluted from a permanent polymer on a metallic platform. In fact, the in-stent obstruction area measured by intravascular ultrasound at six months follow-up is similar to that observed with the permanent everolimus-eluting stent tested in the SPIRIT FIRST trial (respectively 5.77% and 7.9%), and much lower than that observed for the BMS in the control group of the same study(28%)25. Beyond these favourable results, intravascular ultrasound examination six months after BVS implantation showed a significant decrease of calcium score and an increase of fibrous area around the stent, and a sub-study by OCT at the same time in 13 patients (738 struts examined) showed an uncompleted stent strut apposition rate of 5% (already present at baseline).
As for the AMS stent at four months, the intravascular ultrasound examination six months after BVS implantation detected small circular areas with higher echo-reflection than surrounding tissue, without shadowing, in correspondence to the strut’s original position. Moreover, a substudy of the ABSORB trial to assess the temporal degradation process by intravascular ultrasound-backscatter radiofrequency showed a significant reduction of dense calcium in the stented-segment at six months, while the wall-strain values measured by palpography increased after procedure26. This suggests an alarming increase in wall deformation at the time the scaffold properties disappear, with a potentially higher risk of stent thrombosis. These results should be analysed with attention, considering that biodegradable stents are designed to be gradually metabolised, with a bound lost of structural integrity and radial strength that result in an active remodelling and in-size shrinking over time (this latter property has been observed in both the AMS and BVS stents, but not in the Igaki-Tamai stent due to its auto-expansion), specially, when implanted in fibrocellular or fibro-necrotic plaques with less calcium density27. However, recent published intravascular studies with a longer follow-up period provided important reassuring preliminary finding. Two years after BVS implantation, the struts lost their echogeneity, and are not more identifiable by intravascular ultrasound, while only one-third are yet identifiable by OCT28. Moreover, the wall-strain that increased after the procedure to six months did not subsequently change between six months and two years. Beyond these findings, no stent thromboses have been reported until now with both these biodegradable stents. Also if the presence of residual struts within the wall layer detected by OCT two years after its deployment suggests the need of a longer time for the complete degradation, and thrombotic events after this time cannot be yet excluded. However, the absence of late stent thrombosis at four years follow-up in 50 patients (63 lesions) who received the Igaki–Tamai stent is encouraging, considering that it is an auto-expandable stent with major risk of vessel damage to uncontrolled late enlargement compared to the BVS stent29.
Two other concerns need to be briefly discussed. At first, the idea to treat coronary artery disease and restore normal vessel function at the same time when the biodegradable stent is fully degraded appeared fascinating, as well as the possibility to prevent stent thrombosis. The preliminary findings on a restored vasodilatory response to isosorbide-dinitrate and acetylcholine, respectively at four months after AMS implantation (n=5 patients)30 and at two years after BVS implantation (n=9 patients)28 – therefore at the time when both these stents have almost completed their degradation process – is very attractive, but needs to be confirmed by specific studies in a larger population. Second, the similar luminal diameters measured soon after stent implantation by 3-D multislice computed tomography and 2D-quantitative coronary angiography are promising31 and, if confirmed in longer follow-up, add further motives, if not enough, to the need of further larger trials on biodegradable stents.
In conclusion, the main impetus for the development of biodegradable stents was the high incidence of subacute stent thrombosis. In the late 1990s, the Igaki–Tamai stent, a bioabsorbable stent made of a high-molecular-weight polymer of L-lactic acid, was implanted in 15 patients (25 stents), with an acceptable restenosis rate at six months (10.5%). However, when the use of high-pressure deployment of stents and routine aspirin and clopidogrel substantially reduced thrombotic events, results of this first clinical experience rapidly fell into oblivion. The recent resurgence of interest is undoubtedly due to the fear of late stent thrombosis after implantation of drug-eluting stents. Despite reported favourable results on characteristics of recent biodegradable stents, studies until now reported their safety and implantation feasibility, with achieved acceptable results in term of vessel recoil, restenosis and late in-stent late loss. The clinical results on MACE, target lesions revascularisations and stent thrombosis are encouraging, but limited because of the small number of enrolled patients and have to be confirmed in larger studies as well as in high risk patients with more complex lesions. Other concerns could be addressed to the recently tested fully biodegradable stents. If OCT results suggest that BVS biodegradation requires 2–3 years, is there a real advantage over conventional drug-eluting stents, for which we still do not have convincing data for a persistent risk of stent thrombosis beyond 3–4 years? Imaging findings of residual struts at longer follow-up with both AMS and BVS stents indicate that the concern of the risk of late stent thrombosis is yet open, despite the favourable clinical results. However, the absence of late stent thrombosis at four years in 50 patients (63 lesions) who received the Igaki–Tamai stents is encouraging, but these stents promoted a thick rim of intimal thickening separating these struts from the lumen, a result very different from the strut protrusion or malapposition seen with the AMS and BVS stents. Many authors have pointed out that the component of drug-eluting stents which causes inflammation and thrombosis is the polymer rather than the drug. The biocompatibility of poly-L-lactic acid is known from its long standing use in the repair of orbital floor defects, rotator cuff and labral injuries, and in spine surgery, but rare adverse reactions are also reported32.
Definitely, fine-tuning of polymer characteristics and drug-release to obtain persistent mechanical resistance and modulate hyperplasia in the first months after implantation, while maintaining rapid degradation afterwards, is a monumental task. Is the goal worth all this effort, or will the progress of metallic stents with thin struts covered by a film of biodegradable polymer and less aggressive drugs be enough to eliminate late stent thrombosis? Events, as potentially deadly as stent thrombosis, should ideally be eradicated, and radical alternatives to conventional stents, such as biodegradable stents, deserve to be the focus of research investment.

