Introduction
Percutaneous coronary intervention in the last decade has undergone revolutionary progress with reduction of in-stent restenosis to <10% from 35% at nine months following placement of drug-eluting stents (DES) versus bare metal stents (BMS).1-3 These impressive results have unfortunately been tempered by the occurrence of late stent thrombosis (LST), an infrequent (0.6%/year) but potentially deadly complication.4-6 We have reported that signs of delayed healing accompany the reduction of neointimal formation by DES; consisting of persistence of fibrin, decrease in smooth muscle cell and endothelial cells at one month in animals and at six to nine months in man.7,8 These findings constitute the major pathologic substrate underlying all cases of LST. First generation DES, i.e., the sirolimus eluting Cypher® stent (Cordis Johnson & Johnson, Miami, FL, USA) or the paclitaxel eluting Taxus® stent (Boston Scientific Inc., Natick, MA, USA) have both been associated with LST.
While the mechanisms of LST are varied, we have shown, using preclinical models, human autopsies8,9 and, more recently, thrombectomy specimens10, that delayed healing is accompanied by inflammatory cell infiltrate, which in some cases resembles a hypersensitivity reaction. This seems to peak only after full release of the drug, and is thought to be secondary to polymer induced foreign body reaction. These data have raised awareness about the importance of the biocompatibility of permanent polymer implants and their potential role in contributing to LST. Given these issues, more recent focus has been placed upon developing bio-erodible polymers, which degrade over time, and therefore eliminate these issues of polymer biocompatibility. Several new devices have been developed using this approach, and this review will summarise the problems with first generation DES polymers, reviewing the progress that has been made in developing newer DES which incorporate bio-erodible polymers with the goal of eliminating the chances of polymer induced inflammation. The goal of these newer drug-delivery devices is to minimise restenosis while improving long-term outcome by reducing risk for long-term inflammation and LST.
Non-erodible polymers and first generation DES
The first generation of DES are covered by a non-erodible polymer. In the Cypher® stent this is composed of polyethylene-co-vinyl (PEVA) and poly(n-butyl methacrylate) (PBMA) which releases 80% of the loaded dose of sirolimus (approximately 140 mg/cm2) in 30 days and the rest by three months.1 The Taxus® stent is loaded with paclitaxel (1 mg/mm2) on a non-erodible poly(styrene-b-isobutylene-b-styrene) (SIBBS) polymer with a biphasic elution phase, providing a burst release of two days, and subsequently a low-level release over 10 days.11
We have reported the occurrence of a hypersensitivity vasculitis with Cypher® stents associated with extensive chronic inflammation consisting predominantly of eosinophils and T-lymphocytes infiltrating within all layers of the vessel wall without any extension into non-stented segments.12 When this occurs, a hypersensitivity reaction is usually observed beyond the 1-year period, at which time it is believed, from animal studies (RV, unpublished data), that the drug is completely eluted from the stent. It is thought that this hypersensitivity reaction occurs as a result of polymer induced inflammation. PBMA, a component of bone cement, when implanted subcutaneously, induces a macrophage giant cell reaction accompanied by tissue damage and fibrosis. In addition, PEVA, the other component of the polymer employed in the Cypher® stent, when used as an antigen-delivery matrix, has been shown to elicit inflammation in 25% of rabbits. Although sirolimus itself has also been associated with hypersensitivity reaction, the most common adverse reactions are bone marrow suppression, hypercholesterolaemia and hypertriglyceridaemia. Recently Cook et al have reported hypersensitivity in thrombus aspirates in living patients presenting with LST following Cypher® stent placement and stent malapposition with both Cypher® and Taxus® stents.10 Although the SIBBS polymer, which is employed on the Taxus® stent, has not been shown to induce a late hypersensitivity reaction, the polymer is difficult to handle and is associated with webbing and uneven distribution along with macrophage and acute inflammatory infiltrate near the lumen and malapposition, but with no granulomatous reaction.
Both polymers allow the timed release of the drug leading to distinct elution profiles, which are necessary for the anti-restenotic effect. From 1st generation DES, Cypher® and Taxus®, it is clear that non-erodible polymers may also induce inflammation for a longer period –either through hypersensitivity or profound inflammation and malapposition – which contribute to LST and even restenosis. In order to overcome these limitations, it has been suggested that bio-erodible polymers might improve safety by allowing for controlled release of the drug while inducing minimal inflammation with eventual degradation, essentially leaving a bare metal surface behind.
Are bio-erodible polymers better choices for drug delivery?
Biodegradable polymers have been used in the medical industry for a long time (e.g., in surgical sutures since the 1960’s, in orthopaedic implants in the form of pins, rods and tacks, staples for wound closure and dental applications). The earliest and the most commonly used biodegradable polymers are the polyesters, which include poly(lactide), poly(glycolide) and poly(glycolic-co-lactic acid) (Table 1).
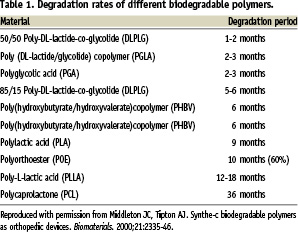
As stated by Middleton and Tipton13, the ideal property of biodegradable polymers are that: i) they should not evoke an inflammatory/toxic response; ii) they be metabolised in the body after fulfilling their purpose; iii) they are easily processed into the final product form; iv) they have an acceptable shelf life; and v) they should be easily sterilised.
Polymer degradation
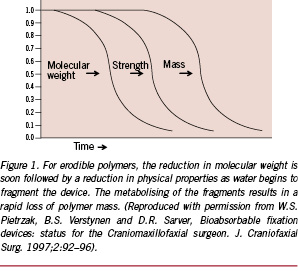
The two principal ways by which semi-crystalline polymers can degrade involves passive hydrolysis and/or enzymatic reactions. Generally, the degradation begins with water penetrating the polymeric device, preferentially attaching the chemical bonds in the amorphous phase and converting the long polymer chains to shorter ones, ultimately forming water-soluble fragments.13 This leads to a reduction in molecular weight – but no loss of physical property (strength) – as the device matrix is still held together by the crystalline region. In the second phase, the enzymatic attack of the fragments occurs, which results in rapid loss of the polymer mass (Figure 1).
The well-known biodegradable copolymers like PLA and PGA, upon hydrolysis of the ester bonds, release lactic acid and glycolic acid respectively as their degradation products, which ultimately convert to water and carbon dioxide through the action of enzymes in the tricarboxylic acid cycle which are excreted through the lungs.14 There are several factors that influence the velocity of the degradation: the chemical bond, pH, presence of catalysts, additives, impurities or plasticisers, copolymer composition and water uptake, are the most important. The degradation is accelerated in “high hydrophilicity of monomer, low or high pH, more reactive hydrolytic group in the backbone, less crystallinity or smaller polymer size”.13 In a vessel with inflammation, pH is expected to be low, which will accelerate degradation. Similarly processing conditions, sterilisation processes, shape of the polymer, size and diffusion and mechanical stress and implantation site also affect degradation. When devices are implanted in poorly vascularised areas this significantly slows degradation.
Biocompatibility
An ideal implant should not elicit an inflammatory or toxic response. The degradation also must be metabolised in the body after fulfilling the purpose that it was implanted for, and should leave no trace. Many studies have been carried out to determine the biocompatibility of implants since the 1960s, and most have shown biocompatibility with a variety of bio-erodible polymers. An important caveat is that most were not implanted within the vasculature and therefore need further scrutiny. Our own experiences with many of the currently approved DES using biodegradable polymers on stents for coronary application have shown promise of safety as well as biocompatibility; these include: BioMatrix (Biosensors International, Singapore), Nobori (TERUMO Europe NV, Leuven, Belgium), bio-erodible polymers by SurModics Inc. (Eden Prairie, MN, USA), Costar (Conor Medsystems, Menlo Park, CA, USA) and BVS (Abbott Vascular, Santa Clara, CA, USA). Many others, during absorption of the polymer, have induced inflammation and been discarded. In some cases, findings from preclinical studies can be misinterpreted, especially in cases where the drug may be toxic, yet the polymer may be blamed for the inflammation or excessive fibrin deposition and lack of endothelialisation, or the drug loading may be in the toxic range. A “polymer only” control is essential in distinguishing culpability between polymer versus drug.
Our experience with the BioMATRIX™ stent (Biosensors International, Singapore), loaded with biolimus A9 (15.6 mg/mm2), a sirolimus analogue on a polylactic acid biodegradable polymer in the pig model at one, three and six months has shown good biocompatibility as well as efficacy without the induction of significant inflammation. Similarly, Nobori™ stent uses the Biolimus A9 (TERUMO Europe NV, Leuven, Belgium), with the same polymer as employed on the BioMATRIX™ stent. In the rabbit model this system has shown good efficacy and long-term biocompatibility with preservation of endothelial function. Both these stents have been approved in Europe, and have shown clinical success in large clinical trials with a decrease in restenosis as well as long-term safety in man.15,16
Other biodegradable polymer drug-eluting stents which have been tested include poly-lactide coglycolide (PLGA) paclitaxel slow-release (12 to 16 weeks), with moderate (0.3 to 0.35 6 mg paclitaxel/mm2) and low dosing (0.156 mg/mm2) on a cobalt-chromium stent. Although in the moderate dose the polymer was shown to be biocompatible, the drug dose was too high and resulted in toxic effects with stent malapposition, excessive fibrin deposition at one month and excessive neointima at three months in the porcine model.17 However, the low dose system did show biocompatibility as well as efficacy of the drug. This combination is currently undergoing a first-in-man trial.
The Conor™ stainless steel and the new cobalt-chromium more flexible Costar Medstent™ (Conor Medsystems, Menlo Park, CA, USA) stents use a reservoir technology to design a paclitaxel, slow-releasing DES with a bioabsorbable PLGA polymer. This polymer has been shown in animal, as well as small clinical studies to be efficacious, not inducing excessive inflammation in the porcine model. However, in larger randomised studies it failed to show a significant reduction in restenosis against the Taxus® stent.18 The same reservoir technology, but modified stent design which elutes sirolimus, has been shown to be efficacious in first-in-man studies.
Two other novel bio-erodible polymers by SurModics Inc. (Eden Prairie, MN, USA) have recently been developed and show promise of safety. The first includes a family of ether-ester urethane multi-block copolymers (MBCP) that comprise blocks of lactide, glycolide, and caprolactone chain-extended with a urethane linkage (SynBiosys™ polymers by OctoPlus N.V. Leiden, The Netherlands; InnoCore Technologies, BV, Groningen, The Netherlands and SurModics, Inc., Eden Prairie, MN, USA). The second, is a family of maltodextrin (MD) modified with fatty acid side chains to yield a hydrophobic polymer (Eureka TN SOLO polymers; SurModics, Inc., Eden Prairie, MN, USA). Both the polymers were loaded with sirolimus on stainless steel stents; the ester-ester MBCP completely released sirolimus in 30 days and completely degraded within 30-60 days, while the hydrophobic MD polymer exhibited a somewhat slower release of sirolimus (~85% in 30 days) within 60 days; with the polymer degrading slower, taking greater than 90 days in PBS. In vivo in the pig model, there was significant reduction in percent stenosis as well as an increase in fibrin deposition and minimal inflammation at 28 days, with both polymers plus sirolimus from the bare metal control, except the hydrophobic MD group, having greater inflammation than ester-ester MBCP (Figures 2 and 3). There were no significant differences in the extent of inflammation at 90 days between groups.
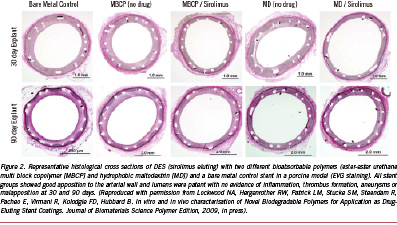
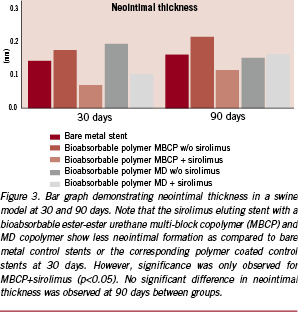
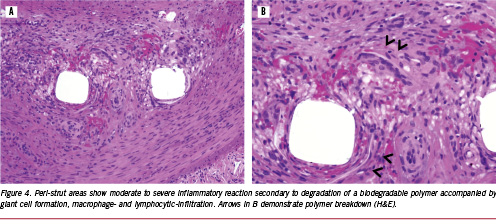
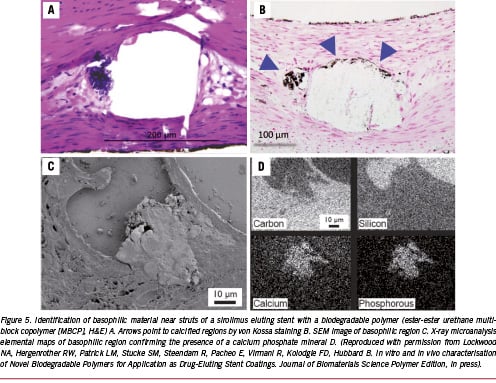
Although the above examples do not show any significant untoward effects, especially inflammation in the pig model at approximately one and three months, it is the balance between drug release kinetics, the rate of degradation of the polymer and the degradation products that are essential for the success of bio-erodible stent systems in the coronary vasculature. In addition to the degradation products, many degradable materials release by-products such as initiators, catalysts and solvents, which are the main initiators of inflammation (Figure 4).19 Similarly, we have noted the presence of calcification around struts in most biodegradable polymers, especially at three months rather than one month, and this is likely the result of partially-degraded polymer or acidic polymer by-products which drop the pH, allowing for the precipitation of calcium phosphate (Figure 5). Usually, we have not observed any significant inflammation where calcium phosphate is deposited. The amount of calcification is minimal, and is likely of no clinical significance since extensive calcification is typically observed in atherosclerotic arteries.
Another novel bioabsorbable salicylate-based polymer with a sirolimus-eluting stent (SA/AA+S) coating has been reported to be successful in 30 days studies in the pig model20. The dose of sirolimus is similar to that employed in the Cypher® stent. Elution kinetics are also very similar to Cypher®, with initial burst over the first 48 hours and a gradual release over the next 30 days, with polymer degradation occurring in 37 days in vitro. The SA/AA+S and Cypher® stents showed significantly less neointimal thickness as compared to BMS, and a trend to reduced inflammation as compared to BMS and Cypher® groups.
Other biodegradable natural coatings that have been used include omega-3 fatty acids (Atrium Medical, Hudson, NH, USA) in the presence of sirolimus analogues on a cobalt chromium stent. In animal studies, this system has shown no untoward effects (Virmani, R. unpublished data). However, no clinical data in man are as yet available.
Fully biodegradable stents
A fully bio-erodible polymeric stent has been suggested to be feasible and attractive alternative to bare metal stents. This concept of a bare metal-like stent that degrades over time is attractive, since we know that even bare metal stents alone induce inflammation and are associated with thrombosis and late restenosis. Also, permanent metal stents make surgical intervention difficult because of the inability to graft to these areas. Ideally, a fully erodible stent would disappear with time and leave the vessel wall in its native state, while during its existence allow for non-invasive imaging. Approximately 15 years ago, Igaki-Tamai (Kyoto Medical Planning Co. Ltd, Kyoto, Japan) were the first to introduced a fully bio-erodible polymeric stent made of poly-L-lactic acid (PLLA) polymer which was successfully deployed in man. The clinical long-term results were equivalent to bare metal stents.20 However, the deployment of the stent required a thermal balloon and was associated with polymer creep, which dampened enthusiasm for this device.
Biotronik (Biotronik, Berlin, Germany) introduced the first metallic bioabsorbable magnesium stent (AMS) composed of 93% magnesium and 7% other rare earth metals with thromboresistant properties. However, structural integrity was only maintained for two months and was replaced by inorganic salts. Initial clinical trials in the periphery as well as in the coronary circulation resulted in higher restenosis rates than bare metal stents21,22. It is currently undergoing modifications for future trials.
An alternative approach by Abbott Vascular (Abbott Vascular, Santa Clara, CA, USA) uses drug-elution along with the bioabsorbable stent (BVS). The BVS stent has a backbone of poly-L-lactic acid (PLLA) providing support and a coating of poly-D,L-lactic acid (PDLLA) that contains and controls the release of the anti-proliferative agent everolimus (Novartis, Basil, Switzerland).23 The strut thickness of the first generation BVS stent was 150 mm. The drug release kinetics is very similar to their DES stent Xience™ V (Abbott Vascular, Santa Clara, CA, USA) – 98 ug for the 12 mm and 153 ug for the 18 mm stent – 80% of the drug is released in 28 days from the polymer coating. The arterial tissue concentration peaks at three hours, with a mean concentration of 15ng/mg (95% CI 13.05 to 17.57), whereas at 28 days the tissue concentration dropped to 0.9 to 2 ng/mg in the pig coronary arteries.23 The PLLA and PDLLA are fully biodegradable. The in vivo polymer degradation profile of a non-clinical PLLA-everolimus eluting stent in the pig coronary artery shows a mass loss of 30% at 12 months with further reduction to 60% by 18 months following implantation.23
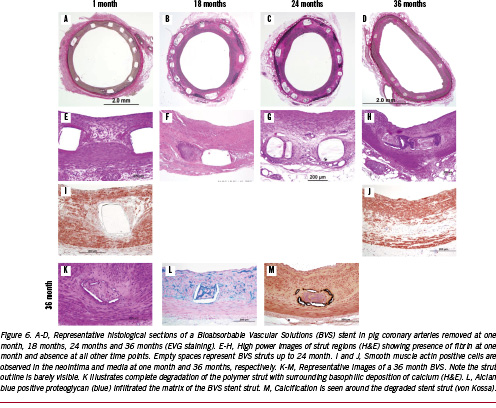
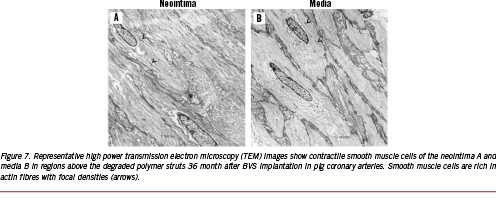
Our laboratory has examined the BVS stent in pig coronary arteries and in rabbit iliac arteries implanted for one month and to three years, comparing these to the Cypher® stent (Figure 6). Neointimal formation is equivalent to the Cypher® stent, but the lumen area is much smaller at one and three months with the BVS stent. However, the inflammation was mild as compared to the Cypher® stent at 6, 12, 18, 24, and 36 months, with significantly fewer giant cells and granulomas seen in the BVS stents. The stent struts of the BVS stent were visible in histologic sections of the pig coronary arteries up to 24 months, however, the interior of the stent was often occupied by proteoglycan, alcian blue positive staining material with some surrounding calcification around the stent struts (Figure 7). There is ‘no inflammation’ to ‘mild inflammation’ present at the 18 and 24 month time-points. Smooth muscle cells were prominent and compactly arranged towards the lumen, but the IEL was often absent. The smooth muscle cells had a contractile phenotype as assessed by electron microscopy (Figure 7). By three years, the strut structure of the BVS stent could not be clearly discerned, however, the stent strut site is occupied by cells which are rich in proteoglycans, but there is almost a complete absence of inflammation. The outline of the PLLA polymer struts are often surrounded by areas of calcification, which stains positive by von Kossa stain, the vague area within it shows impressions of pieces of degraded polymer (voids), which is traversed by cellular infiltrate (Figure 5). The vessel appears to be wide open, with minimal neointimal thickening, which is not significantly different from that seen in the Cypher® stent at three years.
From animal and small clinical trials it appears that the biggest drawback is the early recoil seen soon after implantation due to low radial force as compared to metal stents24, however, in the next generation this is being overcome by better processing and improved stent design. We remain enthusiastic about this system – especially since the problems with early recoil will likely be resolved in the next generation BVS stent – the ABSORB II trial will show even better outcomes than the ABSORB I trial.
The other bioabsorbable stent that is close to clinical trials is the REVA® stent (REVA Medical, Inc., San Diego, CA) made of poly(deaminotyrosyl-tyrosine ethyl ester) carbonate and is radio-opaque due to the incorporation of iodine molecules. The design of the stent is unique in that it has a novel locking mechanism. The polymer degrades into carbon dioxide and water, but also ethanol. Ramcharitar et al report from preclinical studies that complete endothelialisation occurs within 30 days, with low inflammation and a reduction of % stenosis from one to 12 months.25 The paclitaxel REVA stent is under development, and 60 patients have been enrolled in a first-in-man clinical trial.
Conclusion
Drug eluting stents have become the standard of care for the treatment of symptomatic coronary artery disease, however, late stent thrombosis has emerged as a major concern and therefore safer stents are being designed to overcome this hurdle. Replacing non-erodible polymers with erodible polymers appears to be gaining favour. These erodible polymers, fully degrading overtime, leave behind a bare metal surface, which is less likely to induce inflammation and allow for full endothelialisation. Not only have erodible drug-eluting metallic stents been shown to allow a complete return of endothelial function in man and animals within the first year, but they have also reduced the frequency of late stent thrombosis. The concept of fully bio-erodible polymeric stents is even more appealing, as it would return the vessel to its native state once the function of scaffolding has been provided by the stent and the vessel wall has undergone complete healing. With the use of these stents, it is hoped that patients will not need long-term dual antiplatelet therapy. Fully biodegradable stents would also not interfere in cases requiring surgical intervention, allowing for repeat intervention without the fear of overloading the artery with metal caging and obstruction of the side branches. In short, the future for bio-erodible polymers to treat symptomatic coronary disease is bright with the promise of important and novel therapeutic potential.
Appendix
Renu Virmani has company sponsored research support from: 3F Therapeutics/ATS Medical; Abbott Vascular; Ablation Frontiers; Abraxis Bioscience, Inc; AccelLab, Inc; Affinergy, Inc; AGA Medical Corp; AK International Co, LTD; AlchiMedics; Alvimedica Medical Technologies; Amaranth Medical, Inc.; AngioDynamics, Inc; AngioScore, Inc; Angiomed GmbH & Co; Angioslide LTD; Angel Medical Systems, Inc; Angioblast Systems, Inc; Apnex Medical, Inc; Arbor Surgical, Inc; Ardian, Inc.; Atritech, Inc; Atrium Medical Corp; Avantec Vascular; Bard Peripheral Vascular, Inc; B-Balloon Ltd; Biotronik AG; Biogen IDEC; Biotegra, Inc; Biomerix; BioPAL, Inc; Biosensors International; Biomer Technology LTD; Boston Scientific Corp; ByPass Medical Technologies, Ltd; CardioDex, LTD; Cardica, Inc; CardioKinetix, Inc; CardioFocus, Inc; Cardiovascular Research Foundation-Korea; CardioMind, Inc; Cardiovascular Research Foundation; Cierra, Inc; CoAptus Medical Corp; Coherex Medical, Inc; Concentric Medical; Conor Med Systems; CorAssist Cardiovascular LTD; Cordis Corporation; CoRepair, Inc; Correx, Inc; Corindus, Inc; CorNova Inc; CVRx, Inc; CyberHeart Inc; Devax, Inc.; Edwards Lifesciences, LLC; Elixir Medical Corp; Elutex, Inc; ev3, Inc; Evalve, Inc; Gardia Medical Ltd; Gem Biosystems, GlaxoSmithKline; HemCon; InfraReDx, Inc; Invatec Technology Center GmbH; Jerini AG; Kaneka Corp; Laax, Inc; Lumen Biomedical, Inc; Lutonix, Inc.; Maquet Cardiovascular; Medtronic AVE; Medtronic Heart Valves; Meril Life Sciences Pvt Ltd; Microvention, Inc.; Minnow Medical, LLC; Miravant Medical, LLC; Neovasc Medical Ltd; Novartis Pharmaceuticals Corporation; NovoStent Corp; OrbusNeich Medical, Inc; Oregon Medical Laser Center; Paragon Intellectual Properties, LLC; Prescient Medical, Inc.; Probiodrug AG; ReLeaf Medical; Relisys Medical Devices Ltd; ReValve Vascular LTD; Revascular Therapeutics; Sahajanand Medical Technologies Pvt. LTD; Sorin Biomedica Cardio S.r.l; Surmodics, Inc; Takeda Pharmaceuticals North America; Terumo Corp; Theregen, Inc; TissueGen, Inc; Top Spin; Toray Industries, Inc; Transluminal Technologies; Vascular Therapies, LLC; VIA Pharmaceuticals, Inc; Volcano Therapetutics, Inc; X-Cell Medical, Inc; and Xtent, Inc.

