
Not so many years ago we used to say that a drug-eluting stent (DES) is essentially made of a metal platform, a durable polymer, and a limus drug. Today we would say that all three of these components have become quite relative concepts: the platform does not necessarily have to be metallic, the polymer may be temporary or even absent, and the limus drug can have different formulations. In the ever-changing arena of DES, regulatory pathways are evolving as well. In Europe, recommendations for the evaluation of DES now include conditional CE-mark approval by performance of an initial pre-market trial with objective performance criteria benchmarking and invasive imaging follow-up, and unconditional CE-mark approval by a subsequent mandatory large-scale randomised trial with a primary clinical endpoint1. The supplementary appendix to the 2018 guidelines for myocardial revascularisation issued by the European Society of Cardiology lists a number of CE-approved DES recommended for clinical use based on randomised trials with a primary clinical endpoint, of which four are based on durable polymer coating, six are based on biodegradable polymer coatings and two are polymer-free2. It should be noted that this list is always in danger of being incomplete. For example, in 2018, the primary clinical endpoints of at least three more CE-mark approved DES were presented (e.g., the biodegradable-polymer Supraflex [SMT, Surat, India] and Firehawk® [MicroPort, Shanghai, China], and the polymer-free Cre8™ EVO [Alvimedica, Istanbul, Turkey]).
A typical cliché of our times is that the results of contemporary DES can hardly be improved. Stent manufacturers know this well, and the non-inferiority design of their clinical trials reflects the principle of “not getting hurt” or “me too”. Obviously, placing a DES on the market is easier if its clinical results are at least at the level of the most used competitors and the price is lower. The non-inferiority design principle implies for the consumer the theoretical chance to accept a slightly smaller treatment effect than its DES comparator. As such, it is possible that, after a series of trials where the new DES is slightly and acceptably worse than the preceding DES, an ineffective or even harmful device might be incorrectly declared to be effective (a phenomenon known as “bio-creep”)3. For this reason, every new stent should be tested against the current reference standard, and the non-inferiority design accepted only if the new device introduces at least one element of improvement on top of non-inferiority for the primary clinical outcome. Still, what this element of improvement is remains quite a mystery.
DES polymers have been blamed for years as the main element responsible for chronic inflammation, hypersensitivity, delayed healing, and neoatherosclerosis4. Neoatherosclerosis is an increasingly documented pathological entity at the intercept between stent restenosis and thrombosis5. The polymers of current-generation DES are more biocompatible when they are durable, or they are engineered to disappear after a certain time and leave a bare metal stent behind. Some polymers are conformal in that they surround the stent strut throughout its surface, others are abluminal only. These improvements determine less polymer-induced inflammation and better healing compared with older devices, but the rates of neoatherosclerosis of second-generation DES in autopsy series remain stably high at ~50% around one year6. The prevalence of neoatherosclerosis might actually be lower in vivo (e.g., 12-18% at 18 months), as shown by optical coherence tomography in both durable-polymer and biodegradable-polymer DES, but areas for improvement remain7. To date, it is not even clear whether the disappearance of the polymer represents a true long-term advantage8. In a meta-analysis of eight trials comparing durable-polymer and biodegradable-polymer DES with follow-up available at two years, and six trials with follow-up available at three to five years, the pooled odds ratios were 1.01 (95% confidence interval 0.77-1.32) and 1.09 (95% confidence interval 0.93-1.28), displaying no statistically significant difference9. Indeed, there are pros and cons linked to both concepts. On the one hand, some durable polymers may have anti-inflammatory and antithrombotic effects per se, enhancing DES biocompatibility while allowing prolonged drug release and antirestenotic efficacy. On the other hand, prolonged drug retention might be a trigger for endothelial dysfunction and neoatherosclerosis4. These issues are partially addressed in biodegradable-polymer DES, but the effect of polymer degradation on inflammation and whether metal exposure of the residual stent is actually well tolerated by the vessel wall remain uncertain in the long term4.
While we live in a phase of impasse with regard to polymer technology and expected benefits, there is increasing understanding that one of the major drivers for the observed benefits of newer DES is an often neglected aspect, the metallic platform. A meta-analysis of 11,658 patients from 10 trials of three newer-generation ultrathin-strut DES recently showed a 16% relative reduction in the risk of target lesion failure at one year, driven by less target vessel myocardial infarction10. Indeed, stents with thinner struts heal faster and are less likely to trigger thrombus formation. The current design of a metal platform, however, is the necessary compromise between favourable and unfavourable characteristics, and therefore struts which are too small can affect the visibility of the stent or its radial strength. Similarly, stents designed with large cells and less wall coverage (e.g., the so-called “footprint”) have easier side branch access but more plaque prolapses, while stents with a lower number of connectors are more flexible and deliverable, but have lower ability to withstand fracture, elongation, and longitudinal compression caused by a localised force (e.g., a post-dilatation catheter or stent delivery system). Technical aspects such as trackability, crossability and pushability ultimately result in various degrees of device success, which may act as a differentiating characteristic among contemporary DES. Notably, while the definitions of clinical endpoints for stent trials are well standardised11, device success has been inconsistently defined over time.
In aggregate, is there still a need for better DES in 2019, or are we done with progress in this technology? I would humbly say no, and articulate my reasoning as follows (Figure 1). Firstly, newer DES have not eliminated issues such as stent thrombosis and restenosis, as shown by stent trials of the previous year and trials of second-generation DES that now have long-term follow-up available12. Secondly, the outcomes of new-generation DES remain suboptimal in particular clinical and angiographic conditions. For example, in a recent trial comparing second-generation DES, the risks for target lesion failure and target lesion revascularisation were doubled in patients with diabetes compared with those without13. Thirdly, permanent metallic implants are still associated with potential late issues due to fractures and neoatherosclerosis. The idea of overcoming these problems with bioresorbable scaffolds is currently set, hoping for better times with newer upcoming technologies14. Fourthly, the outcomes of patients at high bleeding risk remain suboptimal with long-term dual antiplatelet therapy (DAPT). The currently recommended duration of DAPT is six months or longer in the context of elective percutaneous coronary intervention (PCI), and 12 months or longer in the context of PCI for acute coronary syndromes15. However, patients at high bleeding risk are candidates for shorter DAPT regimens (1, 3 or 6 months), with uncertain net benefit if endothelial coverage is incomplete. Finally, among many good DES the ideal one has probably yet to be developed16. The ideal DES will have a very low profile, thin struts, high flexibility, high radial strength, good visibility under fluoroscopy and will be mounted on a high-pressure balloon that enables direct stenting. It will elute its drug for two or three months, from a very thin surface of durable biocompatible or biodegradable polymer, stimulating early endothelialisation and resisting thrombus formation. It will have a trivial late loss and require four weeks of DAPT. Also, obviously, it will abolish the occurrence of neoatherosclerosis and target lesion failure. Are we asking a little too much?
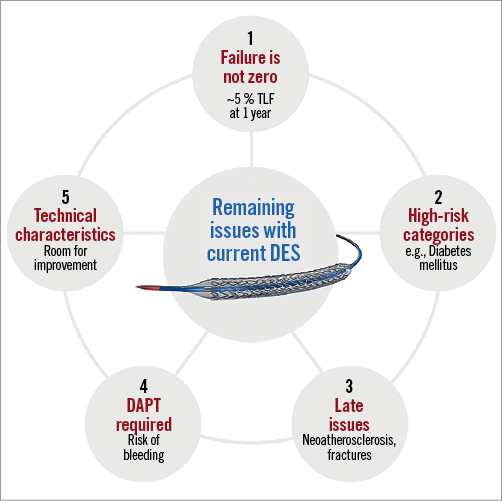
Figure 1. Areas for improvement of currently available coronary stents. DES: drug-eluting stent; TLF: target lesion failure

