Abstract
Advances in coronary stent technology, including refinement of the stent alloy, strut thickness, stent geometry, passive coating, and drug elution, have dramatically enhanced the safety and efficacy of percutaneous coronary intervention (PCI) with stenting. Stents are currently used in over 90% of coronary interventions and the use of drug-eluting stents (DES) has been disseminated to more complex lesion subsets such as total occlusions, long lesions, bifurcation lesions, and for patients with acute myocardial infarction. DES continue to demonstrate reduction in restenosis and the need for repeat revascularisation but are associated with delayed healing and re-endothelialisation, which have led to an increased rates of late stent thrombosis, dependency on prolonged dual antiplatelet therapy, impaired in-vessel reactivity, and chronic inflammation. As scientists and clinicians better understand the mechanism for late restenosis and stent thrombosis, a variety of solutions in regard to stent technology have been proposed, including stent coating, polymer bioabsorption, and fully biodegradable stents. Bare metal stents were improved by the reduction of strut thickness, changes in stent geometry, and the addition of passive coating, which lead to improvements in efficacy and reduction of restenosis. In addition, there is continued improvement in the polymer technology for DES, including new biocompatible, thinner durable polymers, and bioabsorbable polymers that completely bioabsorb within 3-12 months after stent implantation. These features potentially minimise the chronic inflammatory response and late stent thrombosis. Finally, fully bioabsorbable stents, both polymeric and metallic, continue to be developed in order to eliminate any late stenting effects and potentially may enable complete vessel restoration. This manuscript will discuss the wide variety of new stent technologies and compare and contrast durable metallic and polymeric stents to current biodegradable stent technology.
Introduction
First-generation bare metal stents (BMS) eliminated the early and late recoil seen after balloon angioplasty, but were associated with high rates of acute stent thrombosis, which was controlled with antiplatelet therapy, and restenosis, which was reduced significantly with the introduction of the drug-eluting stent (DES).1,2 The significant decrease in restenosis rates, and the need for repeat revascularisation with DES compared to BMS, have led to the speedy, worldwide adoption of DES technology. The enthusiasm for DES, however, has been dampened due to reports of excess late stent thrombosis (ST), incomplete endothelialisation, and abnormal vasomotor function.3-5 Histopathological studies have shown that the durable polymers used for drug delivery in DES can cause localised vascular inflammation, thrombotic reactions, and apoptosis of smooth muscle cells, all of which play a role in ST.6-8 Along with the anti-proliferative drug, the polymer also plays a role in delaying healing.9 Therefore, the focus of stent development was shifted to safer metallic stents, including the passive and active coating of metallic stents, the development of biodegradable polymers, and fully biodegradable stents.
Stent coating
Stent coating is an important factor that influences stent performance and may impact clinical outcome. Stent coating combines the desirable characteristics of different materials. The coating can be either “passive,” which serves only as a barrier between the bloodstream or tissue and metal with good biocompatibility, or “active,” including drug elution which directly interferes with intimal proliferation. Active coatings are primarily designed to elute drugs, they are bonded onto the surface of the stent, or the drug is trapped in polymers that function as sponges. Thus, the polymer coating must provide a platform for appropriate drug elution kinetics, a surface that minimises adverse tissue reactions, or preferably mimic a biologic substrate that can guide stent healing in a favourable pattern.
Passive stent coatings
Passive coatings of polymeric inorganic chemical composition present a biologically inert barrier among the stent surface, vessel wall, and circulating blood, thereby reducing thrombotic and inflammatory reactions and thus preventing ST and reducing neointimal hyperplasia. Some tested passive coatings include gold, heparin, carbon, silicon carbide, and phosphorylcholine (PC). Gold, which provides excellent fluoroscopic visibility with reduced thrombogenicity, is one of the first coatings to be tested, but failed to demonstrate clinical benefit.10 Hepacoat stents (coated with heparin) were well tolerated but had similar ST rates reported from “real world” registry data.11 Though preclinical evaluation of carbon nanocomposite film coating and hydrogen-rich amorphous silicon carbide coating have suggested reduced thrombogenicity, they showed no improvement in angiographic and clinical outcomes.12,13 The PC coating, consisting of methacryloyloxyethyl lauryl methacrylate polymer and a synthetic form of PC, was shown to have favourable effects on reduction of platelet activation and thrombus deposition.14 The PC coating is compatible with a range of chemical compounds, including biomolecules for gene therapy.15 The hydrophilic outer layer of PC coating, combined with a lipophilic inner layer (drug carrier for slow elution), makes PC a favourable, inert, long-term coating for coronary stents after the elution of drugs.16
The case for bare metal stents
Bare metal stents provide smooth surface material contact with the vessel wall, and are associated with positive charges countered by surrounding ions, most likely oxygen in the form of oxide, which promote healing. BMS first proved to prevent recoil, they are overall safer when compared to DES in respect to late effects of stent thrombosis, and although associated with higher rates of restenosis, they are nearly free from late catch-up after 9-12 months. This last advantage reflects complete healing, unlike first-generation DES that reported modest late catch-up in the years following the DES implantation.
Polymers and other coatings on the stent surface may crack and fissure during stent expansion, thereby disrupting the oxygen discharge. Cracks in the coating may lead to an accumulation of charges at sites where the metal is exposed and invariably create dissimilar charges when different parts of the stent allow the flow of electrons from one direction to another, with a larger amount of charges actually resulting in erosion of that area and recruitment of inflammatory cells, which will impair healing.
Active stent coatings
Active coatings are designed to carry and elute drugs to reduce neointima formation, inflammation, and thrombosis. The only drugs identified to actively act biologically on neointima formation are paclitaxel and sirolimus with its analogues: everolimus, zotarolimus, and biolimus. These drugs are eluted from durable or biodegradable carriers that prevent neointima formation and restenosis. Heparin, which was evaluated as a passive coating, has also been studied as an active coating using a DES platform and was shown to be safe.17 The anti-inflammatory dexamethasone-eluting stent was found to be inferior in terms of reduction in neointima formation when compared with other drugs like sirolimus or paclitaxel.18 Coating with anti-proliferative drugs has proven to be highly effective against in-stent restenosis in clinical trials. Two types of polymers are used for drug elution: durable and bioabsorbable. First-generation DES used only durable polymers, which enabled slow and controlled drug release.1,2
Challenges with durable polymers for DES
Polymer coatings are essential for local delivery of the drug from the stent platform. When selecting a durable polymer for DES, it is critical to balance the hydrophilic and hydrophobic components of the system in order to obtain optimal biocompatibility while maintaining controlled drug elution. Permanent polymer platforms such as ethylene vinyl acetate/acrylate or isobutylated have been shown to trigger chronic inflammation and hypersensitivity reactions, which may contribute to the increased risk of stent thrombosis and progressive late catch-up of restenosis.8,19,20 The TAXUS family of stents uses poly (styrene-b-isobutylene-b-styrene), which elutes paclitaxel over a long period of time, while the Cypher stent uses a combination of polyethylene-co-vinyl acetate and poly-n-butyl methacrylate, which is found to be associated with hypersensitivity reactions and inflammation. Some of these polymers require primers like a parylene coating, which can also contribute to the inflammatory process. Other issues with durable polymers are in-homogeneity, webbing, and obstruction of side branches, swelling, and embolisation.
Second-generation DES attempted to overcome these limitations by providing thinner and more biocompatible polymer coatings. (Figure 1) For example, the BioLinx™ polymer used on the Endeavor® Resolute stent (Medtronic, Minneapolis, MN, USA) is a novel polymer that consists of a hydrophobic polymer for retention and drug release and a polyvinyl pyrrolidinone hydrophilic polymer used as an outer coating for drug elution. Enhanced monocyte adhesion was observed with polymers of a more hydrophobic nature, whereas hydrophilic polymers did not induce activated monocyte adhesion. The XIENCE V stent (Abbott, Abbott Park, IL, USA) also has a thin biocompatible durable polymer consisting of acrylic and fluorinated copolymer. These second-generation polymers were found to be more biocompatible and resulted in less inflammation when tested in animal models. A list of durable polymers is shown in Table 1.
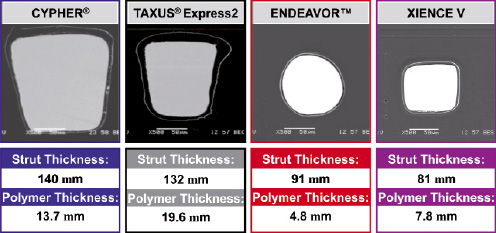
Figure 1. Evolution of DES from first generation to second generation. Note the decreasing stent struts, polymer, and drug dose.
Bioabsorbable polymers
By design, durable polymers act as a permanent barrier between the metal and the vessel wall and pose the risk of chronic inflammation, which may enhance local atherosclerosis, plaque rupture, and stent thrombosis. Bioabsorbable polymers, however, act as a temporary barrier; complete elution of the drug and complete absorption of the polymer leave behind a bare metal stent without the risk of late events often seen with durable polymers. Many factors impact degradation of the polymer, including the polymer size – lengthening the polymer chain increases the resorption time, thus long chain polymers and oligomers take longer to be absorbed compared to monomers. A decrease in hydrophobicity increases resorption time so less hydrophobic polymers take longer to be absorbed. Crystallinity also has an impact on degradation – semi-crystalline polymers have high mechanical integrity and longer resorption times, whereas amorphous polymers have limited mechanical integrity and shorter resorption times. The development of safe bioabsorbable polymers for DES is challenging since the absorption of the polymer can also trigger inflammatory reaction to the vessel wall.21 The principles followed in the development of biodegradable polymer technology are similar to those of durable polymers in terms of biocompatibility and thickness. Second-generation DES were developed with bioabsorbable polymers only on the abluminal side of the stent or by using wells or microdots to depot a mixture of drug and polymer to minimise the overall amounts of polymer and drug. (Figure 2) This approach maintains the DES efficacy with less exposure to the polymer and drug, thereby minimising the risks of inflammation and stent thrombosis. The polymers are targeted to bioabsorb within three to 12 months. The NEVO™ stent (Cordis Corporation, Johnson & Johnson company, Miami Lakes, FL, USA), which uses poly-lactic-coglycolic acid as its biodegradable polymer and sirolimus as its drug, showed superiority over the Taxus stent in the NEVO RES-ELUTION I (RES-I) trial and demonstrated an excellent safety profile.
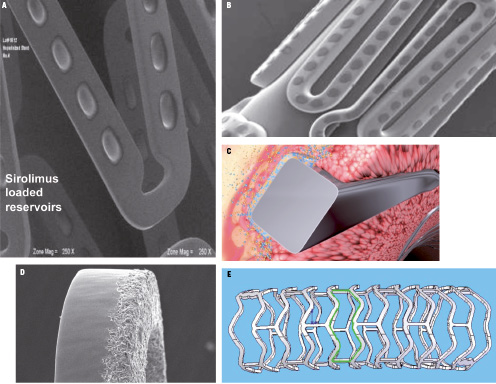
Figure 2. Evolution of DES absorbable polymer on A,B) reservoir technology, C) abluminal side, D) polymer-free, and E) fully bioabsorbable stent.
Currently there is no clinical proof that DES with biodegradable polymers are superior to DES with durable polymers. The only large clinical study, Limus Eluted From A Durable Versus ERodable Stent Coating (LEADERS), which compared the Biomatrix stent with an abluminal coating of poly-lactic acid bioabsorbable-polymer-coated with biolimus A9 to the Cypher stent did not demonstrate differences in overall cardiac event rates, including late stent thrombosis up to two years’ follow-up.22 Other clinical trials (Prospective, Randomised Trial of 3 Rapamycin-Eluting Stents With Different Polymer Coating Strategies For The Reduction of Coronary Restenosis [ISAR-TEST-3] and Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting STents [ISAR-TEST 4]), which examined both novel polymer-free and biodegradable-polymer DES compared to commercially available permanent polymer DES, also demonstrated non-inferiority of biodegradable-polymer DES when compared to DES systems with durable polymers.23,24 Among the other proposed novel bioabsorbable carriers is the bioerodible sol-gel film coated with low-dose paclitaxel. Shinke et al25 showed less toxicity to the coronary tunica media, while retaining effective inhibition of neointima formation at 28 days.
Challenges with bioabsorbable polymers
The concept of bioabsorbable polymers continues to attract physicians and scientists because of the potential to reduce the risks of durable polymers. The development of this technology, however, is associated with tremendous developmental and regulatory challenges. Perhaps the most important question to ask is whether the theoretical advantage of bioabsorbable stents over durable polymers will translate into clinical outcome differences. Among the challenges of the technology are the biocompatibility, composition, and formulation of the polymer, the biodegradable rate of the polymer, and the pharmacokinetics of the drug affected by the degradation of the polymer (potential burst of the drug as the polymer degrades). In addition, sterilisation and other biological environmental factors, including physical factors, can impact on the degradation.
The case for fully biodegradable stents
The concept of fully biodegradable stents was conceived on the premise that stents provide temporary scaffolding and prevent acute and late recoil, but their presence beyond these roles is associated with chronic inflammation and the potential for late thrombosis and restenosis. Also, metallic stents may cage the vessel if smaller sizes are selected and may impact vasoreactivity. Therefore, fully biodegradable stents can provide vessel restoration upon complete degradation in regards to endothelial coverage function, vasomotion, and elimination of stent thrombosis and late catch-up of restenosis. The advantages are lack of dependency on prolonged dual antiplatelet therapy, the vessel gets restored to its natural diameter, and vasomotion capabilities. Potentially, fully biodegradable stents can seal inflammatory plaques and enable the use of non-invasive imaging techniques, such as computed tomography angiography or magnetic resonance imaging without artefacts, as is the case with metallic stents. Finally, the use of biodegradable stents would not restrict any future percutaneous or surgical revascularisation, which is presently the case with DES treatment.
The indications for the use of fully biodegradable stents can be expanded to most coronary lesions in vessels >2.5 mm providing they will demonstrate a similar efficacy performance as DES. Attempts to achieve these results are thus far successful only with drug-eluting biodegradable stents with the bioabsorbable everolimus-eluting stent (BVS) system. This poses the question as to whether a non-drug-eluting fully biodegradable stent could be considered a viable workhorse device for the treatment of coronary artery lesions. Other applications for fully biodegradable stents could be for the treatment of superficial femoral artery (SFA) lesions and for below-the-knee lesions where metallic stents failed to reduce restenosis and are subjected to fractures. The paediatric application, although a niche, is attractive for this technology since metallic stents are not an option and balloon angioplasty is associated with high rates of vessel recoil.
Fully biodegradable polymeric stent systems
While all permanent stents are metallic, fully biodegradable systems are made of polymeric or metallic alloys. The leading polymer for the fully biodegradable stent is poly-L-lactic acid (PLLA). Several distinct forms of polylactide exist: PLLA is the product resulting from polymerisation of L,L-lactide (also known as L-lactide) and has a crystallinity of around 37%. The degree of crystallinity can be modified and plays an important role in defining the radial force and recoil of the stent. The Igaki-Tamai® stent (Igaki Medical Planning, Kyoto, Japan) was the first biodegradable stent to undergo clinical evaluation.26 Made of PLLA monofilament, it has a strut thickness of 0.17 mm. It has two radio-opaque gold markers, and delivery balloon inflation is performed with a heated dye at 80°C. The percent diameter stenosis decreased from 64% before stenting to 12% after stenting. The percentage of acute stent recoil was 22±7% by quantitative coronary angiography (QCA). The initial clinical experience with this stent without drug elution was acceptable and comparable to bare metal stents. Serial intravascular studies suggested favourable remodelling of the stented segment over time. In a 4-year follow-up study of a patient from the initial study, the stent was not visible and the lumen was patent without any adverse findings by angiogram and intravascular ultrasound (IVUS).27
The BVS everolimus-eluting stent backbone is composed of circumferential hoops of highly crystalline poly-lactic acid (PLA), which allows the stent to achieve the radial strength of the MULTI-LINK VISION® metal stents (Guidant, Santa Clara, CA, USA). Two adjacent radio-opaque metal markers mark both ends of the stent. The surface contains a thin coating of a 1:1 mixture of everolimus 8.2 µg/mm and amorphous PLA matrix, allowing 80% of the drug to be released in 30 days. This is similar to the release patterns of the XIENCE V and Cypher stents. The stent is bioabsorbed in approximately 24 months. The feasibility and safety of this stent were examined in humans in the “A bioabsorbable everolimus-eluting coronary stent system” (ABSORB) trial for patients with single de novo coronary artery lesions.28 The acute recoil by QCA was nearly 7% and the clinical outcome reported up to two years, including serial IVUS evaluation, did not detect stent struts at two years’ follow-up with tendency of the vessel to return to its normal dimensions and preservation of the vasomotion capabilities.29 Another polymer used for fully biodegradable stents is the salicylate-based polymer, which was studied in preclinical trials and demonstrated a favourable vascular compatibility and efficacy in a porcine coronary artery model.30
Bioabsorbable metallic stents
Among the metallic alloys proposed for fully biodegradable stents are iron, which was studied only pre-clinically and was associated with very slow degradation, and magnesium, which was initially studied by Heublein.31 These studies detected very fast degradation within 3-4 months with no adverse events related to the stent, but demonstrated a high degree of recoil and neointima formation that have led to higher than expected recurrence rates.32 In the Clinical Performance and Angiographic Results of Coronary Stenting (PROGRESS-AMS) study, the elastic recoil was 7% and in-segment diameter stenosis after four months was 49%. The ischaemia-driven target lesion revascularisation rate was 23.8%. For patients who did not restenose at four months, a durable result was seen at a later time points.33
Challenges with bioabsorbable stents
Fully bioabsorbable stents pose several serious challenges that are critical to the viability of these stents for clinical use. It is important to understand how long the radial force of the stent is required to the vessel undergoing PCI and whether a drug is essential to obtain acceptable patency rates with this technology. The degradation rate post-implantation can determine radial force and the degree of recoil while the biocompatibility of the polymer/metal and the inflammation associated with the degradation will determine whether this technology will be dependent on a drug to obtain comparable patency rates to DES. If a drug is essential, the degradation kinetics may impact on the drug pharmacokinetics. In addition, collecting information on the biodegradable products and the remaining polymer and metal left in the vessel would be required, as they can impact on the degree of inflammation. Other issues related to this technology relate to the visibility of the polymer or to the magnesium alloy under fluoroscopy since both are radio-lucent and will require markers. For SFA and larger vessels, self-expanding materials should be considered and, finally, some of these stents will require special storage conditions and may have shorter shelf lives.
Regulatory considerations for biodegradable polymers and biodegradable stents
Fully biodegradable stents are considered a combination device and will require extensive bench testing and preclinical assessment. The following questions will need to be addressed: What is the mechanism of biodegradation? What are the degradation products and what are their biologic activities? How does the proposed biodegradable stent balance the need for mechanical integrity with the ability to degrade over time?
Preclinical trials will be similar to those of performed with DES and will have to include long-term follow-up at least 180 days prior to initiation of a feasibility trial and a longer follow-up duration, which will determine the complete degradation of the metal or the polymer. The pre-marketing application will have to include a large clinical experience with ≥2000 patients that will get the device either in registries or randomised clinical trials. The comparative group and the study endpoints must also be determined. While fully biodegradable stents seem to be superior to bare metal stents in terms of efficacy, it is not clear if this will be the case when they are compared to DES.
Conclusion
Following PCI, the injured vessel requires scaffolding; however, there is no consensus on how long this scaffolding is required. The currently available DES have proved their capability to provide permanent scaffolding and prevent in-stent restenosis. However, the permanent polymers used in these stents became the grounds for the long-term safety issues raised. These concerns have led to improvements in DES with the use of biocompatible and biodegradable polymers. The limitations of these polymers, and the fact that all these devices will leave a permanent metallic stent, provided the development thrust of biodegradable stents, which provide only temporary scaffolding. This technology is in its infancy and significant hurdles do lie ahead. Because this technology is in its infancy, for the moment bioabsorbable stents are far from their goal of replacing bare metal or DES.

